Abstract
The purpose of the paper is to illustrate the basis of the design of a pilot-scale reactor built to convert putrescent and high-water content biowaste into a stabilized product by using the Hydrothermal Carbonization process (HTC). The hydrothermal carbonization of selected biowaste has been previously studied in a bench-scale reactor to optimize the process parameters such as the temperature, reaction time, water-to-dry matter ratio and then scaled up at a scale 30 times larger. The new pilot-scale reactor has a volume of 0.1 m3 and has been designed and certified to be operated at 300 °C and 86 bar, allowing a wide range of operating conditions. The design has been structured in two steps: process design (a) and mechanical design (b). The main results of the process design step have been: the installed heat power, the method to provide and control the heating, the minimum reaction time necessary to reach a given yield. The mechanical design focused on the scalability of the reactor, the extraction of reaction products from the reactor at the end of process and increasing of reliability and safety. The designed reactor has been then built, commissioned, and operated in such a way to validate the design criteria and hypotheses. The comparison between the experimental results and the design input dataset confirmed the correctness of the design data input but highlighted that the thermal efficiency of the pilot scale plant was low so indicating the need to enhance it for the demonstrative plant.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Statement of Novelty
The research lies in the field of biowaste treatment. The paper is focused on a new pilot plant design that aims to validate the technology and demonstrate the scaling-up potential at industrial scale.
Introduction and Scope
The world energy demand is forecasted to rise up to 22.7 Gtoe by 2040 [1] with the largest increment achieved in Asian countries. The search for reduction of the greenhouse effect and the policies focused on targeting Earth’s temperature increment up to 1.5 °C in 2050, [2] are the key commitments made by many Countries of the World (COP21 and so on). Alternative and more efficient sources of renewable energy are sought to cope with the increasing energy demand and population [3]. If not adequately tackled and surpassed these issues would lead to irreversible loss of most fragile ecosystems and severe concerns for the most vulnerable people and societies.
Bio-based waste represents an important fraction of the waste produced daily both by domestic and industrial activities. The consistent fraction of water contained in such waste like those produced from household kitchen and restaurants, food and beverage industries, sludge from wastewater plant treatment, etc. does not allow to treat this kind of waste in conventional incinerators or used for straightforward energy recovery. The use of biological processes is extensively applied in Europe to treat bio-waste; anyway, all of these processes (composting, anaerobic digestion and mechanical–biological treatment) produce emissions mainly limited to GHG and volatile organic compounds. Mechanical–Biological Treatment (MBT) produces emissions into air that are similar to composting or anaerobic digestion and produces an end product usually contaminated to a such level which hinders its further use [4]. Nevertheless, these techniques have the advantage of purifying the combustible fraction for incineration with energy recovery or mineralise the organic fraction for a safe landfilling. The cited environmental burdens, the increasing economic costs of these biological-based processes together with the intense use of land and the negative social perspective are drivers towards different technologies that guarantee better performances. It is a very severe environmental social and economic problem in both developed and developing Countries, accounting for a production of over 2 Gton/year in 2016 and expected to soar to about 3.4 Gton/year in 2050 within a Business as Usual scenario [5].
The correct management of this waste is a critical issue in the waste system: its landfilling increases the global warming potential by producing methane and carbon dioxide and it has been prohibited in many developed Countries or strongly discouraged; the energy recovery requires a preliminary drying step to remove the excess of moisture (usually larger than 70%), resulting in a negative energy balance; biochemical processes do not require any drying but yields of conversion are very limited, with a corresponding low mass reduction and long processing times. Anaerobic digestion, a bio-technique that involves biowaste fermentation in controlled anaerobic bioreactors, for example, converts around 12–14% of substrates into biogas while the remaining part of the mass needs to be treated with high economic cost to be mineralised [6].
Emerging trends in this field are gaining increasing researchers’ attention. The biocarbon production by HTC is one of the possible alternatives, recently regulated in the framework of End-of-Waste procedure [7]. Hydrothermal carbonization (HTC) offers several advantages, such as: minimization of nasty odour dispersion, destruction of pathogens that can cause crop, soil and water contamination, production of a carbonaceous material, known as hydrochar, HC for short, that can be used to replace soil amendment and/or fertilizer [8, 9].
