Abstract
CotA laccase was successfully expressed from Pichia pastoris. Magnetic reduced graphene oxide (MRGO) nanocomposite was synthesized and functionalized with iminodiacetic acid (IDA-NH2), and then chelated with Cu2+ for effective immobilization with His-tagged CotA laccase. The Cu2+-chelated MRGO (MRGO-IDA-Cu2+) showed 175 mg/g-support adsorption capacity. The immobilization of CotA laccase with MRGO-IDA-Cu2+ nano-hybrid composite was confirmed by Raman spectroscopy, Thermal Gravimetric Analysis, and X-ray diffraction. The use of nano-hybrid MRGO-IDA-Cu2+ composite to improve heavy oil recovery was investigated. The findings revealed that the interfacial tension between oil and water was reduced to ~ 90% of its original value, and the wettability was changed from the oil-wet state [θ = ∼ 115.2 − 124.5°] to the water-wet state [θ = ∼ 8.9 − 30.1°]. The increase of immobilized CotA laccase concentration and the ratio of nano-hybrid MRGO-IDA-Cu2+ composite decreases the value of interfacial tension (IFT) and contact angle (CA). The core-flooding studies revealed that the oil recovery process of 0.3 wt% nano-hybrid MRGO-IDA-Cu2+ composite was enhanced by 82.8%.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For long decades, petroleum was the primary source of energy. Concerns have been raised about the availability of petroleum in the future since it is a nonrenewable resource with high consumption rates. There is a need to enhance oil recovery while appropriate replacements are being developed. It varies considerably from reservoir to reservoir how much hydrocarbon can be recovered, although traditional recovery methods can usually extract only a tiny amount of crude oil; 25% from light oil, 5% from heavy oil, and less than 5% from tar sands [1]. The usage of enzymes in the enzyme enhanced oil recovery (EEOR) field is promising and needs further investigation. These enzymes include thermo-tolerant enzymes, which are well-suited to the high temperatures found in oil wells because of their ability to tolerate heat. Enzyme changes the state of the system from oil-wet to water-wet to free the oil globules into the porous structure of the reservoir rock by reducing the adhesion of the oil to the rock. Moreover, the enzyme attaches to the oil globules, breaking them down into smaller oil drops. Therefore, the smaller droplets will diffuse out of the porous medium (in the flow direction) to make the process easier. As enzymes have both hydrophilic and hydrophobic functional groups, they can have the same mechanism as surfactants, reduce the IFT between oil/water phases and cause emulsions formation which is one of the principal mechanisms of EOR techniques [2]. Enzymes such as lipase (EC 3.1.1.3), amylase (3.2.1.1), and esterase (EC 3.1.1.1) may alter oil's wettability and decrease interfacial tension, which improves petroleum oil mixing and flooding [3]. Lipase and esterase enzymes may be produced by various oil field microorganisms, with bacteria being the majority [4, 5]. The bacterial population in oil wells must be studied since the efficacy of EEOR and MEOR relies on how indigenous microorganisms respond to oil well environmental variables, including temperature, pH, and pressure levels. Despite Enzymes' excellent properties, the expensive cost of manufacturing prevents them from finding widespread use in industry. The use of cheap chemical surfactants or nanoparticles to bind enzymes is a potential approach for improving enzyme interfacial activity while also reducing their manufacturing costs. Because they are activated and stabilized under alkaline circumstances, at higher temperatures, and in media supplemented with high concentrations of copper ions, bacterial laccases have an advantage over fungal laccases. Compared to fungal laccases, CotA laccase from Bacillus subtilis is a thermophilic enzyme with great stability and high catalytic activity. By enhancing the stability and reusability of enzymes, immobilization approaches can improve their performance [6,7,8]. In the literature, only a few cases of CotA laccase immobilization have been addressed. Mukhopadhyay et al. immobilized laccase from E. coli AKL2 using Cu2O nanoparticles, which improved the immobilized laccase's activity and thermal stability [7]. CotA laccase was immobilized on glassy carbon electrodes and functionalized through electrochemical reduction of in situ produced amino-phenylmono-diazonium salts by Beneyton and co-workers. The ideal operating temperature for the immobilized CotA laccase was 50 °C [8].
The usage of P. pastoris for heterologous expression is advantageous for high yield production, better expression, and easier purification when compared with E. coli [9]. Enzyme stability and reusability may be improved using immobilization methods. Many scientists are interested in graphene oxide-based materials (GO) because they can host oxygen functional groups on the center and edges of the graphene nano-sheets, perform well in the bio-compatibility, have high dispersion ability in water, and promising for future functionalization [10]. A variety of functional groups in graphene oxide's backbone allow it to be exfoliated and changed by proteins through physical adsorption or chemical bonding [11]. The benefit of GO was due to its composition of carbon atoms, which does not alter the linked biomolecules' natural biological properties [12]. Enzyme catalysis benefits significantly from the large surface area and strong electrical conductivity of graphene oxide (GO).
In this study, CotA laccase was used for enhanced oil recovery for the first time. CotA laccase was heterologously expressed from P. pastoris to overcome its production as inactive inclusion bodies, which were previously reported about expression from E. coli [13]. Magnetic reduced graphene oxide was functionalized with IDA-NH2 chelated with copper ion for efficient binding with His-tagged CotA laccase, which covalently reacts with MRGO carboxyl groups [14]. The produced MRGO was extensively attached to IDA-Cu2+ in our system and exhibited a high capacity for adsorption with CotA laccase. Immobilization of CotA laccase on MRGO-IDA-Cu2+ nanosheets significantly enhanced the catalytic activity and stability of the enzyme. Besides, the recovery of the heavy oil was significantly enhanced by 82.8%. The usage of immobilized enzymes for heavy crude oil recovery is urgently needed for sustainable energy to save every oil drop.
