Abstract
Carbonation of biomass ash allows for the final storage of CO2 as solid carbonates and may therewith contribute to supply energy with net negative greenhouse gas emissions. Accelerating the reaction under ambient temperature and pressure requires presence of water as reaction space. Therefore, dry-discharged ashes need to be humidified. Here we developed and tested a rotating drum reactor integrating hydration and carbonation of biomass bottom ash (BBA). The bed motion was evaluated with moist quartz sand (QS) as a model material. In the BBA carbonation experiments, liquid-to-solid ratios (L/S) between 0.1 and 0.3 were adjusted with two-fluid nozzles. The reactant gas (10 vol% CO2) was fed either simultaneously with or subsequently to humidification. The CO2 uptake was determined gravimetrically as well as using a gas balance and was compared to results obtained under fixed-bed conditions. In the rotating drum, a favorable slumping motion of the QS was identified at a rotation rate of 7 rpm and a fill level of 20 vol%. Thus, BBA carbonation tests were carried out under these conditions yielding a CO2 uptake between 22 and 31 g/kg within 2 h. Uptake was highest at L/S 0.1 and lowest at L/S 0.3. These results indicate that the rotating drum reactor reduces the required moisture content compared to fixed-bed carbonation. The CO2 feeding mode (simultaneous or subsequent) had only a minor effect on the cumulative CO2 uptake but provided valuable insight into the heat production by hydration and carbonation of BBA in the rotating drum system.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Statement of Novelty
Carbonation of biomass ash allows for the final storage of CO2 as solid carbonates and may therewith contribute to supply energy with net negative greenhouse gas emissions. At ambient temperature, the process requires the presence of a liquid water film around the solid particles. Thus, dry discharged ashes need to be humidified prior to carbonation. Here, a novel setup was developed that integrates hydration and carbonation into one reactor system. Reactant gas and liquid water were supplied to a rotating drum reactor via two-fluid nozzles directed on the moving material bed. The system allows for a customized humidification of the solid and is favorable in terms of ash handling as well as workspace safety.
Introduction
Mitigating climate change requires a substantial reduction of greenhouse gas emissions from the energy supply sector. To meet the global warming target of 1.5 °C at maximum, the energy supply from biomass would need to increase from 10 to 28% between 2020 and 2050 [1]. If climate-friendly biomass combustion were combined with carbon capture and storage, it could become a CO2-neutral or -negative energy source. However, many biomass combustion plants are rather small as compared to hard coal or lignite incinerators. This results in disproportionally high costs for the CO2 capture units and the transport of captured CO2 from decentralized sources to disposal sites [2].
Worldwide, the production of mostly alkaline ashes from combustion of solid biomass is estimated to be more than 450 million tons [3]. Its major share is disposed of in landfills [4, 5]. Considering that these ashes act as CO2 absorbents [6], upon disposal they may be regarded as a final CO2 sink. The CO2 uptake is driven by the carbonation reaction of readily available alkaline metal (hydr-)oxides with CO2 to form solid carbonates. Besides capturing CO2, biomass ash carbonation may result in an easier handling of the material due to granulation [7] as well as in lower disposal costs due to reduced leachability of some trace metals [8]. When complying with environmental regulations, biomass ash could also be used as fertilizer or for soil liming [9].
At ambient temperature and pressure, the carbonation reaction requires the presence of a free water phase within which alkaline components and CO2 dissolve, combine, and precipitate as carbonates [10]. In the presence of water, hydration of metal oxides (i.e. calcium oxide, Eq. 1) precedes the actual carbonation reaction (Eq. 2).
Available carbonation routes include the so-called slurry carbonation at liquid-to-solid ratios (L/S) > 2 or the moist carbonation where the reaction takes place in a water film (L/S < 1) [10, 11]. Depending on the incinerator design (e.g. inclusion of a quenching step) and the ash origin (fly ash, bottom ash etc.) the material is discharged in wet or dry state. The latter is the case for fly ash from combustion plants with a dry flue gas cleaning system (cyclones, filters). Dry discharge also holds for bottom ash from most of the smaller-scale biomass combustion plants [3, 12, 13].
Carbonation is rate-limited due to the mass transfer of reactants through the ash or liquid layer and/or the liquid–solid interface [14,15,16]. The former is influenced by moisture conditions as a high L/S results in longer diffusion distance. Dependence of the CO2 uptake on the L/S has been shown for various alkaline waste materials [8, 16,17,18]. Reaction temperature has also been shown to influence the carbonation as at higher temperatures the diffusion velocity of the educts increases, while the solubility of CO2 decreases and pore water evaporates [16].
Biomass ashes produced from biomass incineration are highly variable and their composition depends on the type of biomass. They can be classified into four types (“S”, “C”, “K” and “CK”) based on their acidity and their content of mineral forming components (mainly CaO, MgO, SiO2, Al2O3, K2O, phosphates, chlorides and sulfates) [3]. Of these components only the alkaline (earth) oxides significantly contribute to CO2 uptake, while anionic components like phosphates, chlorides and sulfates lower the uptake capacity [8]. The absolute content of alkaline (earth) oxides of the ash types increases in the order of “C” > “CK” > “K” > “S”, resulting in higher CO2 sequestration by “C” and “CK” type ashes [19]. Such ashes are produced from the incineration of woody biomass (type “C”) or animal biomass (type “CK”), while the incineration of herbaceous and agricultural biomass produces “K” type ashes [3]. An uptake capacity of up to 705 g CO2/kg ash based on the theoretical full conversion of all alkaline (earth) oxides has been reported for woody biomass ashes [6]. Despite of the high theoretical capacity, experimental results have been considerably lower. Uptakes in the range of 30 to 200 g/kg have been achieved when passing a CO2 rich gas (35–40 vol% CO2) for 5 to 876 h through a fixed bed of ash [20,21,22]. Even after atmospheric storage for up to 10 years only 50% of the theoretical capacity has been used [19, 23].