HTC is a thermochemical process in which biomass is converted into hydrochar by several different steps with mild reaction conditions at low temperatures (180–250 °C), absence of oxygen and sub-critical water conditions, under saturation pressure for several hours. The HTC process is convenient when biomass is wet since it can carbonize substrate with a water content up to 90% by weight without prior drying, which results in an expensive and long process [10,11,12]. In the HTC process, water plays a key role for both physical and chemical reasons: It promotes the heat transfer throughout the bulk of biomass during the process and it is essential for the cleavage of chemical bonds of the feedstock. Chemical dehydration is another exothermal step which decomposes carbohydrates. In the decarboxylation reactions the carboxyl and carbonyl groups (radicals) are degraded, at temperatures around 150 °C. Other typical reaction mechanisms that take place in the HTC process are polymerization and aromatization, the last one can takes place for high temperatures. Overall, the exothermal steps are important in the HTC process, but the heat release does not seem sufficient for overcoming the heat losses from the reactor to the ambient [13]. Thus, the appropriate heating system is required in order to attain the set-up temperature. In addition, the reduction of heat losses is obtained by using a proper insulation system [14, 15].
Many studies focused on the hydrochar phase, but liquid and gas products have received in the past years limited attention. These by-products require more detailed analyses for a better understanding of the whole hydrothermal process in relation to the hydrochar formation. The liquid products can be generally treated by wet oxidation [16] or recovered for production of biogas (methane) and fertilizer [17,18,19]. In particular, the post-treatment process can be essential for recovering nutrients such as Nitrogen and Phosphorous [20].
The solid product is very interesting because it can be used to produce high added-value products such as solid fuels, activated carbon, carbon-based catalysts and other useful carbonaceous materials, and it can also be used as boost for biogas production [8, 21,22,23]. Several possible uses of HC are as, among others, soil improver, environmental absorber, dry solid fuel [24]. The resulting aqueous fraction (bio-oil or HTC liquor) is rich in organic acids, among others acetic, formic, levulinic, and glycolic acid, and Hydroxy-methyl-furfural showing high value of total organic carbon (TOC). The handling and disposal of this liquid may outweigh the advantages of the HTC process from an economic and environmental point of view. The combination of aqueous-phase recirculation and its use as a fertilizer can be an appropriate method to reuse the liquid phase and return nutrients to support plant growth, thus increasing the HTC efficiency and economic feasibility.
Recently, Ischia and Fiori, deployed a review on HTC plant and reactor modeling [25]. A thorough description of the kinetic mechanism is reported in the paper, together with a heat transfer model. More recently, Zhuang et al. [26] provided a comprehensive overview with the aim to highlight the actual knowledge of the technical mechanisms, application advantages, and economic benefits of hydrothermal carbonization.
Several HTC reactors have been built, the vast majority in lab or bench scales, as it can be found in [27]. Very few attempts were focused on large, pilot or industrial level, reactors. To the authors’ knowledge, very few HTC reactors are now in operation at an industrial scale. One with the longest operational time started since 2016, is the plant of TerraNova® [28], with a daily capacity of 1.1 tons per day of sludge.
The yield of carbonaceous products is affected by several process parameters. Reaction temperature and residence time are the main process parameters affecting the yield together with the feedstock composition, whereas reactor pressure and water/biomass ratio show weak impact on the process. Reaction temperature and residence time comprise the severity index, which is a main factor influencing the process [13]. The purpose of the paper is to illustrate the basis of design of a pilot-scale HTC reactor built with the aim to convert putrescent and high-water content biowaste into a stabilized product; the process is intended to be realized as a final treatment process for recovery of material (hydrochar) or as a pre-treatment before the disposal, depending on the properties of the biowaste itself. The design, development and tests for checking the compliance of the reactor to the project are reported in this paper. The design has been driven by the need of an easy-to-use reactor, for both operation and maintenance, and mechanically enough reliable to avoid even small leakages and odour release as well as an easy scale-up of the plant. The single unit has been therefore designed and realised by looking at modularity as possible scale-up method. The unit is equipped with a monitoring and control system able to keep the reactor at the desired temperature by modulating the heating, no cooling system is installed. The preliminary tests carried out with biowaste are reported and analysed in order to verify the effectiveness of the reactor design criteria and provide for the data need for the demonstrative scale-up.
Scope of the HTC Research and the Related Applied Methodology
The project started with the basic studies about hydrothermal carbonization of a selected biowaste carried out on a bench scale apparatus. The possible applications of the process were: (a) the inertization of biologically active contaminated biowaste (such as sludges) prior the final disposal (landfill/incineration) and (b) the hydrochar production for agricultural purposes starting from clean biowaste such as kitchen waste, food, digestate (intermediate of anaerobic digestion plant), etc. The main scope of the HTC research to which this paper refers is the pre-treatment of high moisture content biowaste with the aim to transform it into a product with high carbon content, low nitrogen content, with a substantially hydrophobic behavior and having a low moisture content (< 30%).