Materials and Methods
Wizard® HMW DNA Extraction kit, PureYield™ Plasmid Miniprep System, Wizard® SV Gel and PCR Clean-Up System were bought from Promega, USA. Taq DNA polymerase, chemically competent E. coli DH5α cells, T4 DNA ligase and restriction enzymes were purchased from New England Biolabs GmbH, USA. pMD18-T vector was purchased from Takarabio, Japan. pPICZαB vector was purchased from Thermo Fisher Scientific, USA. Zeocin was purchased from InvivoGen, France. P. pastoris was purchased from Invitrogen, USA. B. subtilis 168 bacterial strains were got from China General Microbiological Culture Collection Center (China). Shanghai Aladdin Biochemical Technology Company supplied the reduced graphene oxide RGO (China). Sigma-Aldrich, USA, provided 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonicacid) (ABTS), N-hydroxyl succinimide (NHS), IDA-NH2, and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC). Sino Pharm Chemical Reagent Company provided 1,2 diaminoethane, ferric oxide chloride hexahydrate, and ferrous oxide chloride tetrahydrate (China). All chemicals and reagents used in this work were analytical grade. Table 1 shows the physicochemical parameters of used asphaltic crude oil supplied from Petrobel Petroleum Egyptian Company.
Cloning of B. subtilis 168’s CotA Laccase Gene into P. pastoris
B. subtilis 168 genomic DNA was isolated according to the instructions of Wizard® HMW DNA Extraction Kit, Promega. The isolated CotA laccase gene was used as a template for polymerase chain reaction (PCR) amplification. The forward primer, AACTGCAGAACCAATGCATTGGATGACACTTGAA AAATTTG, includes PstΙ restriction site and the reverse primer TCCCCGCGGGGACTTTATGGGGATCAG TTATATC includes SacΙΙ restriction site were used to amplify the CotA laccase gene. The PCR amplification program was as following: 4 min at 95 °C, 30 s at 95 °C, 30 s at 55 °C, 2 min at 72 °C, step two repeated for 32 ×, and final extension at 72 °C for 10 min. Wizard® SV Gel and PCR Clean-Up System were used to purify the produced PCR products before being inserted into the pMD18-T vector. PstΙ and SacΙΙ restriction enzymes were used to digest the recombinant pMD18-T-CotA plasmid and then ligated between matching sites of the digested pPICZαB vector. E. coli DH5α strain was utilized for the ligation mixture’s transformation. Low-salt LB medium containing 25 μg/mL Zeocin was used for positive colonies selection. pPICZαB-CotA plasmid was sequenced to confirm the successful cloning.
Over-expression and Purification of P. pastoris’s CotA Laccase
The recombinant expression plasmid pPICZαB-CotA was transformed into P. pastoris via electroporation after linearization with SacΙ restriction enzyme. The genomic DNA from the P. pastoris was isolated and purified and then used as a PCR template. CotA gene was PCR-amplified with the previously mentioned forward primer and reverse primer. The expression and transformation of CotA laccase were conducted in accordance with Lu et al. [15]. YPDS agar plates containing 100 mg/mL Zeocin were used to screen the positive clones. Positive colony was used to inoculate 30 mL buffered glycerol-complex medium (BGC), and the culture was incubated till OD600 value of 2–6, at 30 °C and 200 rpm. Cells were harvested by centrifugation at 3600×g, 4 °C for 15 min. Harvested cells were inoculated on buffered minimal methanol medium (BMM) amended with 0.2 mM CuSO4, and then incubated at 30 °C and 200 rpm. 0.5% methanol was added daily to the culture for enzyme induction. After 10 days, the cultured supernatant was collected by centrifugation at 7000×g (4 °C for 30 min). Polyethylene glycol 2000 was used to concentrate the cell-free culture media (molecular weight 30,000 cutoff). The concentrated CotA laccase was then placed onto a DEAE Sepharose FF column (Sigma Aldrich, Missouri, USA) equilibrated with citrate–phosphate buffer (20 mM pH 7.5). The absorbed proteins were eluted using a linear NaCl gradient (300 mL, 0–1 M) at 1 mL/min after the column was washed with the same buffer. Ultrafiltration was used to combine and concentrate the laccase-active fractions (30 kDa cutoff; Sigma Aldrich, Missouri, USA). The resultant sample was subsequently passed through a Sephadex G-75 column (Sigma Aldrich, Missouri, USA) equilibrated with the same buffer at 0.2 mL/min, and the laccase-active fractions were concentrated as previously described. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) was performed to confirm the molecular weight and purity of the produced CotA laccase. Enzyme concentration was detected using Thermo Scientific Pierce Coomassie (Bradford) Protein Assay Kit (Fisher Scientific GmbH, Schwerte, Germany).
Synthesis of Magnetic Reduced Graphene Oxide (MRGO)
The co-precipitation method [16] was used to synthesize the magnetic RGO nano-hybrid composite. Under vigorous stirring under nitrogen conditions, FeCl3.6H2O (0.4 g) and FeCl2·4H2O (0.15 g) in 100 mL H2O were added with care to a 40 mL dispersion solution of RGO (10 mg/mL) in 100 mL H2O, then add ammonia solution (25%) at 85 °C. After a 45-min reaction, the dark precipitate was washed three times with Milli-Q water.
Preparation of MRGO-IDA-Cu2+ Nano-sheets
In the presence of ethylene-diamine, IDA-NH2 interacted with MRGO. EDC (0.2 g) and NHS (0.2 g) solutions were added to the IDA-NH2 solution with constant stirring for 2 h for IDA carboxyl groups activation [17]. Following that, MRGO dispersion and 1,2 diaminoethane were reacted for 4 h at ambient temperature before being stirred for 6 h at 80 °C [18]. Milli-Q water was used to rinse the MRGO-IDA product three times. Finally, the pH of the MRGO-IDA suspension was raised to 11 in order to enhance the Cu2+ binding ratio [19]. After that, the suspension was immersed for 1 h in 0.1 M CuSO4 solution, washed three times, and kept at room temperature in ultra-pure water. Scheme 1 indicates the fabrication and functionalization of MRGO nano-sheets, and the immobilization of CotA laccase enzyme.
Characterization of the Synthesized Nano-sheets
Raman spectra were measured on a Dispersive Raman Microscope laser (Senterra, Bruker); 542 nm wavelength and 10 MW power were used. X-ray diffraction (XRD) (PANalytical X'Pert PRO MPD) with Cu K-radiation (λ = 1.540598 nm) was utilized to identify the crystal structure. Thermo-gravimetric analysis (TGA) was carried out in a nitrogen environment at a heating rate of 10 °C/min from 20 to 800 °C. Field Emission Scanning Electron Microscope (SEM) was utilized to investigate the surface morphology and structure (JEOL, JSM-6700F, Japan).