For alkaline materials other than biomass ash, it has been demonstrated that continuous mixing of the solid in rotating drum reactors substantially increases the level and the reaction speed of carbonation [18, 24,25,26]. In such systems, the solid’s bed motion follows characteristic patterns. For slow to medium rotation rates these have been denoted as slipping, slumping, and rolling modes. In the slipping mode, the bulk material moves as whole along the drum wall. This motion may be permanent or intermittent and provides only slight mixing, i.e. the contact between gas and solid is comparable to a fixed bed. Slumping and rolling bed motion are related to conditions where an upper bed fraction slides over a lower bed fraction either periodically (slumping) or continuously (rolling). Compared to slipping, these motion patterns occur at higher fill levels and improve the heat and mass transfer [27] thus promoting carbonation [26].
The mode of bed motion depends on reactor operation (chiefly on fill level and rotation rate [28]) and on the properties of the solid (particle size and shape [28], as well as the particle surface structure [29]). The moisture content of the solid acts on the inter-particle cohesion [30]. Thus, models or experimental results derived for dry materials cannot be applied to a wet carbonation set-up [26, 31].
Carbonation of alkaline waste in rotating drums under ambient conditions has so far been studied with materials that had already hydrated in a quenching process [25, 26] or that had been artificially hydrated before conducting the carbonation experiments [18, 24]. However, from a practical perspective (e.g. workplace safety during handling of the material, processing time and design) it would be desirable to integrate hydration and carbonation in-situ. This would also cut investment and operation costs (e.g. no additional mixing). Water in such a system has so far only been supplied in form of steam [32]. In such a setup, humidification is related to heat supply and therefore affects the carbonation reaction by increasing the temperature. To the best of our knowledge, a more straightforward humidification approach with liquid water has not been described, yet.
Here, we developed and tested a lab scale batch rotating drum reactor with an integrated supply of liquid water for the carbonation of initially dry alkaline waste such as biomass bottom ash (BBA). Favorable process conditions regarding the bed motion were empirically derived from experiments with quartz sand (QS) as a model material. Favorable conditions were then applied in combined hydration/carbonation experiments with BBA where effects of a simultaneous vs. a subsequent CO2-feeding mode were studied.
Materials and Methods
Waste and Model Materials
Carbonation experiments were performed with BBA originating from the incineration of waste wood chips and green waste on a grate furnace. Samples were taken directly from the discharge container and manually screened with a mesh size of 5 mm to remove metal particles like nails, screws or brackets. Until experimental testing, the BBA was stored in air-tight clamping ring drums. For details regarding the origin, pretreatment and composition please refer to [8]. The BBA was used after the manual screening (hereafter called “native sample”). Quartz sand (QS) was used as model material to investigate process conditions conducive to an effective moist carbonation in a rotating drum. Prior to use, the QS was dried at 105 °C.
Moisture content of the BBA was 0.3 wt% as determined gravimetrically after drying subsamples at 105 °C for 24 h. The loose bulk density of the native BBA and dry QS was determined by weighing a graduated cylinder holding 300 mL of each material. The compacted bulk density (tap density) was determined in a graduated cylinder holding initially 1000 mL of the corresponding material. The volume after compaction was determined after tapping the cylinder three times vertically from 10 cm height. The loose bulk densities were 0.90 and 1.53 kg/L, and the tap densities were 1.14 and 1.64 kg/L for BBA and QS, respectively. The particle size distribution (Fig. 1) as determined by sieving with stainless-steel screens (mesh size 2 mm, 1 mm, 0.5 mm, 0.25 mm, 0.1 mm [33]) classifies both materials as sand [34] (see Fig. 2 for a visual impression).
A previous study [8] with BBA from the same incineration plant indicated a moisture-dependent CO2 uptake when the material was exposed to pure CO2 in a fixed bed setup. The BBA sample used in this study showed the same optimum moisture corresponding to L/S 0.3 or 0.4 (maximum uptake of 32 g/kg, see section S1 of the supplementary material).
Experimental Setup
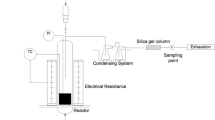
The carbonation experiments were conducted in a rotating drum reactor. Figure 3 shows the experimental setup and pictures are presented in the supplementary material section S8. The reactor was a stainless-steel cylinder with an inner diameter of 0.3 m and a length of 1.5 m running within v-seal rings in static front plates. The drum was driven by a variable-speed motor with a chain drive attached to a sprocket on the outer reactor mantle.