The research plan has been developed by aiming to a specific target: building a prototype to demonstrate the feasibility of the application as a suitable pre-treatment of many types of biowaste. Virtually, all the biowaste with a high-water fraction can be stabilized to obtain a biofuel or a non-hazardous waste. In this latter case, the inertization impedes releasing odors during storage/transportation/landfilling, promotes high bulk density, increases the water removal efficiency, sequestrates the carbon in a mineral matrix, etc.
The sequence of steps that characterized the research development is sketched in the Fig. 1. The step to which the paper refers is the Development one. More specifically, the results of the commissioning task are presented with the scope to move forward to the Industrialization phase, after the validation and optimization of plant design.
The Bench-Scale HTC Apparatus and Experimental Results
The experimental campaign carried out using the bench-scale apparatus aimed to investigate the effect of operating parameters on the HTC products and obtain useful information to proper design the pilot-scale HTC system. The bench-scale apparatus used for the experimental tests is composed of an externally heated stirred batch reactor having a reaction volume of 3 L. A more accurate description of the bench-scale apparatus can be found in [29]. The feedstock utilized for the experimental tests was digestate obtained by anaerobic digestion of food and kitchen waste (biowaste). The experimental tests were carried out by modifying the water-to-dry feedstock ratio, the temperature of the reactor and the reaction time. After each test, samples of remaining solid (hydrochar), liquid and gas phases were characterized. The composition of the biowaste and the results obtained with the HTC bench-scale apparatus were published in [11, 29].
Methodology
The combined effect of time and temperature of the process is indicated by the severity factor f, defined as in [10]:
The R value is defined as the ratio between the mass of water used in the process and that of the dry feedstock. Thus, it reads as:
The reactor experimental design has been also verified in terms of the efficiency of the thermochemical conversion. For this reason, typical yield indices have been calculated.
The efficiency of conversion is reported by the mass yield (MY) which reports the mass of the hydrochar and feedstock, both dried, Eq. (3)
The carbon yield (CY) is focused only on the carbon content of the mass involved, Eq. (4). It is an essential physical quantity since the HTC process is designed to increment the carbon content of the processed substrate.
Related to the mass yield, the energy yield is written in Eq. (5):
where feed is the initial feedstock and HC represents the hydrochar quantities. HHV are the higher heating values of the two matters.
Gas Analysis
The gas produced during the HTC process is analysed with an Agilent 490 Micro gas chromatograph equipped with two capillary columns. A MolSieve 5A column is used to separate H2, O2, N2, CH4 and CO; the injector and column temperature are respectively set at 90 °C and 110 °C with argon as gas carrier. A Poraplot Q column is used for CO2 and C2–C4 hydrocarbons measurements; the injector and column temperatures are set at 90 °C and 85 °C, respectively and helium is the gas carrier.
TKN Method
The analysis is carried out through three phases of the Kjeldahl method: digestion, distillation and titration, as reported in the Standard Methods [30]. A volume of 20 mL is used for each sample and inserted inside the cuvette. No preliminary filtration or dilution is performed. Digestion of the samples takes about 4 h. Over this time interval, the temperature is gradually raised until reaching the maximum value of 350 °C. The progressive increase in temperature is performed to avoid the formation of foams. At the end of the 4 h, the cuvettes are cooled and used for the subsequent distillation step. By adding deionized distilled water, the digested sample was diluted, furthermore with the addition of sodium hydroxide (35%) the pH is raised to favour the complete conversion of NH4+ into gaseous NH3. The boric acid solution containing the distilled ammonia is automatically titrated until reaching a pH 4.7.
TOC Method
The samples are analysed by using the Shimadzu TOC-L instrument equipped with an autosampler. The samples to be analysed are preliminarily diluted according to 3 different dilution ratios (1:200, 1:500, 1:1000) and subsequently inserted into the vials for the determination of the TOC. The method employed by the instrument is a 680 °C combustion oxidation with a non-dispersive infrared detection (NDIR) method.
Ultimate Analysis
The elemental composition, i.e. the mass fraction of carbon, hydrogen, nitrogen, sulphur and oxygen (evaluated by difference), of the reaction mixture and hydrochar are determined using a LECO CHN-S 628. The measurement method is based on the complete and instantaneous oxidation using pure oxygen (dynamic flash combustion) of the samples with their conversion into gaseous products. Their quantitative estimation is obtained either by non-dispersive IR or thermal conductivity cells.
The Pilot-Scale Apparatus: Bases of Design
Design Criteria and Input Data
The pilot-scale reactor has been designed by considering the following input data and requirements:
-
(a)
use of optimised set of operating conditions as obtained from the experimental campaign on the bench-scale;
-
(b)
avoiding the mechanical mixing to minimise the gas leakage risk
-
(c)
utilising an external heating with accurate, fast and easy control of the internal reactor temperature;
-
(d)
favouring the scale-up based on modularity instead of increasing the vessels’ volume;
-
(e)
minimization of site footprint;
-
(f)
targeting to easy and low-cost maintenance.
