Abstract
Agroindustrial wastes is an interesting opportunity for enzymes production, reducing operational costs and strengthening the biorefineries concept. The conversion of lignocellulosic waste to fermentable sugars is current challenge for biorefineries industries. Enzyme cocktail used in delignification and saccharification platform are produced by a combinatory of several lignocellulolytic enzymes. The aim of this study was to develop filamentous fungi compatible-consortia isolated from pineapple wastes for cellulose-degrading enzymes production using pineapple crown waste in solid-state cultivation and its potential for saccharification of pineapple crown waste. Isolates were screened in agar-plate and in solid-state cultivation for cellulases, xylanase and pectinases productions. Six compatible consortia of Trichoderma strains with Aspergillus niger or Pleurotus ostreatus were evaluated for SSC using pineapple crown. All consortia increased the enzymes production compared to monoculture. Consortia of Trichoderma asperellum PEC-6 and P. ostreatus increased 1.60-fold the xylanase and 1.42-fold the β-glucosidase productions. T. asperellum PEC-17 and A. niger increased 1.16-fold endoglucanase and 1.28-fold pectinase. Saccharification of pineapple crown waste using enzyme cocktail produced by filamentous fungi consortia increased the total reducing sugar released in 12.50–13.93% compared to enzymes produced by monocultures. This study provides an alternative model to the cocktail enzyme production by mixed cultures development with lower cost of on-site enzyme manufacture by use of agroindustrial wastes eliminating an enzyme blended step. Moreover, the procedure used in this work can be potentially cost saving and environmentally friendly and should be explored on other bioenergy feedstocks and feed production.
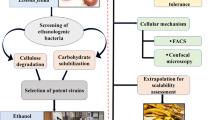
Graphic Abstract


Adapted from Sthal and Christensen et al.


Similar content being viewed by others
References
Zainuddin, M.F., Shamsudin, R., Mokhtar, M.N., Ismail, D.: Physicochemical properties of pineapple plant waste fibers from the leaves and stems of different varieties. BioResources 9, 5311–5324 (2014)
Roda, A., Lambri, M.: Food uses of pineapple waste and by-products: a review. Int. J. Food Sci. Technol. 54, 1009–1017 (2019)
Sadh, P.K., Duhan, S., Duahn, J.S.: Agro-industrial wastes and their utilization using solid state fermentation: a review. Bioresour. Bioprocess (2018). https://doi.org/10.1186/s40643-017-0187-z
Daud, Z., Hatta, M.Z.M., Kassim, A.S.M., Aripin, A.M.: Analysis of the chemical compositions and fiber morphology of pineapple (Anans comosus) leaves in Malaysia. J. Appl. Sci. 14, 1355–1358 (2014)
Asim, M., Abdan, K., Jawaid, M., Nasir, M., Dashtizadeh, Z., Ishak, M.R., Hoque, M.E.: A review on pineapple leaves fibre and its composites. Int. J. Polym. Sci. 2015, 1 (2015)
Banerjee, S., Ranganathan, V., Patti, A., Arora, A.: Valorisation of pineapple wastes for food and therapeutic applications. Trends Food Sci. Technol. 80, 60–70 (2018)
Hansen, G.H., Lübeck, M., Frisvada, J.C., Lübeck, P.S., Andersen, B.: Production of cellulolytic enzymes from ascomycetes: comparison of solid state and submerged fermentation. Process Biochem. 50, 1327–1341 (2015)
Druzhinina, I.S., Chenthamara, K., Zhang, J., Atanasova, L., Yang, D., Miao, Y., et al.: Massive lateral transfer of genes encoding plant cell wall-degrading enzymes to the mycoparasitic fungus Trichoderma from its plant-associated hosts. PLoS Genet. 14, e1007322 (2018)
Zhao, C., Deng, L., Fang, H.: Mixed culture of recombinant Trichoderma reesei and Aspergillus niger for cellulase production to increase the cellulose degrading capability. Biomass Bioenergy 112, 93–98 (2018)
Goswami, D., Thakker, J.N., Dhandhukia, P.C.: Portraying mechanics of plant growth promoting rhizobacteria (PGPR): a review. Cogent Food Agric. (2016). https://doi.org/10.1080/23311932.2015.1127500
Poulsen, H.V., Wolfgang, F.W., Ingvorsen, K.: Aerobic and anaerobic cellulase production by Cellulomonas uda. Arch. Microbiol. 198, 725–735 (2016)