Setup of the carbonation experiments with axial section of the reactor. (1) CO2 and compressed air supply, (2) gas mixing station for constant flux and concentration of CO2, (3) water reservoir on a scale for acquisition of the water flow rate and adjustment of peristaltic pump, (4) flowmeters to adjust an equal supply to both nozzles, (5) static front plates equipped with v-seals and gas outlets, (6) two-fluid nozzles for supply of water and reactant gas, (7) bearing wheels, (8) variable-speed motor with drive sprocket, (9) scale measuring the mass of the reactor, (10) temperature and relative humidity sensor, (11) CO2 sensor, (12) biomass bottom ash or quartz sand
Four stainless steel pipes were conducted through one front plate feeding two two-fluid nozzles (MC GmbH, Type Z-F 4, Ostfildern, Germany). The nozzles served for spraying water and feeding gas into the reactor. They were positioned at 0.38 m and 1.13 m from the reactor front-end and directed to the material bed with a flat spraying characteristic (spraying angle 80° to 130°, depending on the fluid pressure). Gas outlets were integrated in the reactor front and back ends. The exhaust was routed to a flow-through cell equipped with sensors for temperature, relative humidity (AREXX, TSN-TH70E, RS Zwolle, The Netherlands) and CO2 (MRU, VarioPlus Industrial, Neckarsulm, Germany) with tolerances of ± 0.5 °C, ± 4.5% RH and ± 0.5 vol% CO2. Temperature and humidity were recorded every minute and CO2 concentration was logged every 10 s.
The reactant gas was continuously supplied by a gas mixing station (HiTec Zang GmbH, Gmix, Herzogenrath, Germany) mixing CO2 (N4.5) and compressed air. Water was supplied by a peristaltic pump to both nozzles. The water supply was determined gravimetrically by weighing the water reservoir (Kern & Sohn GmbH, FCB30K1, Balingen, Germany; accuracy of ± 1 g) and recording the mass every 10 s. The water supply to the nozzles was monitored with two flowmeters (Biotech e.K., FCH-m-PP-LC, Vilshofen, Germany) and adjusted by a valve. The latter ensured equal flow through each nozzle. The reactor and measurements were controlled with an automation controller (Measurement Computing, PMD-1208FS, Middleboro, Massachusetts, USA) and a software interface in National Instruments LabView 2017.
The mass of the reactor was logged every 5 min with an accuracy of ± 10 g (Kern & Sohn GmbH, DE300K5DL, Balingen, Germany) after stopping the rotation for 10 s to achieve a stable value.
Experimental Procedure
Characterization of Bed Motion in the Rotating Drum Reactor
The influence of rotation rate, fill level, and moisture content on the bed motion was studied with QS as model material for the sandy BBA. This avoided the formation of hazardous dust during the humidification of the solid as well as the alteration of material properties (e.g. due to uncontrolled carbonation) during the experiments.
For the bed behavior study, the reactor was equipped with a transparent acrylic glass front plate and the bed motion was recorded with a video camera (Logitech c920 HD, resolution 1920 × 1080 pixels). The videos were visually analyzed in terms of the mode of bed motion (slipping, slumping, rolling, or transitional). Results were used to construct so-called bed behavior diagrams [28] of the model substrate that related the bed motion to the operation parameters. The latter included fill level (in vol% of the void volume), reactor rotation rate (in rpm), and moisture content of the bed (expressed as liquid to solid ratio, L/S). The named parameters were varied between 5 and 20 vol%, 0.5 and 7 rpm, and L/S 0.0 to 0.2, respectively. Humidification of QS was performed prior to reactor loading in a cement mixer by slowly adding the water with a spray flask, which ensured a homogeneous moisture content distribution in the QS.
Carbonation Experiments
The carbonation of BBA was studied varying (i) the degree of humidification (L/S of 0.1, 0.2 and 0.3) and (ii) the mode of CO2 feeding. The latter included a CO2 supply simultaneous with or subsequent to humidification. A total of 10 experiments was conducted including two replications and two blank tests with QS as an inert material with nil CO2 uptake capacity (see Table 1 for a list of all experiments).
All carbonation experiments with BBA were conducted with a reactor loading of 19.1 kg dry matter corresponding to a fill level of 20 vol%. A rotation rate of 7 rpm was adjusted, throughout. Based on the findings of the bed behavior study (see Sect. “Bed Motion of Dry and Moist Quartz Sand”) a favorable material motion was expected under these conditions.
Water was added over a period of 45 min at the beginning of all experiments to ensure a slow humidification and adequate mixing. The water flow rate was adjusted such as to yield the desired L/S within the above timeframe. In the simultaneous feeding mode, the two-fluid nozzles were synchronously supplied with water and the CO2-rich reactant gas. In the subsequent feeding mode, compressed air was supplied together with water to maintain equal spraying characteristics. After the humidification period, compressed air was changed for the reactant gas. The gas flow rate was kept at 50 L/min (referring to standard conditions, i.e. dry gas, 101.325 kPa, 0 °C), throughout. Based on the typical composition of flue gas from wood combustion [35] the CO2 concentration was adjusted to 10 vol% and fed for 120 min. The latter is in accordance with the conditions of a previous study performed with the same BBA in a fixed bed reactor setup [8] and is a realistic residence time for potential full-scale applications [36]. Considering the flow rate, CO2-level, duration of feed, and reactor loading the cumulative specific CO2 supply was ~ 62 g CO2/kg BBA.
Experiments with empty reactor served to characterize the gas phase dynamics. With onset of CO2 feeding the exhaust CO2 concentration responded almost immediately and reached > 95% of the input concentration within 6 min (approx. three times the mean residence time). This observation conforms to the characteristic of an ideal reactor (continuous stirred-tank reactor without reaction) absent of a concentration gradient in the gas phase.
The bed motion of BBA was visually analyzed before humidification and after 120 min of CO2 feeding through a sight hole. Five samples of the solid material were taken after each experiment at the axial positions of approx. 0.00, 0.38, 0.75, 1.12 and 1.50 m.
Quantification of CO2 Uptake
The CO2 uptake by BBA was determined from (i) a gas balance where lacking CO2 was considered as taken up by BBA and (ii) the BBA mass increase due to CO2 uptake. A comparison of the CO2 uptake as calculated by the two independent approaches is given in the supplementary material S6. For additional quality control experimental replications were also included (cf. Sect. “Carbonation Experiments”).