The reactor has been sized on the basis of the biowaste data reported in Table 1; the reference biowaste is a mixture of the putrescible waste known as “kitchen waste”, coming from the urban separate collection, and the sludge resulting from the wastewater biological treatment.
The operating conditions that can be utilised in the pilot-scale reactor are summarised in the Table 2. It is noteworthy that the operating conditions are conceptually different by the design conditions (300 °C, 86 bar); these latter are the values at which the mechanical resistance of the reactor is guaranteed without risk of the structure collapse for a given time; the operating conditions refer to the range of values at which the process is carried out.
The most important operating parameters that characterises the batchwise process are: the reaction temperature, the transient heating time, the reaction time and, only for industrial application, the number of cycles per day (24 h) for each reactor and the down-times such as that necessary for reactor’s emptying after the completion of the process as well as its cleaning and filling time.
Hydrodynamic Assumptions
A batch reactor has been chosen instead of a continuous one in order to increase the flexibility in term of reaction time and input characteristics and reduce the risk of leakage. In fact, it is not easy find in the market reliable sealing systems working properly with an acceptable lifetime in a mid-temperature and high-pressure environment. The same reasons suggested to avoid the installation of mechanical agitator; this choice has advantages as well as drawbacks. The absence of mixing was expected to induce a segregation of reacting solids toward the bottom of the reactor with the establishment of a vertical gradient of concentration; moreover, the absence of mixing reduces the heat transfer rate in both vertical and radial direction. The segregation at bottom of the heavier fraction such as inert and the floating of lighter fraction such as foams is unavoidable, as well as the non-uniformity of temperature and matter concentrations. The goal of the next optimization task is the reduction of the axial gradients before the industrialization phase. In order to avoid negative effects of segregation the mixture between reactive solids, inert material and water must be as much uniform as possible, and this condition has been reached by using a pulper. The uniformity and the establishment of a stable mixture between the solids and the water medium would allow neglecting the negative effects of the segregation of reacting matter. Similarly, to the above cited stratification of solids that can be established in absence of mixing, the heat transfer rate from the walls to the centreline of the reactor is poor. Thus, the internal diameter of reactor has been maintained enough small to obtain an acceptable nearly uniform radial temperature profile.
Description of the Pilot-Scale HTC Plant and Its Scale-Up to Full-Scale
The HTC plant is basically composed of a 0.10 m3 cylindrical batch reactor and a 3 m3 condenser (Fig. 2). The reactor, made of AISI 316 L, is heated-up by three sets of electrical heating elements indicated as Rs1, Rs2 and Rs3. Each heating element is composed of three ceramic insulated band heaters wired in parallel. The heating element Rs1, located at the bottom of the reactor, i.e. in correspondence of the dense phase of the reaction mixture, has a total power of 8.1 kW (3 × 2.7 kW). Instead, the heating elements Rs2 and Rs3, placed respectively in the middle and upper section of the reactor, have a power of 6 kW each (3 × 2.0 kW). The nominal power of the heating system is 20.1 kW. Each heating element is equipped with a type K thermocouple (TC R1-3) and an ON/OFF temperature controller for the setting of the set point temperature (Table 3).
The unit reactor has been then designed enough small in diameter to limit the extension of radial temperature profile, so considering the process rate nearly uniform along the radius. The non-uniform axial profile of solids fraction and density as well as that of temperature is expected due to the absence of mixing. The choice to avoid the mixing is due to a specific target to minimise the odour release that was detected during the past experimental campaign with the stirred lab-scale reactor. The mixing can be obviously added in a second step if considered necessary based on the results of tests in term of hydrochar quality, non-uniformity of the produced hydrochar, etc. Anyway, the target is proposing and validating a new technological concept, different from that already present in the market, that allows to mineralise the organic carbon of high-water content biowaste in a sustainable way, where sustainable means low economic cost, low-emission (even diffuse) and highly safe.
The reactor top ends with a 4 inches’ flange which allows the feedstock charging. Above the flange, on a 4 inches’ manifold, a 2 inches’ valve for the gas exit (MV1), a safety valve and a pressure sensor (PT) are located. On the bottom of the reactor is positioned a 4 inches’ valve (MV2) for the discharge of hydrochar and liquids produced.