Cardoso, W.S., Queiroz, P.V., Tavares, G.P., Santos, F.A., Soares, F.E.F., Kasuya, M.C.M., Queiroz, J.H.: Multi-enzyme complex of white rot fungi in saccharification of lignocellulosic material. Braz. J. Microbiol. 49, 879–884 (2018)
Farinas, C.S.: Developments in solid-state fermentation for the production of biomass degrading enzymes for the bioenergy sector. Renew. Sustain. Energy Rev. 52, 179–188 (2015)
Abdel-Rahman, M.A., El-Din, M.N., Refaat, B.M., Abdel-Shakour, E.H., Ewais, E.E.-D., Alrefaey, H.M.A.: Biotechnological application of thermotolerant cellulose-decomposing bacteria in composting of rice straw. Ann. Agric. Sci. 61, 135–143 (2016)
Liu, J., Shi, P., Ahmad, S., Yin, C., Liu, X., Liu, Y., Zhang, H., Xu, Q., Yan, H., Li, Q.: Co-culture of Bacillus coagulans and Candida utilis efficiently treats Lactobacillus fermentation wastewater. AMB Express 9, 15 (2019)
Unban, K., Khatthongngam, N., Shetty, K., Khanongnuch, C.: Nutritional biotransformation in traditional fermented tea (Miang) from north Thailand and its impact on antioxidant and antimicrobial activities. Food Sci. Technol. 56, 2687–2699 (2019)
Fang, H., Zhao, C., Song, X.Y., Chen, M., Chang, Z., Chu, J.: Enhanced cellulolytic enzyme production by the synergism between Trichoderma reesei RUT-C30 and Aspergillus niger NL02 and by the addition of surfactants. Biotechnol. Bioprocess. Eng. 18, 390–398 (2013)
Kolasa, M., Ahring, B.K., Lübeck, P.S., Lübeck, M.: Co-cultivation of Trichoderma reesei RutC30 with three black Aspergillus strains facilitates efficient hydrolysis of pretreated wheat straw and shows promises for on-site enzyme production. Bioresour. Technol. 169, 143–148 (2014)
Vogel, H.J.: A convenient growth medium for Neurospora crassa (medium N). Microbiol. Genet Bull. 13, 42–43 (1956)
Castellani, A.: Maintenace and cultivation of the common pathogenic fungi of man in sterile distilled water. J. Trop. Med. Hyg. 70, 181–184 (1967)
Raper, K.B.: Fennel, D.I.: The genus Aspergillus. Robert & Krieger, Florida, (1977)
Pitt, J.I.: A Laboratory Guide to Common Penicillium Species. Commonwealth scientific and industrial research organization. Div. Food Proces, North Wales (1988)
Rifai, M.A.: A revision of the genus Trichoderma. Mycology 116, 1–56 (1969)
Raeder, U., Broda, P.: Rapid preparation of DNA from filamentous fungi. Lett. Appl. Microbiol. 1, 17–20 (1985)
Kumar, S., Stecher, G., Tamura, K.: MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874 (2016)
Jukes, T.H., Cantor, C.R.: Evolution of protein molecules. In: Munro, H.N. (ed.) Mammalian Protein Metabolism. Academic Press, New York (1969)
Saitou, N., Nei, M.: The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4, 406–425 (1987)
Felsenstein, J.: Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39, 783–791 (1985)
Ghose, T.K.: Measurement of cellulase activities. Pure Appl. Chem. 59, 257–268 (1987)
Miller, G.L.: Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31, 426–428 (1959)
Molla, A.H., Fakhru’l-Razi, A., Abd-Aziz, S., Hanafi, M.M., Alam, M.Z.: In vitro compatibility evaluation of fungal mixed culture for bioconversion of domestic wastewater sludge. World J. Microbiol. Biotechnol. 17, 849–856 (2001)
Alam, M.D.Z., Fakhru’l-Razi, A., Abd-Aziz, S., Molla, A.H.: Optimization of compatible mixed cultures for liquid state bioconversion of municipal wastewater sludge. Water Air Soil Pollut. 149, 113–126 (2003)
Skidmore, A.M., Dickinson, C.M.: Colony interactions and hyphal interference between Sepatoria nodorum and phylloplane fungi. Trans. Br. Mycol. Soc. 66, 57–64 (1976)
Stahl, P.D., Christensen, M.: In vitro mycelial interactions among members of a soil microfungal community. Soil Biol. Biochem. 24, 309–316 (1992)
Latif, F., Rajoka, M.I., Malik, K.A.: Saccharification of Leptochloa fusca (kallar grass straw) using thermostable cellulases. Bioresour. Technol. 50, 107–111 (1994)
Passos, D.F., Pereira Jr., N., Castro, A.M.: A comparative review of recent advances in cellulases production by Aspergillus, Penicillium and Trichoderma strains and their use for lignocellulose deconstruction. Curr. Opin. Green Sustain. Chem. 14, 01860–01866 (2018)