Using the gas balance, the specific CO2 uptake (ζbalance, in kg/kg) was calculated from the recorded input and output concentrations of CO2 accounting for the gas flow rate (Eq. 3).
where ρCO2 is the density of CO2 (1.98 kg/m3 at 101,325 Pa and 0 °C), mmat is the dry mater of the material in the reactor (19.1 kg), \(\dot{V}\) is the volumetric gas flow under standard conditions, c is the concentration of CO2 and Vr is the free volume of the reactor considering the density of the sample (0.09 m3). Indices in and out denote the input and output, respectively.
The volumetric gas flow at the output could not be determined, because the v-seal system did not ensure gas-tightness at slight overpressures that would have been induced by an exhaust hose and flow measurement. Therefore, the volumetric output flow was calculated according to Eq. 4 (for the derivation of the equation see the supplementary material S2).
In addition to the gas balance, the CO2 uptake by BBA was determined gravimetrically (ζgravimetric, in kg/kg). The mass difference of the reactor (Δmreactor) before and after the experiment minus the mass difference of the water reservoir (ΔmH2Oin) gives the absolute mass increase of the reactor system including water losses to the exhaust. The latter was calculated according to Eq. 5 assuming water saturation of the exhaust gas at the measured exhaust gas temperature (ϑ, in °C). Water vapor saturation was indicated by condensation of water in the exhaust system. The equation uses the vapor pressure of water (pH2O in Pa) and the absolute air pressure (ptotal, in Pa) as well as the molar mass of water (MH2O = 0.01802 kg/mol) and the molar volume of an ideal gas (Vm = 0.022 414 m3/mol at 101,325 Pa and 0 °C) following Dalton’s law (for the derivation of the equation and the used vapor pressure data see the supplementary material S3).
The mass increase by carbonation ζgravimetric was obtained by adding the absolute mass increase of the reactor minus the water addition [\(\Delta {m}_{r}\left(t\right)-\Delta {m}_{H2Oin}\left(t\right)\)] to the integral flux of water vapor over the reaction time (Eq. 6). The time course of the mass increase is exemplarily shown in section S5 of the supplement.
Analytical
The native material and bed samples taken from the reactor were analyzed for further evaluation of the carbonation process. Moisture content was determined gravimetrically after drying subsamples at 105 °C for 24 h. Batch leachates were prepared with demineralized water at a liquid-to-solid ratio (L/S) of 10 and immediately analyzed for pH and electrical conductivity (EC). The native material and samples of one replication each L/S were subjected to thermogravimetric analysis (TGA) and calcimetry.
Thermogravimetric analysis (TGA) was employed to quantify the content of carbonates. The mass loss between 600 and 750 °C observed while heating the samples with 10 K/min under inert (N2) atmosphere was attributed to the decomposition of carbonate [8, 37] and expressed as content of CO2 in g/kg.
Volumetric calcimetry was performed by measuring the volume of CO2 gas released upon acidification of the BBA samples according to DIN EN ISO 10693 [38]. In brief, approx. 1 g of the sample was acidified with 7 mL of hydrochloric acid (4 mol/L) in a closed system. The sample was stirred until no more gas evolved. The gas volume was determined with an accuracy of ± 0.1 mL and converted to the content of CO2 in g/kg.
Results and Discussion
Bed Motion of Dry and Moist Quartz Sand
Prior to the carbonation experiments the bed motion of the model material QS was studied at varied fill level (5 to 30 vol%), rotation rate (0.5 to 7 rpm), and moisture content (L/S 0.0 to 0.2). The modes of bed motion identified under these conditions are given in Fig. 4.
Overall, all relevant modes of bed motion (slipping, slumping and rolling) were identified. Irrespective of bed moisture and rotation rate, slipping predominated at a relatively low fill level while slumping and rolling occurred at higher fill levels. Depending on rotation rate, slumping (slower rotation) or rolling (faster rotation) was favored. This is in accordance with findings for various other materials [26, 28]. The transition between slumping and rolling varied with the L/S.
Dry QS exhibited a rolling bed motion across a wide range of fill levels and rotation rates. Pure slipping was identified at a fill level of 5%, only and was independent of the rotation rate. The transition between slumping and rolling happened at 0.5 rpm for fill levels above 10%. The transition is in accordance with findings by Henein et al. [28]. However, the authors did not observe any slipping because their rotating drum was lined with abrasive paper.
The behavior of dry material differed greatly from the pattern of bed motion of moist QS with L/S 0.1 and 0.2 where slipping predominated over a wide range of conditions. Only at high fill levels and rotation rates slumping or rolling was achieved while their fields of occurrence shrank with increasing moisture. This indicates increased cohesion of the particles which promoted the movement of the bed as a whole rather than the movement of individual particles.
Slumping and rolling bed motion favor carbonation [26] since they provide better mixing and enhance the exposure of the solid’s surface to the gas phase. Thus, based on the findings obtained with QS the carbonation experiments with BBA were conducted at a fill level of 20 vol% and a rotation rate of 7 rpm, throughout.
Integrated Carbonation and Humidification of Biomass Bottom Ash
In the carbonation experiments three moisture levels (L/S of 0.1, 0.2, and 0.3) and two modes of CO2 feeding (simultaneous and subsequent to humidification) were studied.