Adsorption Efficiency of CotA Laccase
Adsorption of CotA laccase enzyme with MRGO and MRGO-IDA-Cu2+ nano-sheets was studied using different pH conditions (pH 2–11) and different enzyme concentrations (0.05–0.25 mg/mL), according to Xia et al. [20]. For different pH ranges, 0.1 M glycine–HCl (pH 3–5), 0.1 M potassium phosphate (pH 6–8), and 0.1 M glycine–NaOH (pH 9–11) buffers were used to dissolve ABTS. The free enzyme was incubated under shaking (130 rpm) for 1 h at 25 °C in order to perform adsorption equilibrium.
Activity Measurement of Free and Immobilized CotA Laccase
5 mM of ABTS (molar absorption coefficient: 36,000 M−1 cm−1) was used as a substrate to measure the activity of free and immobilized CotA laccase at 420 nm using a spectrophotometer (Shanghai Precision & Scientific Instrument, China). To detect the enzymatic activity at different pH ranges, 0.1 M glycine–HCl (pH 3–5) and 0.1 M potassium phosphate (pH 6–7) buffers were used to dissolve ABTS. One unit of enzymatic activity was correlated to the enzyme amount needed to oxidize 1 µmol of ABTS. The relative activity of free and immobilized enzymes was measured at different pH range (2–11) and temperatures (20–100 °C). Activity recovery of immobilized laccase was performed according to Xia et al. [20].
Interfacial Tension (IFT) and Contact Angle (CA) Measurements
A pendant droplet of crude oil was maintained at a constant temperature for IFT (oil–brine/fluid) measurements (according to ASTM ISO 19403-4). The values of dynamic Interfacial tension were also determined by connecting the Interfacial tension equipment to a computer and running picture analysis software. The findings revealed that the values of Interfacial tension vary as a function of time until they approach a constant value with the passage of time, which is referred to in this research as the equilibrium IFT. For the contact angle measurements, a droplet of crude oil was put on the rock (core) for 24 h. The crude oil/fluid-rock contact angles were calculated using the Sessile Method (ASTM ISO/19403-5) [21]. The Attension Theta High-pressure Chamber was used to calculate the IFT and contact angle (Biolin Scientific, Finland). All calculations were investigated at a temperature of 25 and 60 °C and a pressure up to 500 psi.
Oil Recovery Measurements
Chemical flooding tests were performed using a Chemical flood system integrated with a sandstone model in one-dimension, as illustrated in Fig. 1 to evaluate the Fluid's efficiency. To obtain the required permeability and porosity (28.6%), the utilized sand was packed in various mesh sizes (~ 1.5 and 3.5 meshes) as a porous medium. Then, two days of saturation with brine solution were performed. After that, approximately 150 cm3 of oil was injected at a rate of 2 cm3/min under reservoir conditions (60 °C and pressure up to 500 psi), and it was aged for 24 h at 60 °C. The water saturation (Swr) and initial Oil saturation (Sor) were 11.09 and 89.91, respectively. In order to calculate the percentage of oil recovery (%), the recovery of the secondary stage was followed by the tertiary recovery flooding mechanism after flooding with brine solution [22].
Results and Discussion
Cloning and Expression of CotA Laccase in P. pastoris
The successful construction of the pPICZαB-CotA expression plasmid was confirmed using the sequence analysis. The volumetric activity of the expressed CotA laccase was found in the range of 3500 ± 206 U/L (specific activity 11.8 ± 0.3 U/mg). As shown in Fig. 2, the purified CotA laccase showed a molecular weight of ~ 65 kDa with 90% purity. Bradford assay revealed that the enzyme was expressed with a concentration of 295 ± 4.1 μg/mL. CotA laccase was expressed from E. coli in a previous study performed at our lab, but the enzyme yield and activity were lower than the expressed CotA laccase from P. pastoris [13].
Characterization of MRGO-IDA-Cu2+ Nano-hybrid Composite
Raman spectrum reveals substantial differences in the structure of MRGO-IDA-Cu2+ nano-hybrid composite compared to RGO structure (Fig. 3a). The spectra revealed significant peaks at 1350 and 1580 cm−1 corresponding to the D and G bands, respectively [23]. The MRGO-IDA-Cu2+ nano-hybrid composite spectra had a lower wave number and a smaller band expansion than RGO due to the presence of Fe3O4 and many functional groups on the RGO surface during the functionalization process, which disrupts it. The XRD patterns of MRGO-IDA-Cu2+ nano-hybrid composite (Fig. 3b) showed diffraction peaks at 35.6, 43.07, 57.4, and 62.8° (2θ), which are linked to Fe3O4 [24]. The XRD data indicates that the IDA-NH2 binding had no effect on the crystalline structure of magnetic Fe3O4. The XRD pattern of MRGO shows a single peak at 10° (2θ), which couldn’t be observed with MRGO-IDA-Cu2+ nano-hybrid composite pattern. These results confirmed that RGO and MRGO-IDA-Cu2+ nano-hybrid composites were successfully synthesized [24]. TGA analysis was used to examine the thermal properties of RGO, MRGO-IDA-Cu2+ nano-hybrid composite, as indicated in Fig. 3c, in order to validate RGO's functionalization. Due to the loss of residual water at the start, the RGO curve indicates weight loss between 30 and 150 °C. At 150–350 °C, further weight loss was detected due to the degradation of oxygen-containing groups in RGO [25]. The decomposition of the carboxyl groups in RGO caused the final weight loss seen from 300 to 700 °C. The wight loss in case of MRGO-IDA-Cu2+ curve was below 200 °C because of the loss of water and absorbed solvent. The weight loss observed from 200 to 450 °C is owing to the burning of MRGO-IDA-Cu2+ nano-sheets' remaining oxygen functional groups such as epoxy, carboxylic acid, and hydroxyl groups. The last weight loss seen between 450 and 500 °C was linked to graphene carbon frame collapse [26]. According to SEM analysis, the wrinkles on the surface of MRGO-IDA-Cu2+ (Fig. 4) are rougher than RGO sheets. These results indicate that a large number of magnetic Fe3O4 particles have been effectively bonded to the surface of RGO sheets. The average diameter of MRGO-IDA-Cu2+ nano-sheets is less than 1 µm, as determined by SEM images, indicating that the nano-sheets have micro-and nanoporous morphologies. These grooves and holes improved the surface area of MRGO, enhanced enzyme attachment, and improved the interaction between enzyme and substrate [27].