Ten, L.N., Im, W.T., Kim, M.K., Kang, M.S., Lee, S.T.: Development of a plate technique for screening of polysaccharide degrading microorganisms by using a mixture of insoluble chromogenic substrates. J. Microbiol. Methods 56, 375–382 (2004)
Shelley, A.W., Deeth, H.C., MacRae, I.C.: Review of methods of enumeration, detection and isolation of lipolytic microorganisms with special reference to dairy applications. J. Microbiol. Methods 6, 123–137 (1987)
Teather, R.M., Wood, P.J.: Use of Congo Red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl. Environ. Microbiol. 43, 777–780 (1982)
Ruegger, M.J.S., Tauk-Tornisielo, S.M.: Atividade da celulase de fungos isolados do solo da Estação Ecológica de Juréia-Itatins, São Paulo. Brasil. Rev. Bras. Bot. 27, 205–211 (2004)
Babu, C.R., Ketanapalli, H., Beebi, S.K., Kolluru, V.C.: Wheat bran-composition and nutritional quality: a review. Adv. Biotechnol. Microbiol. 9, 555754 (2018)
Valencia, E.Y., Chambergo, F.S.: Mini-review: Brazilian fungi diversity for biomass degradation. Fungal Gen. Biol. 60, 9–18 (2013)
Delabona, P.S., Pirota, R.D.P.B., Codima, C.A., Tremacoldi, C.R., Rodrigues, A., Farinas, C.S.: Using amazon forest fungi and agricultural residues as a strategy to produce cellulolytic enzymes. Biomass Bioenergy 37, 243–250 (2012)
Florencio, C., Couri, S., Farinas, C.S.: Correlation between agar plate screening and solid-state fermentation for the prediction of cellulase production by Trichoderma strains. Enzyme Res. 2012, 7 (2012)
Taher, I.B., Bennour, H., Fickers, P., Hassouna, M.: Valorization of potato peels residues on cellulase production using a mixed culture of Aspergillus niger ATCC 16404 and Trichoderma reesei DSMZ 970. Waste Biomass Valor. 8, 183–192 (2017)
Troiano, D., Orsat, V., Dumont, M.J.: Status of filamentous fungi in integrated biorefineries. Renew. Sustain. Energy Rev. 117, 109742 (2020)
Marx, I.J., Wyk, N., Smit, S., Jacobson, D., Viljoen-Bloom, M., Volschenk, H.: Comparative secretome analysis of Trichoderma asperellum S4F8 and Trichoderma reesei Rut C30 during solid-state fermentation on sugarcane bagasse. Biotechnol. Biofuels 6, 172 (2013)
Wu, Q., Sun, R., Ni, M., Yu, J., Li, Y., Yu, C., et al.: Identification of a novel fungus, Trichoderma asperellum GDFS1009, and comprehensive evaluation of its biocontrol efficacy. PLoS ONE 12, e0179957 (2017)
Inoue, H., Kitao, C., Yano, S., Sawayama, S.: Production of b-xylosidase from Trichoderma asperellum KIF125 and its application in efficient hydrolysis of pretreated rice straw with fungal cellulase. World J. Microbiol. Biotechnol. 32, 186 (2016)
Abdel-Ghany, T.M., Ganash, M., Bakri, M.M., Al-Rajhi, A.M.H.: Molecular characterization of Trichoderma asperellum and lignocellulolytic activity on barley straw treated with silver nanoparticles. BioResources 13, 1729–1744 (2018)
Victoria, J., Odaneth, A., Lali, A.: Importance of cellulase cocktails favouring hydrolysis of cellulose. Prep. Biochem. Biotechnol. 47, 547–553 (2017)
Ma, K., Ruan, Z.: Production of a lignocellulolytic enzyme system for simultaneous bio-delignification and saccharification of corn stover employing co-culture of fungi. Bioresour. Technol. 175, 586–593 (2015)
Acknowledgements
The authors thank the Pró-Reitoria de Pesquisa e Pós-Graduação (PROPESQ/UFT) for the financial support (Edital PROPESQ/PROEX no 24/2013), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001, Laboratory of Microorganism Biochemical-UNESP, Rio Claro, São Paulo, Brazil and Dra. Sâmia Maria Tauk-Tornisielo, CEA-UNESP, Rio Claro, São Paulo, Brazil.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Teixeira, W.F.A., Batista, R.D., do Amaral Santos, C.C.A. et al. Minimal Enzymes Cocktail Development by Filamentous Fungi Consortia in Solid-State Cultivation and Valorization of Pineapple Crown Waste by Enzymatic Saccharification. Waste Biomass Valor 12, 2521–2539 (2021). https://doi.org/10.1007/s12649-020-01199-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-020-01199-8