Bed Motion and Humidification
The modes of BBA bed motion were visually inspected before and after the experiments. As anticipated from the physical properties, BBA showed a bed motion similar to QS. Whilst rolling prevailed under dry conditions the bed motion changed to slipping/slumping at L/S 0.1 and to slumping/rolling at L/S 0.2. Unlike QS, BBA could hold a water volume corresponding to a L/S of 0.3. That L/S caused mere slipping of the bed indicating strongly reduced wall friction due to the formation of a water film on the inner reactor mantle. As observed at the end of the experiments, the material partially granulated (see Fig. 5). Granulation as a side effect of the combined humidification and carbonation may be beneficial in view of geotechnical properties and is deemed useful in terms of material handling. Delineating process conditions to further enhance and control granulation is therefore part of ongoing research.
Samples collected after the experiments showed that the material’s moisture content was distributed unevenly along the reactor axis (cf. Figure S4 of the supplementary material). The samples taken at position 0.0 m (where the rotating steel cylinder connects to the static front plates) were significantly drier than the other samples indicating lower humidification and less effective mixing of the BBA in this section. For further experiments in a batch setup, this could be avoided by installing lifting devices which can also generate some axial movement of the material. In a continuously operated rotating drum, the axial movement and dispersion can prevent the formation of sections with less mixing and humidification. The mean moisture content (arithmetic mean of all samples for each experiment) was 0.02 to 0.05 kg H2O/kg dry BBA lower than the L/S calculated from the adjusted water addition. This indicates the evaporation of water which was also directly observed in experiments with CO2 feed subsequent to humidification. There, the absolute mass increase of the reactor system was negative until the feed of CO2 began (see supplementary material S5). The water vapor balance (cf. Sect. “Quantification of CO2 Uptake”) yielded cumulated losses of 0.01 to 0.02 kg H2O/kg dry BBA, thus partially explaining the discrepancy between calculated and measured L/S. Further, water is incorporated into the ash in a non-volatile form as shown in an earlier study [8]. In respect of future continuous drum reactors, an initial humidification with subsequent drying seems possible, which could reduce transportation costs as compared to moist material.
Cumulative CO2 Uptake
The cumulative CO2 uptake over 120 min of CO2 supply was quantified using the gas balance and, alternatively, using the gravimetric approach. Figure 6 shows the results of both approaches, where the gas balance (Fig. 6a) yielded slightly higher CO2 uptakes as compared to the gravimetric approach (Fig. 6b). Experimental replications indicated by the double bars in Fig. 6 yielded similar results. A further comparison of the quantification approaches and more detailed information on the replications is presented in section S6 of the supplementary material.
Overall, the CO2 uptake by BBA varied between 22 and 31 g/kg, which is far from full conversion of the alkaline components predicted from the elemental composition of the material (273 g/kg, [8]). The BBA had a significant carbonate content already before the carbonation (31–40 g/kg, see section S6 of the supplementary material), which increased to 42–76 g/kg after carbonation. The BBA sequestered 1/3 to 1/2 of the total CO2 supply (~ 62 g/kg). These results indicate, that the described setup may be suitable for purification of a CO2-rich gas like biogas [25]. At L/S 0.3 the CO2 uptake was slightly lower than at L/S 0.1 and 0.2. This is consistent with the slipping bed motion that prevailed at L/S 0.3 and which has been recognized as hampering carbonation compared to slipping and slumping modes [26].
After carbonation for 120 min, the leachates of the BBA yielded pH values between 12.3 and 12.7 (see section S7 of the supplementary material). Such values (pH > 10) indicate the presence of reactive alkaline (earth) oxides which have not yet undergone carbonation. In a previous study [8], the pH dropped below 10 when 92 g/kg CO2 were sequestered after contacting a fixed bed with pure CO2 for 168 h. However, such reaction times would exceed the possible residence time in continuously operated reactors.
Unlike the L/S-dependent CO2 uptake observed in a fixed bed set-up (cf. section S1 of the supplementary material) no clear maximum of the CO2 uptake can be discerned in Fig. 6. Also, no clear influence of the CO2 feeding modes on the cumulative CO2 uptake was observed, although in the simultaneous feed mode the carbonation was delayed until the material was humid enough (cf. Section “Time-Course of Carbonation”). Only, for L/S 0.1 an effect of the feeding mode can be recognized where the simultaneous feeding mode yielded a comparably lower CO2 uptake. This may have been due to the slow water addition at this particular L/S. When CO2 and water are added simultaneously, a critical moisture content must be exceeded before carbonation starts. This is not the case in the subsequent feed mode where humidification is accomplished before the material is contacted with CO2.
The maximum CO2 uptake observed in the rotating drum setup (31 g/kg) was similar to the results obtained in a fixed bed setup (32 g/kg; cf. section S1 of the supplementary material). This is remarkable for two reasons. First, the CO2 feed concentration in the rotating drum setup was substantially lower than in the fixed bed setup (10 vs. 100 vol%). Second, the specific CO2 supply was finite in the rotating drum experiments (62 g CO2/kg of BBA considering gas flow rate and concentration) while in the fixed bed experiments it was fully uptake-controlled and therefore, in principle, infinite.
In view of these facts, we attribute the achieved CO2 uptake and the reduced sensitivity towards moisture to the mixing process in the rotating drum. The latter is known to improve the mass transfer between gas and solids by permanently renewing the solid’s surface exposed to CO2 [25, 26, 39].
Other studies reported uptakes of 30 to 200 g CO2/kg woody biomass ash in fixed-bed setups for reaction times of 5 to 876 h [20,21,22]. These uptakes are considerably higher than those observed here in a similar fixed-bed setup ([8] and section S1 of the supplementary material) and in the rotating drum. Therefore, the differences in CO2 uptake may be mainly attributed to differences in ash composition.