CotA Laccase Immobilization
CotA laccase immobilization capacity was studied in the pH range of 3–11 (Fig. 5a, b). The greatest CotA laccase adsorption on the two distinct types of nano-sheets was seen at the iso-electric point (PI) of CotA laccase, pH 7, out of all the pH values tested. Adsorption of CotA laccase at pH 7 occurred due to the fact that the largest adsorption of protein from an aqueous solution through metal affinity adsorption occurs at the protein's PI, where the protein has no net charge [28, 29]. After 1 h of incubation at 25 °C, the MRGO-IDA-Cu2+ nano-hybrid composite had the highest CotA laccase adsorption of 100% among the other nano-sheets. To investigate the optimal adsorption capacities of CotA laccase on MRGO-IDA-Cu2+ nano-hybrid composites, as well as the activity recovery of immobilized CotA laccase, varied starting quantities of the protein concentrations were immobilized. The equilibrium adsorption capacity increased when the initial concentration of CotA laccase increased, as seen in Fig. 5b. After 1 h of incubation, the adsorption capacities of the MRGO-IDA-Cu2+ nano-hybrid composite were 175 mg CotA laccase per gram support. The numerous amino and carboxyl groups in the MRGO-IDA-Cu2+ nano-hybrid composite likely contributed to its high immobilization capacity by providing additional active adsorption sites for binding with the enzyme. The maximal activity recovery of CotA laccase immobilized on MRGO-IDA-Cu2+ nano-hybrid composite reached 108% when 0.15 mg/mL of CotA laccase was used. After that, when the enzyme’s concentration exceeded 0.15 mg/mL, the activity recovery rapidly reduced. The activity recovery reduction is due to the fact that an excessive quantity of CotA laccase adsorption on the nano-hybrid composite surface causes intermolecular steric hindrance, which prevents substrate and product contact [30]. The explanation for MRGO-IDA-Cu-CotA laccase's excellent activity recovery might be due to RGO catalytic characteristics, which increase reaction rate while leaving the product unchanged [31], providing large surface area and optimized pore size, which promote enzyme loading and promote enzyme activity [32]. Additionally, the presence of the IDA ligand, which is attached with copper and CotA laccase, binds directly to the functionalized MRGO in a particular orientation determined by the poly-His tag location. Furthermore, owing to the presence of histidine residues, copper ion which acts as strong Lewis acid has a high binding ability with Lewis bases such as CotA laccase [33], and may increase CotA laccase activity [34].
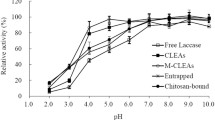
MRGO-IDA-Cu-CotA laccase's stability was investigated to confirm the direct effect of the immobilization process. The activity of the immobilized and free CotA laccase toward ABTS was studied at 2–7 pH range (Fig. 6a). The immobilized enzyme showed maximum activity at pH 5, while the free enzyme showed its highest activity at pH 4. This pH shift in case of the free enzyme could be attributed to the inhibition of the enzyme’s activity at high pH by the OH−-binding at the type 2 and type 3 copper ion sites in the enzyme’s structure. On the other hand, the copper metal affinity in case of MRGO-IDA-Cu-CotA laccase reduced the conformational changes between the different pH values and enhanced the enzyme’s activity. It has been reported that laccase enzyme showed the highest activity at pH 4 after immobilization with bimodal mesoporous carbon support because of the electrostatic interaction produced by the immobilization support [35]. The catalytic activity for both immobilized and free CotA laccase was also studied at different temperatures (20–100 °C), as shown in Fig. 6b. The free enzyme showed the highest catalytic activity at 60 °C, while the immobilized enzyme showed the highest catalytic activity at 80 °C. This temperature shift is because of the multipoint chelate interaction, which enhances the activation energy and re-arranges an optimal conformation of the enzyme, allowing a better binding with the substrate [36].
Interfacial Tension and Wettability Alteration Measurements
IFT Measurements
In the experiments, the crude oil and MRGO-IDA-Cu-CotA laccase solution were applied as drop and bulk phases, respectively. Table 2 indicates that the interfacial tension value between the untreated crude oil and formation water was calculated to be about 18.53 and 15.94 mN m−1. The treated system with free CotA laccase enzyme was 6.04 and 4.87 mN m−1 at 25 and 60 °C, respectively and the pressure was up to 500 psi. As noticed in Fig. 7, the IFT value reaches to ∼ 2.13 and 1.56 mN m−1 at 25 and 60 °C, respectively and the pressure was up to 500 psi at the concentration of 0.1 wt%, MRGO-IDA-Cu-CotA laccase; suggesting that in the presence of MRGO-IDA-Cu-CotA laccase, the interfacial tension value may be reduced more than 90%. Figure 7a, b and Table 2 show the variation of interfacial tension values with increasing the concentration of the MRGO-IDA-Cu-CotA laccase ratio. It is apparent that the IFT reduces as the concentration of MRGO-IDA-Cu-CotA laccase increases, which may be attributed to MRGO-IDA-Cu-CotA laccase adsorption at the oil/water interface. Figure 7b indicates the dynamic IFT at 25 and 60 °C, respectively. The system takes 5–20 min to obtain IFT equilibrium, and the dynamic IFT variation includes different patterns. Firstly, the IFT was decreased with time and then flattened due to equilibrium achievement. This is attributed to the adsorption and desorption of surfactant species at the oil/water interface. In reality, MRGO-IDA-Cu-CotA laccase forms an additional layer at the interface of oil and solution, extending the interface by acting as an amphiphilic surfactant. As a result, the addition of MRGO-IDA-Cu-CotA laccase significantly lowers capillary forces while increasing capillary number [37]. Moreover, in the case of MRGO-IDA-Cu-CotA laccase (0.3 %wt), the interfacial tension values were reduced more than MRGO-IDA-Cu-CotA laccase (0.1 %wt) and MRGO-IDA-Cu-CotA laccase (0.2 %wt), as shown in Table 2. This reduction is owing to the greater concentration of functional groups in the sample, which results in improved dispersion and stability. Indeed, functional groups on the MRGO edge plane and surface may hinder particle–particle interaction by lowering nanoparticle aggregation; as a result, more MRGO-IDA-Cu-CotA laccase can migrate to the oil–water interface, reducing IFT [38]. In addition, the enzyme (CotA laccase) has three components: H+, OH−, and an "active site" that represents the proprietary DNA component. The polar portion is formed by the H+ and OH− components, whereas the non-ionic part is formed by the active site. When the enzyme (CotA laccase) comes into contact with a hydrocarbon (Crude oil), the non-ionic component enables the enzyme to penetrate the hydrocarbon (Crude oil), allowing the polar H+ and OH− components to work together to release micro droplets of oil. As a consequence of this unusual action, oil is released cleanly by reducing interfacial tension, decreasing contact angle, and alleviating capillary pressures. The best IFT result was obtained by MRGO-IDA-Cu-CotA laccase (0.3 %wt); the IFT value reaches to ∼ 0.64 and 0.38 mN m−1 at 25 and 60 °C, respectively and pressure was up to 500 psi.