The headspace at the top of the reactor, that varies between 10 and 20% of the reactor volume, hosts the gases generated during the process and the vapor in equilibrium with the liquid water; its value affects the total pressure in the reactor. Once the hydrothermal process is completed, the valve installed on the top opens by releasing gas and steam to the condenser where the pressure is 1 bar at beginning of the reactor emptying process. The evaporation process continues until the equilibrium at the new process pressure is reached. The experimental texts showed that about 45% of the initial mass of water is discharged with the hydrochar by opening the valve located at bottom of the reactor. Then, the wet hydrochar is dried thanks to use of a dewatering process (filtering under pressure).
The condenser includes two internal cooling coils, in which cold water is circulated to accelerate the condensation of the hot vapours coming from the top of the reactor. The condenser is equipped with two type K thermocouples and an analogic manometer for temperature and pressure monitoring. On the top of the condenser is positioned a valve for gas sampling/discharge and at the bottom a valve for the sampling/discharge of condensate.
The HTC full-scale plant has been conceptually designed as a parallel configuration of a certain number of single reactor units installed in a unique heating case constituting a module. The module would contain x reactor units and can treat about x*0.090 m3 of water-biowaste mixture. Each module has its own dedicated systems for filling/extraction, heating and temperature control, condensation of steam, gas exhaust, safety and control. The filling section of the module will be connected to the outlet of a pulper, where a homogenised water-biowaste mixture is prepared, through a conveying pipe while the humid hydrochar will be removed by connecting the bottom outlet of each module to a dedicated vacuum system. The treatment capacity can be increased by adding new modules without modifications of auxiliary systems and piping except those related to the feeding of water-biowaste mixture, removal of hydrochar and water from the condenser. This configuration allows to consider the plant as modular, strongly simplifying the scale up design and the flexibility of operations with different biowaste. The reactor unit has been then made and tested to verify the data related to the HTC process; in a second step, a full module will be accomplished to test the efficiency of heating system and of the filling/extraction sections.
Experimental Procedures and Results
Commissioning Procedure
The commissioning has been accomplished in two steps:
-
(a)
tests with pure water at 260 °C, carried out in order to verify the perfect sealing of the connections, gaskets, flanges, etc.
-
(b)
tests carried out with a mixture of sludge produced in a wastewater treatment plant and water at a given ratio (R) and set of operating conditions, namely reaction time and temperature, i.e. the severity factor f.
The tests have been carried out to confirm, or not, the hypotheses made during the design procedure. In particular, the heating time, the reaction time, the vertical concentration profile and the mass balance have been verified.
In the following paragraphs, the tested data have been reported and compared with the design data.
Experimental Results
Two different tests were run to check the assembly of the reactor.
The first test (a) used water as the substance inside the reactor to verify the sealing, the heating system and the safety valve for the pressure threshold. The second test employed sludge as substrate for testing the development of the hydrothermal carbonization process inside the reactor.
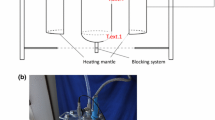
The water and resistance temperatures as a function of the time are reported in Fig. 3, for the K2 thermocouple and the surface resistance thermocouples R1, R2 and R3, together with the average temperature value of the last three thermocouples, indicated as TC Rave. The K2 temperature evolution with time is nearly linear, indicating a typical constant heat flux imposed on the boundaries of the fluid inside the reactor. The temperature of the electric resistances increases fast in the first part of the heating time, up to twenty minutes, with a time gradient of about 15 °C/min, then the increment slows down with a temperature variation of about 1 °C/min after about 40 min when its temperature attains 180 °C. This is due to the increase of resistance temperatures which implicates an increment of the heat losses toward the environment, both as convective and radiative heat transfer modes. The water temperature displays a time increment nearly constant of around 1.5 °C/min. It can be observed that the three resistance temperatures show different values, with maximum thermal gradient of about 50 °C up to 60 min from the starting of the process. Then, the temperature differences reduce attaining close values when the heating time is attained. The average temperature of the three resistance thermocouples TC Rave resembles the TC R2 time evolution. The maximum pressure attained in the reactor is equal to about 50 bar.
HTC experimental test type (b) was carried out using a reaction mixture composed of dried municipal wastewater sewage sludge and water. The feed was prepared in order to obtain an R value of 11. The test was conducted fixing the set point temperature at 260 °C for 6 h at steady state conditions, with a severity index f = 0.457. The reactor autogenous pressure stabilized at about 50 bar (Table 4).
Temperatures as a function of the time are reported in Fig. 4. The recording time of the temperature evolution is displayed when the value of TC K2 attains 220 °C, which occurs about 1.5 h from the start of the heating time. The temperature evolution is displayed for a time interval of six hours. The thermocouple K2 shows large temperature value than the set point temperature after about one hour. This can be ascribed to the exothermal reactions occurring in the bulk of the sludge. Instead, the thermocouple K3 located in the gas phase shows smaller values than the set point. This occurs because of the larger heat dissipation towards the ambient. This occurrence indicates that the temperature set point control method should be improved.