Time-Course of Carbonation
The instrumentation of the rotating drum reactor allowed for a time-resolved analysis of the integrated humidification and carbonation of BBA under dynamic conditions. As an example, Fig. 7 depicts the time-course of the CO2 concentration along with the relative gas phase temperature (ΔT as difference between reactor temperature and ambient temperature) for the L/S 0.2 moisture condition. The upper section (Fig. 7a) shows the evolution of the named parameters for the simultaneous feeding mode, while the lower section (Fig. 7b) shows the results for the subsequent feeding mode.
Irrespective of the mode the breakthrough of CO2 was delayed indicating CO2 sequestration by BBA. The integral between the cin and cout signals is indicative of the CO2 consumption by carbonation. Initially, BBA carbonation led to a drop of the input CO2 down to a concentration below 1 vol%. Over time, cout increased following a sigmoidal characteristic. After 120 min of CO2 supply, cout was 9 vol% and thus close to the feed concentration (cin) in either feeding mode. However, cout did not equal cin after 120 min indicating ongoing CO2 uptake. Similarly in previous fixed-bed experiments [8], the carbonation continued even after 168 h without reaching full conversion of the alkaline components (see also Sect. “Cumulative CO2 Uptake”).
In the simultaneous feeding mode (Fig. 7a), the CO2 output peaked shortly after the start of feeding. This is explained by the initially dry BBA that is not capable of taking up CO2. Carbonation was therefore delayed until the material had sufficiently hydrated. After 8 min cout rebounded to a low level. This underlines that hydration and carbonation run consecutively, while the latter depends on the first.
In the subsequent feed mode where CO2 was added after humidification of the BBA, no initial CO2 peak appeared (Fig. 7b). Since the BBA was already moist when contacted with CO2, carbonation started immediately upon CO2 addition. At this point a small step increase of cout to 1.1 vol% was observed. Obviously, the residence time of the reactant gas was too low to fully suppress CO2 breakthrough.
The temperature course in Fig. 7a shows a steep increase of ΔT which peaked at 18 K after 35 min. This pronounced initial self-heating period coincides with the timeframe of humidification and the maximum CO2 uptake (cout values below 2.5 vol%). The temperature increase is explained by the superimposition of the reaction heat released by hydration and carbonation (cf. Eqs. 1 and 2). Until the end of the experiment, ΔT gradually decreased reaching 10 K after 120 min. This is mirrored by the concomitant decrease of carbonation intensity shown by the course of cout.
Also, in the subsequent CO2 feeding mode, ΔT peaked at 19 K (Fig. 7b). However, two distinct phases of self-heating were observed upon the feed of water and CO2, respectively. The lower self-heating during humidification is consistent with the lower enthalpy of hydration compared to carbonation (cf. Eqs. 1 and 2). Moreover, self-heating due to hydration was weakened by the presence of already hydrated CaO in the native BBA [8]. For future applications the temperature could be used as indicator to monitor the progress of hydration and carbonation reactions.
Conclusions
In this study a rotating drum reactor was successfully used for the humidification and carbonation of BBA. The effect of operating conditions (moisture content, rotation rate, fill level) on the pattern of bed motion was evaluated with a model material. Favorable bed motion was identified at a rotation rate of 7 rpm and a fill level of 20 vol%. These findings may also be useful for the operation of continuously fed reactors since parameters like length and inclination act on the axial transport but do not affect the transverse bed motion.
Carbonation of the dry-discharged BBA was performed by feeding a reactant gas with 10 vol% CO2. The maximum CO2 uptake of 31 g/kg was achieved at a moisture content of L/S 0.1 within a reaction time of 2 h, only. Effects of the gas feeding mode were marginal. In contrast to BBA carbonation under fixed-bed conditions where a clear optimum L/S could be discerned, the CO2 uptake in the rotating drum was rather insensitive towards the L/S highlighting the importance of substrate mixing for the CO2-uptake. Ongoing work aims at determining the effect of process conditions on the BBA granulation and at evaluating the CO2 uptake at L/S below 0.1. Further work should also evaluate the carbonation of other dry alkaline waste materials like fly ashes and slags in the integrated reactor. Also, the greenhouse-gas balance of the process including the necessary logistics must be evaluated in future studies.
Overall, the results confirm that the integrated humidification and carbonation of BBA in a single rotating drum reactor is feasible. This approach offers advantages in terms of occupational safety (dust emissions) and investment costs (one integrated reactor). Ongoing work is devoted to the implementation in a continuous-feed rotating drum reactor with respect to both, the solid and the gas phase. With such a process design, future full-scale applications should be possible.