Wettability Alteration Measurements
Wettability and contact angle plays a significant role in detecting the interaction between rocks (core) and liquids (brine, crude oil) in reservoirs. The effect of CotA laccase enzyme and MRGO-IDA-Cu-CotA laccase on wettability alteration was studied by evaluating contact angles (θ), as listed in Table 2. The final angles for MRGO-IDA-Cu-CotA laccase (0.1 %wt) were 30.08°, 21.47°, and the treated system with free Cota laccase enzyme was 49.58° and 38.11°, while for blank was 124.53°, 115.20° at temperatures of 25 and 60 °C, respectively and pressure was up to 500 psi. The results showed a decrease in diffusion of oil droplets on the surface after adding MRGO-IDA-Cu-CotA laccase. The data also shows that the contact angle decreases by increasing the ratio of MRGO-IDA-Cu-CotA laccase. Furthermore, in the case of MRGO-IDA-Cu-CotA laccase (0.3 %wt), the CA values were reduced more than MRGO-IDA-Cu-CotA laccase (0.1 %wt) and MRGO-IDA-Cu-CotA laccase (0.2 %wt). This indicates that the rock sample was changed from an oily to a watery condition. As shown in Table 2, capillary forces were decreased, which aids the displacement process in an oil recovery process when smaller viscous forces are required to separate oil droplets from the rock surface and mobilize the oil, improving the EOR process' efficiency [39, 40]. Figure 8 shows the conversion of the system from oil-wet to water-wet after adding MRGO-IDA-Cu-CotA laccase enzyme. The best CA results was obtained by MRGO-IDA-Cu-CotA laccase enzyme (0.3 %wt); the CA value reached to ∼ 15.78° and 8.93° at temperatures of 25 and 60 °C, respectively and pressure was up to 500 psi. Recent investigations have shown that even little changes in the water chemistry have a significant impact on the amount of oil displaced in the crude-oil-rock-brine system. Wang et al. [41] examined the capability of enzymes to modify their wettability by examining contact angle alterations, imbibition processes, and adhesion work. They found that enzymes can quickly change the wettability of sandstone from being oil-wet to being water-wet, while enzymes have a much slower effect on limestone's wettability. They also found that enzymes may enhance water imbibition driving force in water-wet reservoirs while reducing oil-wet reservoir draining resistance force. The enzyme also decreased oil work of adhesion, increasing oil desorption from the rock surface. On the other hand, Khusainova et al. [42] examined the wettability alteration potential of enzymes using adhesion and contact angle studies on calcite surfaces. Oil droplet adherence was detected in the majority of the enzymes' aqueous solutions, although absolute non-adhesion was seen with one of them. Furthermore, a range of 38 ± 7° angles was found from their contact angle measurements, whereas a decrease of about 15° in contact angle was investigated with the same enzyme application. This was due to the enzyme's ability to change the wettability of the surface.
Effect of MRGO-IDA-Cu-CotA Laccase Enzyme on EOR
Three core flooding experiments were performed using a sandstone packed model as a porous medium using MRGO-IDA-Cu-CotA laccase enzyme slugs (0.3 wt% RGO) at three immobilized CotA laccase concentrations (0.1, 0.2 and 0.5%) (up to 3 PV). The different concentrations of immobilized CotA laccase were injected into the model at a fixed flow rate of 2 mL/min at temperature of 60 °C and injection pressure up to 500 psi. Following injection of the free CotA Laccase and MRGO-IDA-Cu-CotA laccase enzyme slug separately, oil droplets trapped in pores are released as the IFT between displacing fluid and oil decreases. For four hours, the slug was allowed to settle in the sandstone model to decrease the IFT and change the wettability. To study tertiary recovery, all fluid tests were taken after water flooding and secondary recovery. The water saturation (Swr) and initial Oil saturation (Sor) were 11.09 and 89.91, respectively. At the same time, the flooding test for free CotA laccase enzyme was calculated to be about 10.3, 16.8, and 23.1% at concentrations of 0.1, 0.2, and 0.5%, respectively. Figure 9a, b illustrated the relationship between cumulative oil recovery (%) and injected pore volume (PV). The results showed that the oil recovery from MRGO-IDA-Cu-CotA laccase enzyme flooding test was calculated to be about 42.5, 48.6, and 82.8% at concentrations of 0.1, 0.2 and 0.5% MRGO-IDA-Cu-CotA laccase enzyme, respectively. In comparison, the improvement in sweep efficiency when MRGO-IDA-Cu-CotA laccase enzyme is present can be observed by increasing the quantity of functional groups containing oxygen (from 0.1 to 0.3 %wt MRGO-IDA-Cu-CotA laccase). The results show that the oil recovery from MRGO-IDA-Cu-CotA laccase enzyme flooding test was calculated to be about 52.6, 64.8, and 82.8% for 0.1, 0.2 and 0.3 wt% MRGO-IDA-Cu-CotA laccase enzyme (concentration 0.5%) respectively, and that confirming the impact of oxygen containing functional groups on improving the EOR performance. According to the IFT findings discussed before, the quantity of oxygen-containing functional groups affects the IFT between oil/water. The low IFT in highly functionalized 0.3 wt% MRGO-IDA-Cu-CotA laccase enzyme is because of the hydrophilicity effects of the highly functionalized graphene nanosheets (RGO) groups, making them amphiphilic particles; solute in water and oil. Consequently, the higher functional groups (RGO) embedded, the lower the interfacial tension and, therefore, higher oil recovery. A predicted