Table 5 reports the ultimate analysis and the ash content on dry basis of the reaction mixture utilized for the HTC test. The ultimate analysis shows that the reaction mixture has a carbon content of 33.2% while the oxygen amount is about 13.2%. Lower amounts of hydrogen and nitrogen are found. The inorganic fraction (ash) content of the mixture adds up to 45.7%.
The operating parameters selected for the HTC test affect the characteristics of the obtained hydrochar. In particular, the ultimate analyses of the reaction mixture and of the produced hydrochar (Tables 5 and 6) show that the contents of carbon, hydrogen, nitrogen and oxygen decrease of 12.1, 13.9, 57.2 and 71.7%, respectively. On the other hand, the ash fraction rises of 33.7%.
This behavior is due to the development of the HTC reactions which lead to the breaking and the reorganization of the chemical bonds of the starting feedstock. This process causes the migration of organic elements from the solid fraction to the liquid and gaseous phases and, consequently, the depletion from hydrochar of carbon, hydrogen, nitrogen and oxygen. At the same time, due to its refractoriness to the reaction environment, the inorganic fraction of hydrochar increases [31, 32].
Table 7 reports the mass, carbon and energy yields of hydrochar (as defined in Eqs. 3–5). The results indicate that the 75% of the reaction mixture is converted into hydrochar and that it preserves the 66% of the carbon content and the 67% the feedstock energy. These results are consistent with those obtained in the experimental tests with the bench-scale reactor by processing a similar biowaste [32].
A sample of liquid produced during the HTC test was collected from the bottom of the reactor and characterized by determining the Total Organic Carbon (TOC) and the Total Kjeldahl Nitrogen (TKN) (Table 8). The results indicate that the TOC is about 66,000 mg/l and that the TKN is about 1260 mg/l, confirming that a significant amount of carbon and nitrogen moves from the solid starting feedstock to the liquid phase. This liquid phase is a mixture of water and oxygenated hydrocarbons similar to bio-oil but with a massive dilution in water.
The main gaseous compound produced during the HTC test is CO2, as reported in Table 9. Other gases generated in lower quantities are CO, H2, CH4 and light hydrocarbons, with CmHn, representing the sum of traces of C2–C4 hydrocarbons.
Conclusion
This work is included in the framework of a technology development program based on the Hydrothermal Carbonization reactor design. The paper refers mainly to the Commissioning phase results of a pilot-scale reactor.
The data previously obtained at lab-scale size have been used for the design of this reactor.
The Commissioning phase allowed the demonstration of the process reliability and scalability as well as the check of the design criteria.
The pilot-scale unit is a batch-wise reactor having a volume enough small to avoid the mechanical mixing and a tubular geometry allowing an almost uniform temperature profile along the radius of the reactor, while the axial temperature profile is expected to occur.
The Commissioning tests demonstrate that in about 6 h after the attainment of the set point temperature, hydrochar yield of 75% is obtained with a feedstock energy yield of about 67%. The carbon dioxide represents about 90% of the produced gases.
The total processing biowaste time, also considering the feeding and discharge phases, takes about 8 h processing about 20 kg of biowaste per each reactor unit. This could allow to operate 3 cycles per day for each unit, processing a total of 60 kg of biowaste per day.
The experimental data have been compared with the design input data set. Operating conditions such as temperature and pressure attained in the reactor, and mass and energy parameters such as yields of gas, liquid and hydro-char and absorbed power, are in good agreement with the design dataset. The main disagreement is related to the heating time. The heating time resulted larger than the expected one due to the poor bottom insulation that is negatively affected by the system for hydrochar retrieval. As expected, an axial temperature profile occurs. Thus, in the upgraded version of the reactor this occurrence will be modified by using a heating system divided into the three modules, each of which separately controlled by a dedicated monitoring and control system.
The experimental dataset obtained by the commissioning tests confirmed the process design criteria and suggested how to optimise the efficiency of the system by an energetic point of view.
Data Availability
None.
Code Availability
None.