Data Availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Rogelj, J., Shindell, D., Jiang, K., Fifita, S., Forster, P., Ginzburg, V., Handa, C., Kheshgi, H., Kobayashi, S., Kriegler, E., Mundaca, L., Séférian, R., Vilariño, M.V.: Mitigation pathways compatible with 1.5°C in the context of sustainable development. In: Global warming of 1.5°C. An IPCC special report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty. (2018)
Gough, C., Upham, P.: Biomass energy with carbon capture and storage (BECCS or Bio-CCS). Greenh. Gas Sci. Technol. 1, 324–334 (2011). https://doi.org/10.1002/ghg.34
Vassilev, S.V., Baxter, D., Andersen, L.K., Vassileva, C.G.: An overview of the composition and application of biomass ash. Part 1. Phase–mineral and chemical composition and classification. Fuel 105, 40–76 (2013). https://doi.org/10.1016/j.fuel.2012.09.041
Hope, E.S., McKenney, D.W., Allen, D.J., Pedlar, J.H.: A cost analysis of bioenergy-generated ash disposal options in Canada. Can. J. For. Res. 47, 1222–1231 (2017). https://doi.org/10.1139/cjfr-2016-0524
Voshell, S., Mäkelä, M., Dahl, O.: A review of biomass ash properties towards treatment and recycling. Renew. Sustain. Energy Rev. 96, 479–486 (2018). https://doi.org/10.1016/j.rser.2018.07.025
He, Q., Shi, M., Liang, F., Xu, L., Ji, L., Yan, S.: Renewable absorbents for CO2 capture: from biomass to nature. Greenh. Gas Sci. Technol. 9, 637–651 (2019). https://doi.org/10.1002/ghg.1902
Illikainen, M., Tanskanen, P., Kinnunen, P., Körkkö, M., Peltosaari, O., Wigren, V., Österbacka, J., Talling, B., Niinimäki, J.: Reactivity and self-hardening of fly ash from the fluidized bed combustion of wood and peat. Fuel 135, 69–75 (2014). https://doi.org/10.1016/j.fuel.2014.06.029
Schnabel, K., Brück, F., Pohl, S., Mansfeldt, T., Weigand, H.: Technically exploitable mineral carbonation potential of four alkaline waste materials and effects on contaminant mobility. Greenh. Gas Sci. Technol. 11, 506–519 (2021). https://doi.org/10.1002/ghg.2063
Bachmaier, H., Kuptz, D., Hartmann, H.: Wood ashes from grate-fired heat and power plants: evaluation of nutrient and heavy metal contents. Sustainability 13, 5482 (2021). https://doi.org/10.3390/su13105482
Pan, S.-Y., Chang, E.-E., Chiang, P.-C.: CO2 capture by accelerated carbonation of alkaline wastes: a review on its principles and applications. Aerosol Air Qual. Res. (2012). https://doi.org/10.4209/aaqr.2012.06.0149
Bodor, M., Santos, R., van Gerven, T., Vlad, M.: Recent developments and perspectives on the treatment of industrial wastes by mineral carbonation—a review. Cent. Eur. J. Eng. 3, 566–584 (2013). https://doi.org/10.2478/s13531-013-0115-8
da Costa, T.P., Quinteiro, P., Tarelho, L.A.C., Arroja, L., Dias, A.C.: Environmental assessment of valorisation alternatives for woody biomass ash in construction materials. Resour. Conserv. Recycl. 148, 67–79 (2019). https://doi.org/10.1016/j.resconrec.2019.04.022
Steenari, B.-M., Karlsson, L.G., Lindqvist, O.: Evaluation of the leaching characteristics of wood ash and the influence of ash agglomeration. Biomass Bioenergy 16, 119–136 (1999). https://doi.org/10.1016/S0961-9534(98)00070-1
Pan, S.-Y., Liu, H.-L., Chang, E.-E., Kim, H., Chen, Y.-H., Chiang, P.-C.: Multiple model approach to evaluation of accelerated carbonation for steelmaking slag in a slurry reactor. Chemosphere 154, 63–71 (2016). https://doi.org/10.1016/j.chemosphere.2016.03.093
Um, N., Nam, S.-Y., Ahn, J.-W.: Effect of accelerated carbonation on the leaching behavior of Cr in municipal solid waste incinerator bottom ash and the carbonation kinetics. Mater. Trans. 54, 1510–1516 (2013). https://doi.org/10.2320/matertrans.M-M2013809
Sun, J., Fernández Bertos, M., Simons, S.J.R.: Kinetic study of accelerated carbonation of municipal solid waste incinerator air pollution control residues for sequestration of flue gas CO2. Energy Environ. Sci. 1, 370 (2008). https://doi.org/10.1039/b804165m
Dong, F., Kirk, D.W., Tran, H.: Biomass ash alkalinity reduction for land application via CO2 from treated boiler flue gas. Fuel 136, 208–218 (2014). https://doi.org/10.1016/j.fuel.2014.07.059
dos Reis, G.S., Cazacliu, B.G., Artoni, R., Torrenti, J.-M.: Effect of the accelerated carbonation treatment on the recycled sand physicochemical characteristics through the rolling carbonation process. J CO2 Util 39, 101181 (2020). https://doi.org/10.1016/j.jcou.2020.101181
Vassilev, S.V., Vassileva, C.G., Petrova, N.L.: Mineral carbonation of biomass ashes in relation to their CO2 capture and storage potential. ACS Omega 6, 14598–14611 (2021). https://doi.org/10.1021/acsomega.1c01730
Lombardi, L., Costa, G., Spagnuolo, R.: Accelerated carbonation of wood combustion ash for CO2 removal from gaseous streams and storage in solid form. Environ. Sci. Pollut. Res. 25, 35855–35865 (2018). https://doi.org/10.1007/s11356-018-2159-z
Andersson, J., Nordberg, Å.: Biogas upgrading using ash from combustion of wood fuels: laboratory experiments. EER 7, 38 (2017). https://doi.org/10.5539/eer.v7n1p38
Fernández-Delgado Juárez, M., Mostbauer, P., Knapp, A., Müller, W., Tertsch, S., Bockreis, A., Insam, H.: Biogas purification with biomass ash. Waste Manage. 71, 224–232 (2018). https://doi.org/10.1016/j.wasman.2017.09.043