mechanism illustrating the action of MRGO-IDA-Cu-CotA laccase enzyme is shown in Fig. 10. As a result of our calculations and experimental findings, 0.3 wt% MRGO-IDA-Cu-CotA laccase enzyme (concentration 0.5%) improves EOR performance compared to others. Another factor was observed after applying MRGO-IDA-Cu-CotA laccase enzyme, altering the viscosity of heavy crude oil. When there is no additive in the reaction system, a reduction in the viscosity of heavy oil was noticed just by applying heat. The reduction of heavy oil viscosity was happened when the MRGO-IDA-Cu-CotA laccase enzyme was injected to the system at 25, 60 and 90 °C and up to 500 psi, as shown in Table 3 using PVS rheometer (Brookfield). The results revealed that by increasing ratio of RGO sheets the viscosity decreased; this is due to the adsorption over the MRGO nanosheets promotes a breakdown of the viscoelastic network that favors the reduction of their viscosity. The maximum viscosity reduction rate was exhibited by MRGO-IDA-Cu-CotA laccase enzyme (0.3 %wt) at concentration of 0.5% as 68.6, 70, and 74.6% at 25, 60, and 90 °C, respectively, while for free CotA laccase enzyme 10.5, 4.8, and 6.5% under the same conditions compared to the results of untreated crude oil. The resultant low molecular weight hydrocarbon can help dissolve the heavy fractions, and consequently, the viscosity is reduced. The chromatographic analysis (GC) shows the change in crude oil after EOR process using immobilized CotA laccase. A chromatogram of crude oil before and after treatment is shown in Fig. 11.
In both laboratory investigations and reservoir field testing, Feng et al. [43] reported about enzyme applications in oil recovery field. Improved recoveries of 12.4–16.3%, 13.9–20%, and 15.7–21.1% were found in their core flooding studies on core samples aged for seven days using 3, 6, and 10% changed enzyme doses, respectively. They also tested the modified enzyme's EOR potential in a micro-model displacement procedure. Improved oil mobility was seen with the use of a modified enzyme, and the successful enzyme application was linked to the conversion of oil-wet into water-wet sections and the emulsification process. Zhang et al. [44] also explored the effect of the enzyme on de-pressurization modification of ultra-low permeability Daqing Chaoyanggou Oilfield with low water flooding oil recovery. They discovered that adding an enzyme to the system increased the water absorption capacity of the water wells and stimulated de-pressurization. The application of enzyme was likewise related to a 2208t increase in cumulative oil recovery; however, there was an increase in ion concentration and salinity of the associated generated water. This was regarded as a sign of the formation of a new production sector and the displacement of the oil layer. Recent literatures have reported that the oil recovery factor was raised up to 16% after applying enzymes on the laboratory scale and up to 269 barrels of oil/day after applying enzymes on the field scale [45,46,47,48,49,50]. On the other hand, the immobilized CotA laccase enhanced the oil recovery to 82.8% on the laboratory scale.
The Economics of Using Immobilized CotA Laccase
Enzyme immobilization is very promising for enzyme-enhanced oil recovery fields compared with using free enzymes. Enzyme immobilization allows continuous use and re-use of the biocatalyst. Some advantages of the immobilized laccase over their free forms are: increased enzyme stability, reduced enzyme costs, greater ease of enzyme separation and recovery for re-utilization, possibility of operating continuously, ease of product separation, reduced effluent problems, and increased activity. Bacterial laccases are not available in the market due to the difficult challenges of their production, especially in the active form. So, we compared the production cost of the enzyme and the immobilization technology with fungal laccase from Trametes versicolor. The price of 10 g from the enzyme is 638 USD from Sigma Aldrich. The preparation cost of about 5 g immobilized CotA laccase is about 1241 USD, and for 10 g, it will be about 2482 USD for one-time use. Immobilized CotA laccase can be used for ten cycles efficiently. The cost of the free enzyme for 10 cycles is 6380 RMB, so; immobilized laccase will save 3898 USD for ten cycles. The developed crude oil recovery in this study could also be applied by using the unpurified enzyme with a cheaper cost (~ 1000 USD) but with lower efficiency.
Conclusion
CotA laccase was expressed from P. pastoris actively. Magnetic reduced graphene oxide (MRGO) nanocomposite was synthesized and functionalized with iminodiacetic acid (IDA-NH2), and then chelated with Cu2+ for effective immobilization with His-tagged CotA laccase. The Cu2+-chelated MRGO (MRGO-IDA-Cu2+) showed an effective adsorption capacity of 175 mg/g-support. Immobilization of CotA laccase on MRGO-IDA-Cu2+ nano-sheets helped to retain 108% of the enzyme’s activity. Subsequently, the potential application of MRGO-IDA-Cu-CotA laccase enzyme for enhanced oil recovery was perfectly investigated. The results reveal that IFT values can be reduced by more than 90% in the presence of MRGO-IDA-Cu-CotA laccase enzyme, and the wettability was modified from an oily state [θ = ∼ 115.2 − 124.5°] to a watery state [θ = ∼ 8.9 − 30.1°]. The values of IFT and CA decrease by increasing the concentration of MRGO-IDA-Cu-CotA laccase enzyme. The 0.3 wt% MRGO-IDA-Cu-CotA laccase enzyme exhibited the best results toward enhancing the oil recovery process by 82.8%.