Abbreviations
- CY :
-
Carbon Yield, Eq. (4)
- EY :
-
Energy Yield, Eq. (5)
- F :
-
Severity factor, Eq. (1)
- HC:
-
Hydro Char
- HHVHC (kJ/kg):
-
Higher Heating Value of HC
- HHVfeed,dry (kJ/kg):
-
Higher Heating Value of dry feedstock
- HTC:
-
Hydrothermal Carbonization
- m feed,dry (kg):
-
Dry feedstock mass
- m HC (kg):
-
Hydro Char mass
- m H2O (kg):
-
Water mass
- MY :
-
Mass Yield, Eq. (3)
- P (bar):
-
Pressure
- R:
-
Water-to-dry mass ratio, Eq. (2)
- t (min or h):
-
Time
- T (°C):
-
Temperature
- TC:
-
Thermocouple
References
Kahan, A.: EIA projects nearly 50% increase in world energy usage by 2050, led by growth in Asia, https://www.eia.gov/todayinenergy/detail.php?id=41433 (2019). Accessed Nov 2021
IPCC: Global Warming of 1.5°C. An IPCC Special Report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change (2018)
Sharma, S., Basu, S., Shetti, N.P., Kamali, M., Walvekar, P., Aminabhavi, T.M.: Waste-to-energy nexus: a sustainable development. Environ. Pollut. (2020). https://doi.org/10.1016/j.envpol.2020.115501
Unlocking new VALUE from urban bioWASTE – VALUEWASTE Project. Grant agreement number 818312. Ref. Ares (2019) 4112037 - 28/06/2019
Kaza, S., Yao, L., Bhada-Tata, P., Van Woerden, F.: What a waste 2.0. A global snapshot of solid waste management to 2050. World Bank Publications, Washington (2018)
Mastellone, M.L.: Waste Management and Clean Energy Production from Municipal Solid Waste. Nova Science Publ, New York (2015)
UNI 11853:2022. Specifications of hydrocahr prepared from hydrothermal carbonization treatment (HTC) of residues from municipal wastewater treatment plants or industrial sludge of organic matrix. UNI - Italian National Unification Body (2022)
Ischia, G., Fiori, L., Gao, L., Goldfarb, J.L.: Valorizing municipal solid waste via integrating hydrothermal carbonization and downstream extraction for biofuel production. J. Clean. Prod. (2021). https://doi.org/10.1016/j.jclepro.2021.125781
Lucian, M., Volpe, M., Merzari, F., Wüst, D., Kruse, A., Andreottola, G., Fiori, L.: Hydrothermal carbonization coupled with anaerobic digestion for the valorization of the organic fraction of municipal solid waste. Bioresour. Technol. (2020). https://doi.org/10.1016/j.biortech.2020.123734
Wang, T., Zhai, Y., Zhu, Y., Li, C., Zeng, G.: A review of the hydrothermal carbonization of biomass waste for hydrochar formation: process conditions, fundamentals, and physicochemical properties. Renew. Sustain. Energy Rev. (2018). https://doi.org/10.1016/j.rser.2018.03.071
Mastellone, M.L., Zaccariello, L., Lotito, R., Battaglia, D.: An experimental study on hydrothermal carbonization of anaerobic digestion residue. In: Sardinia Symposium 2019 - 17th Int. Waste Management and Landfill Symposium, p. 6 (2019)
Scrinzi, D., Bona, D., Denaro, A., Silvestri, S., Andreottola, G., Fiori, L.: Hydrochar and hydrochar co-compost from OFMSW digestate for soil application: 1. Production and chemical characterization. J. Environ. Manag. (2022). https://doi.org/10.1016/j.jenvman.2022.114688
Funke, A., Ziegler, F.: Heat of reaction measurements for hydrothermal carbonization of biomass. Bioresour. Technol. (2011). https://doi.org/10.1016/j.biortech.2011.05.016
Pecchi, M., Patuzzi, F., Benedetti, V., Di Maggio, R., Baratieri, M.: Thermodynamics of hydrothermal carbonization: assessment of the heat release profile and process enthalpy change. Fuel Process. Technol. (2020). https://doi.org/10.1016/j.fuproc.2019.106206
Ischia, G., Cazzanelli, M., Fiori, L., Orlandi, M., Miotello, A.: Exothermicity of hydrothermal carbonization: determination of heat profile and enthalpy of reaction via high-pressure differential scanning calorimetry. Fuel (2022). https://doi.org/10.1016/j.fuel.2021.122312
Reza, M.T., Freitas, A., Yang, X., Coronella, C.J.: Wet air oxidation of hydrothermal carbonization (HTC) process liquid. ACS Sustain. Chem. Eng. (2016). https://doi.org/10.1021/acssuschemeng.6b00292
De la Rubia, M.A., Villamil, J.A., Rodriguez, J.J., Borja, R., Mohedano, A.F.: Mesophilic anaerobic co-digestion of the organic fraction of municipal solid waste with the liquid fraction from hydrothermal carbonization of sewage sludge. Waste Manag. (2018). https://doi.org/10.1016/j.wasman.2018.02.046