Vassilev, S.V., Vassileva, C.G.: Extra CO2 capture and storage by carbonation of biomass ashes. Energy Convers. Manage. 204, 112331 (2020). https://doi.org/10.1016/j.enconman.2019.112331
Łączny, J.M., Iwaszenko, S., Gogola, K., Bajerski, A., Janoszek, T., Klupa, A., Cempa-Balewicz, M.: Study on the possibilities of treatment of combustion by-products from fluidized bed boilers into a product devoid of free calcium oxide. J. Sustain. Min. 14, 164–172 (2015). https://doi.org/10.1016/j.jsm.2015.12.002
Lombardi, L., Carnevale, E.A., Pecorini, I.: Experimental evaluation of two different types of reactors for CO2 removal from gaseous stream by bottom ash accelerated carbonation. Waste Manage. 58, 287–298 (2016). https://doi.org/10.1016/j.wasman.2016.09.038
Brück, F., Schnabel, K., Mansfeldt, T., Weigand, H.: Accelerated carbonation of waste incinerator bottom ash in a rotating drum batch reactor. J. Environ. Chem. Eng. 6, 5259–5268 (2018). https://doi.org/10.1016/j.jece.2018.08.024
Mellmann, J.: The transverse motion of solids in rotating cylinders—forms of motion and transition behavior. Powder Technol. 118, 251–270 (2001). https://doi.org/10.1016/S0032-5910(00)00402-2
Henein, H., Brimacombe, J.K., Watkinson, A.P.: Experimental study of transverse bed motion in rotary kilns. MTB 14, 191–205 (1983). https://doi.org/10.1007/BF02661016
Liu, X.Y., Specht, E., Mellmann, J.: Experimental study of the lower and upper angles of repose of granular materials in rotating drums. Powder Technol. 154, 125–131 (2005). https://doi.org/10.1016/j.powtec.2005.04.040
Nase, S.T., Vargas, W.L., Abatan, A.A., McCarthy, J.J.: Discrete characterization tools for cohesive granular material. Powder Technol. 116, 214–223 (2001). https://doi.org/10.1016/S0032-5910(00)00398-3
Ding, Y.L., Forster, R.N., Seville, J.P.K., Parker, D.J.: Scaling relationships for rotating drums. Chem. Eng. Sci. 56, 3737–3750 (2001). https://doi.org/10.1016/S0009-2509(01)00092-6
Librandi, P., Costa, G., Stendardo, S., Baciocchi, R.: Carbonation of BOF slag in a rotary kiln reactor in view of the scale-up of the wet route process. Environ. Prog. Sustain. Energy 38, e13140 (2019). https://doi.org/10.1002/ep.13140
DIN EN ISO 17892-4:2017-04, Geotechnical investigation and testing—Laboratory testing of soil—Part 4: Determination of particle size distribution (ISO 17892-4:2016); German version of EN ISO 17892-4:2016, Beuth Verlag GmbH, Berlin (2017). https://doi.org/10.31030/2362539
DIN EN ISO 14688-1:2018-05, Geotechnical investigation and testing—Identification and classification of soil—Part 1: Identification and description (ISO 14688-1:2017); German version EN ISO 14688-1:2018, Beuth Verlag GmbH, Berlin (2018). https://doi.org/10.31030/2748424
Johansson, L.S., Leckner, B., Gustavsson, L., Cooper, D., Tullin, C., Potter, A.: Emission characteristics of modern and old-type residential boilers fired with wood logs and wood pellets. Atmos. Environ. 38, 4183–4195 (2004). https://doi.org/10.1016/j.atmosenv.2004.04.020
Schnabel, K., Brück, F., Mansfeldt, T., Weigand, H.: Full-scale accelerated carbonation of waste incinerator bottom ash under continuous-feed conditions. Waste Manage. 125, 40–48 (2021). https://doi.org/10.1016/j.wasman.2021.02.027
Rocca, S., van Zomeren, A., Costa, G., Dijkstra, J.J., Comans, R.N.J., Lombardi, F.: Mechanisms contributing to the thermal analysis of waste incineration bottom ash and quantification of different carbon species. Waste Manage. 33, 373–381 (2013). https://doi.org/10.1016/j.wasman.2012.11.004
DIN EN ISO 10693:2014-06, Soil quality—determination of carbonate content—Volumetric method (ISO 10693:1995); German version EN ISO 10693:2014, Beuth Verlag GmbH, Berlin (2014). https://doi.org/10.31030/2143356
Brück, F., Fröhlich, C., Mansfeldt, T., Weigand, H.: A fast and simple method to monitor carbonation of MSWI bottom ash under static and dynamic conditions. Waste Manage. 78, 588–594 (2018). https://doi.org/10.1016/j.wasman.2018.06.042
Acknowledgements
This work is part of a dissertation project that is being conducted by the first author at the Graduate Centre of Engineering Sciences of the Research Campus of Central Hessen under lead of the Technische Hochschule Mittelhessen (THM) University of Applied Sciences. It was partially supported by the German Industrial Research Foundation (Stiftung Industrieforschung) under Grant S0234/10267/2018. We thank Jürgen Henkel for his technical expertise and Niklas Rumpel for his assistance with the experiments.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
KS: conceptualization, investigation, writing—original draft. FB: conceptualization, methodology development, resources. SP: conceptualization, funding acquisition, supervision. HW: conceptualization, writing—review & editing, supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schnabel, K., Brück, F., Pohl, S. et al. Development and Test of a Rotating Drum Reactor for the Simultaneous Hydration and Carbonation of Dry Biomass Bottom Ash. Waste Biomass Valor 13, 4319–4330 (2022). https://doi.org/10.1007/s12649-022-01784-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-022-01784-z