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Denbina, E.S., Boberg, T.C., Rotter, M.B.: Evaluation of key reservoir drive mechanisms in the early cycles of steam stimulation at cold lake. SPE Reserv. Eng. 6(2), 207–211 (1991)
Dake, L.P.: Fundamentals of Reservoir Engineering. Elsevier BV, Oxford (1978)
Nasiri, H.: Enzymes for Enhanced Oil Recovery (EOR). Department of Chemistry, University of Bergen, Norway (2011)
Kato, T., Haruki, M., Imanaka, T., Morikawa, M., Kanaya, S.: Isolation and characterization of psychrotrophic bacteria from oil-reservoir water and oil sands. Appl. Microbiol. Biotechnol. 55, 794–800 (2001)
Mishra, S., Singh, S.N.: Microbial degradation of n-hexadecane in mineral salt medium as mediated by degradative enzymes. Bioresour. Technol. 111, 148–154 (2012)
Brander, S., Mikkelsen, J.D., Kepp, K.P.: Characterization of an akali-and halide-resistant laccase expressed in E. coli: CotA from Bacillus clausii. PLoS ONE 9, e99402 (2014)
Mukhopadhyay, A., Dasgupta, A.K., Chakrabarti, K.: Thermostability, pH stability and dye degrading activity of a bacterial laccase are enhanced in the presence of Cu2O nanoparticles. Bioresour. Technol. 127, 25–36 (2013)
Beneyton, T., El Harrak, A., Griffiths, A.D., Hellwig, P., Taly, V.: Immobilization of CotA, an extremophilic laccase from Bacillus subtilis, on glassy carbon electrodes for biofuel cell applications. Electrochem. Commun. 13, 24–27 (2011)
Damasceno, L.M., Huang, C.J., Batt, C.A.: Protein secretion in Pichia pastoris and advances in protein production. Appl. Microbiol. Biotechnol. 93, 31–39 (2012). https://doi.org/10.1007/s00253-011-3654-z
Shi, J., Zhang, H., Snyder, A., Wang, M.-X., Xie, J., Porterfield, D.M., Stanciu, L.A.: Anaqueous media based approach for the preparation of a biosensor platform composed of graphene oxide and Pt-black. Biosens. Bioelectron. 38, 314–320 (2012)
Wang, Y., Li, Z., Wang, J., Li, J., Lin, Y.: Graphene and graphene oxide:biofunctionalization and applications in biotechnology. Trends Biotechnol. 29, 205–212 (2011)
Zhang, H., Gruner, G., Zhao, Y.: Recent advancements of graphene in biomedicine. J. Mater. Chem. B 1, 2542–2567 (2013)
Samak, N.A., Hu, J., Wang, K., Guo, C., Liu, C.: Development of a novel micro-aerobic cultivation strategy for high potential CotA laccase production. Waste Biomass Valoriz. (2017). https://doi.org/10.1007/s12649-016-9824-6.)
Graff, R.A., Swanson, T.M., Strano, M.S.: Synthesis of nickel-nitrilotriacetic acid coupled single-walled carbon nanotubes for directed self-assembly with polyhistidine-tagged proteins. Chem. Mater. 20, 1824–1829 (2008)
Lu, L., Wang, T.N., Xu, T.F., Wang, J.Y., Wang, C.L., Zhao, M.: Cloning and expression of thermo-alkali-stable laccase of Bacillus licheniformis in Pichia pastoris and its characterization. Bioresour. Technol. 134, 81–86 (2013)
Hu, X.J., Liu, Y.G., Wang, H., Chen, A.W., Zeng, G.M., Liu, S.M., Guo, Y.M., Hu, X., Li, T.T., Wang, Y.Q., Zhou, L., Liu, S.H.: Removal of Cu(II) ions from aqueous solution using sulfonated magnetic graphene oxide composite. Sep. Purif. Technol. 108, 189–195 (2013)
Guo, F., Liu, Y., Wang, H., Zeng, G., Hu, X., Zheng, B., Li, T., Tan, X., Wang, S.: Adsorption behavior of Cr(VI) from aqueous solution onto magneticgraphene oxide diaminocyclohexanetetraacetic acid. RSC Adv. 5, 45384–45392 (2015)
Wang, H., Liu, Y.G., Zeng, G.M., Hu, X.J., Hu, X., Li, T.T., Li, H.Y., Wang, Y.Q., Jiang, L.H.: Grafting of _-cyclodextrin to magnetic graphene oxide viaethylenediamine and application for Cr(VI) removal. Carbohydr. Polym. 113, 166–173 (2014)
Graff, R.A., Swanson, T.M., Strano, M.S.: Synthesis of nickel-nitrilotriacetic acidcoupled single-walled carbon nanotubes for directed self-assembly withpolyhistidine-tagged proteins. Chem. Mater. 20, 1824–1829 (2008)
Xia, T.T., Liu, C.Z., Hu, J.H., Guo, C.: improved performance of immobilized laccase on amine-functioned magnetic Fe3O4 nanoparticles modified with polyethylenimine. Chem. Eng. J. 295, 201–206 (2016)
Berry, J.D., Neeson, M.J., Dagastine, R.R., Chan, D.Y., Tabor, R.F.: Measurement of surface and interfacial tension using pendant drop tensiometry. J. Coll. Interface Sci. 454, 226–237 (2015)
Samak, N.A., Mahmoud, T., Abdelhamid, M.M., Aboulrous, A.A., Xing, J.: Enhanced biosurfactant production using developed fed-batch fermentation for effective heavy crude oil recovery. Energy Fuels J. 11(34), 14560–14572 (2020)
Guo, S., Zhang, G., Guo, Y., Yu, J.C.: Graphene oxide-Fe2O3 hybrid material as highly efficient heterogeneous catalyst for degradation of organic contaminants. Carbon 60, 437–444 (2013)
Travlou, N.A., Kyzas, G.Z., Lazaridis, N.K., Deliyanni, E.A.: Functionalization of graphite oxide with magnetic chitosan for the preparation of a nano composite dye adsorbent. Langmuir 29, 1657–1668 (2013)
Yang, J., Hu, Y., Jiang, L., Zou, B., Jia, R., Huang, H.: Enhancing the catalytic properties of porcine pancreatic lipase by immobilization on SBA-15 modified by functionalized ionic liquid. Biochem. Eng. J. 70, 46–54 (2013)
Li, Z., Xie, K., Slade, R.C.T.: High selective catalyst CuCl/MCM-41 for oxidative carbonylation of methanol to dimethyl carbonate. Appl. Catal. A 205, 85–92 (2001)
Sill, T.J., Von Recum, H.A.: Electrospinning: applications in drug delivery and tissue engineering. Biomaterials 29, 1989–2006 (2008)