Pagés-Díaz, J., Cerda Alvarado, A.O., Montalvo, S., Diaz-Robles, L., Curio, C.H.: Anaerobic bio-methane potential of the liquors from hydrothermal carbonization of different lignocellulose biomasses. Renew. Energy (2020). https://doi.org/10.1016/j.renene.2020.05.025
Mau, V., Neumann, J., Wehrli, B., Gross, A.: Nutrient behavior in hydrothermal carbonization aqueous phase following recirculation and reuse. Environ. Sci. Technol. (2019). https://doi.org/10.1021/acs.est.9b03080
Huang, R., Fang, C., Zhang, B., Tang, Y.: Transformations of phosphorus speciation during (hydro)thermal treatments of animal manures. Environ. Sci. Technol. (2018). https://doi.org/10.1021/acs.est.7b05203
Sevilla, M., Fuertes, A.B.: The production of carbon materials by hydrothermal carbonization of cellulose. Carbon (2009). https://doi.org/10.1016/j.carbon.2009.04.026
Sevilla, M., Fuertes, A.B., Mokaya, R.: High density hydrogen storage in superactivated carbons from hydrothermally carbonized renewable organic materials. Energy Environ. Sci. (2011). https://doi.org/10.1039/c0ee00347f
Zhang, S., Zhu, X., Zhou, S., Shang, H., Luo, J., Tsang, D.C.W.: Hydrothermal carbonization for hydrochar production and its application. In: Ok, Y.S., Tsang, D.C.W., Bolan, N., Novak, J.M. (eds.) Biochar from Biomass and Waste, Fundamentals and Applications, pp. i-iii. Elsevier (2019)
Jain, A., Balasubramanian, R., Srinivasan, M.P.: Hydrothermal conversion of biomass waste to activated carbon with high porosity: a review. Chem. Eng. J. (2016). https://doi.org/10.1016/j.cej.2015.08.014
Ischia, G., Fiori, L.: Hydrothermal carbonization of organic waste and biomass: a review on process, reactor, and plant modeling. Waste Biomass Valoriz. (2021). https://doi.org/10.1007/s12649-020-01255-3
Zhuang, X., Liu, J., Zhang, Q., Wang, C., Zhan, H., Ma, L.: A review on the utilization of industrial biowaste via hydrothermal carbonization. Renew. Sustain. Energy Rev. (2022). https://doi.org/10.1016/j.rser.2021.111877
Robbiani, Z.: Hydrothermal carbonization of biowaste/fecal sludge, Master Thesis, ETHZ Zurich, Swisse (2013)
Terranova Energy, https://terranova-energy.com/en/ (2021). Accessed 9 Sept 2021
Zaccariello, L., Mastellone, M.L., Amelia, L.I.D., Catauro, M., Morrone, B.: Assessment of integration between lactic acid, biogas and hydrochar production in OFMSW plants. Energies (2020). https://doi.org/10.3390/en13246593
APHA: Standard Methods for the Examination of Water and Waste Water, 21st edn. APHA, Washington, DC (2005)
Vallejo, F., Díaz-Robles, L., Cubillos, F., Espinoza, A.P., Espinoza, L., Pinilla, F., Pino-Cortes, E.: Valorization of municipal solid waste using hydrothermal carbonization and gasification: a review. Chem. Eng. Trans. (2020). https://doi.org/10.3303/CET2081175
Zaccariello, L., Battaglia, D., Morrone, B., Mastellone, M.L.: Hydrothermal carbonization of digestate and leachate in a lab-scale batch reactor. Chem. Eng. Trans. (2021). https://doi.org/10.3303/CET2186016
Funding
Open access funding provided by Università degli Studi della Campania Luigi Vanvitelli within the CRUI-CARE Agreement. V:ALERE 2019 grant support from Università degli studi della Campania “L. Vanvitelli” of CHIMERA project is gratefully acknowledged for financial support of the experimental activity.
Author information
Authors and Affiliations
Contributions
MLM, LZ, BM Conceptualization; MLM, LZ, BM Methodology; LZ, DB Investigation; LZ, DB, BM Data Curation; MLM, LZ, BM Writing—original draft preparation; MLM, LZ, BM Writing—review and editing; LZ, BM Visualization; MLM, BM Project administration; MLM, BM Funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zaccariello, L., Battaglia, D., Morrone, B. et al. Hydrothermal Carbonization: A Pilot-Scale Reactor Design for Bio-waste and Sludge Pre-treatment. Waste Biomass Valor 13, 3865–3876 (2022). https://doi.org/10.1007/s12649-022-01859-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-022-01859-x