Gunzer, G., Hennrich, N.: Purification of _1-proteinase inhibitor by triazine dye affinity chromatography, ion-exchange chromatography and gel filtration on fractogel TSK. J. Chromatogr. 296, 221–229 (1984)
Samak, N.A., Tan, Y., Sui, K., Xia, T.-T., Wang, K., Guo, C., Liu, C.: CotA laccase immobilized on functionalized magnetic graphene oxidenano-sheets for efficient biocatalysis. Mol. Catal. 445, 269–278 (2018)
Wang, F., Guo, C., Liu, H., Liu, C.: Immobilization of Pycnoporus sanguineus laccase by metal affinity adsorption on magnetic chelator particles. J. Chem. Technol. Biotechnol. 84, 97–104 (2008)
Dong, S., Xiao, H., Huang, Q., Zhang, J., Mao, L., Gao, S.: Graphene facilitated removal of labetalol in laccase-ABTS system: reaction efficiency, pathways and mechanism. Sci. Rep. 6, 21396 (2016)
Singh, R.K., Tiwari, M.K., Singh, R., Lee, J.: From protein engineering toimmobilization: promising strategies for the upgrade of industrial enzymes. Int. J. Mol. Sci. 14, 1232–1277 (2013)
Arnold, F.: Metal-affinity separations: a new dimension in protein processing. Nat. Biotechnol. 9, 151–156 (1991)
Hatvani, N., Mécs, I.: Effects of certain heavy metals on the growth, dyedecolorization, and enzyme activity of Lentinula edodes. Ecotoxicol. Environ. Saf. 55, 199–203 (2003)
Liu, Y., Zeng, Z., Zeng, G., Tang, L., Pang, Y., Li, Z., Liu, C., Lei, X., Wu, M., Ren, P., Liu, Z., Chen, M., Xie, G.: Immobilization of laccase on magnetic bimodal mesoporouscarbon and the application in the removal of phenolic compounds. Biores. Technol. 115, 21–26 (2012)
Sari, M., Akgo, S., Karatas, M., Denizli, A.: Reversible immobilization of catalase by metal chelate affinity interaction on magnetic beads. Ind. Eng. Chem. Res. 45(9), 3036–3043 (2006)
Rusli, S., Grabowski, J., Drews, A., Kraume, M.: A multi-scale approach to modeling the interfacial reaction kinetics of lipases with emphasis on enzyme adsorption at water–oil interfaces. Processes 8(9), 1082 (2020)
Shi Y., Liu L., Ding Y., Yang D.: Improvement of Transient Interfacial Tension and Displacement Efficiency with Enzyme: An Application of Enzyme on the Heavy Oil Recovery. In: Lin J. (eds) Proceedings of the International Field Exploration and Development Conference 2019. IFEDC 2019. Springer Series in Geomechanics and Geoengineering. Springer, Singapore. (2020) https://doi.org/10.1007/978-981-15-2485-1_82
Betiha, M.A., El-Henawy, S.B., Al-Sabagh, A.M., Negm, N.A., Mahmoud, T.: Experimental evaluation of cationic-Schiff base surfactants based on 5-chloromethyl salicylaldehyde for improving crude oil recovery and bactericide. J. Mol. Liq. 316, 113862 (2020)
Pei, H., Zhang, G., Ge, J., Jin, L., Ma, C.: Potential of alkaline flooding to enhance heavy oil recovery through water-in-oil emulsification. Fuel 104, 284 (2013)
Wang, Y., Kantzas, A., Li, B., Li, Z., Wang, Q. and Zhao, M.: New agent for formation-damage mitigation in heavy-oil reservoir: Mechanism and application. In SPE International Symposium and Exhibition on Formation Damage Control held in Lafayette, Louisiana, U.S.A., 1315 February, 2008. Society of Petroleum Engineers (2008).
Khusainova, A., Shapiro, A.A., Stenby, E.H. and Woodley, J.M.: Wettability improvement with enzymes: Application to enhanced oil recovery under conditions of the North Sea reservoirs. Graduate Schools Yearbook, p. 125 (2013)
Feng, Q.X., Ni, F., Shao, D., Ma, X.P., Qin, B., Zhou, L.H. and Ji, C.F.: Eor pilot tests with modified enzyme in china. In EUROPEC/EAGE Conference and Exhibition. Society of Petroleum Engineers (2007).
He, L., Zhonghong, Z.: Biology enzyme eor for low permeability reservoirs. In SPE Enhanced Oil Recovery Conference. Society of Petroleum Engineers (2011).
Feng, Q.X., Ma, X.P., Zhou, L.H., Shao, D.B., Wang, X.L., Qin, B.Y.: EOR pilot tests with modified enzyme in China. In: Proceedings of the SPE 107128 Presented at SPE Europe/EAGE Annual Conference and Exhibition, London, United Kingdom, June 11–14 (2007).
Moon, T.: Using Enzymes to Enhance Oil Recovery. JPT online, SPE (2008)
Nasiri, H., Spildo, K., Skauge, A.: Use of Enzymes to Improve Waterflood Performance. In: Presented at the International Symposium of the Society of Core Analysis, Noordwijk, Netherlands, September 27–30 (2009)
He, L., Zhonghong, Z.: Biology Enzyme EOR for Low Permeability Reservoirs. In: Proceedings of the SPE 114281 Presented at the SPE Enhanced Oil Recovery Conference, Kuala Lumpur, Malaysia, July 19–21 (2011)
Ott, W.K., Nyo, T., Aung, W.N., Khaing, A.T.: EEOR Success in Mann Field, Myanmar. In: SPE 114231 Presented at the SPE Enhanced Oil Recovery Conference, Kuala Lumpur, Malaysia, July 19–21 (2011).
Mahmoud, T., Samak, N.A., Abdelhamid, M.M., Aboulrous, A.A., Xing, J.: Modification wettability and interfacial tension of heavy crude oil by green bio-surfactant based on Bacillus licheniformis and Rhodococcus erythropolis strains under reservoir conditions: microbial enhanced oil recovery. Energy Fuels 35(2), 1648–1663 (2020)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have not disclosed any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mahmoud, T., Liu, C. & Samak, N.A. Immobilized CotA Laccase for Efficient Recovery of HEAVY OIL. Waste Biomass Valor 14, 127–144 (2023). https://doi.org/10.1007/s12649-022-01850-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-022-01850-6