Abstract
Purpose
This study aims to employ five marine Pseudoalteromonas strains, belonging to Psa. arctica DSM 18437T, Psa. carrageenovora DSM 6820T, Psa. issachenkonii LMG 19697T, Psa. rubra DSM 6842T and Psa. tunicata DSM 14096T to ferment brown crab processing side streams.
Methods
The generated hydrolysates after the fermentation were screened for their antioxidant, anthelmintic and anti-biofilm activities.
Results
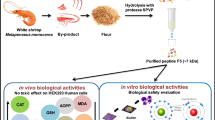
After 72 h, the highest degree of hydrolysis (DoH) of 14.92% was reached by Psa. issachenkonii. The highest 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging ability was obtained by hydrolysates of Psa. rubra with an IC50 of 2.23 mg/mL while for the 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging ability, the maximum was reached by the hydrolysates of Psa. tunicata with an IC50 of 1.23 mg/mL. Anthelmintic activity, as tested on Caenorhabditis elegans, revealed a 99% mortality rate at 1 mg/mL with ethyl acetate extracts of the hydrolysates of Psa. arctica, Psa. issachenkonii and Psa. rubra. In terms of antibiofilm activity, aqueous extracts of the hydrolysates of Psa. arctica and Psa. issachenkonii at 500 µg/mL could dramatically decrease the biofilm formation index of Staphylococcus epidermidis RP62A from 1.35 to 0.37 and 0.36, respectively.
Conclusions
Brown crab processing side streams could be valorized by marine Pseudoalteromonas bacteria fermentation for the production of a mixture of bioactive compounds.
Graphic Abstract



Similar content being viewed by others
References
Barrento, S., Marques, A., Teixeira, B., Mendes, R., Bandarra, N., Vaz-Pires, P., Nunes, M.L.: Chemical composition, cholesterol, fatty acid and amino acid in two populations of brown crab Cancer pagurus ecological and human health implications. J. Food Compos. Anal. 23, 716–725 (2010). https://doi.org/10.1016/j.jfca.2010.03.019
Ervik, H., Lierhagen, S., Asimakopoulos, A.G.: Elemental content of brown crab (Cancer pagurus)—is it safe for human consumption? A recent case study from Mausund, Norway. Sci. Total Environ. 716, 135175 (2019)
Hamed, I., Ozogul, F., Regenstein, J.M.: Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): a review. Trends Food Sci. Technol. 48, 40–50 (2016). https://doi.org/10.1016/j.tifs.2015.11.007
Healy, M.G., Romo, C.R., Bustos, R.: Bioconversion of marine crustacean shell waste. Resour. Conserv. Recy. 11, 139–147 (1994). https://doi.org/10.1016/0921-3449(94)90085-X
Yen, M.T., Yang, J.H., Mau, J.L.: Physicochemical characterization of chitin and chitosan from crab shells. Carbohydr. Polym. 75, 15–21 (2009). https://doi.org/10.1016/j.carbpol.2008.06.006
Abdou, E.S., Nagy, K.S., Elsabee, M.Z.: Extraction and characterization of chitin and chitosan from local sources. Bioresour. technol. 99, 1359–1367 (2008). https://doi.org/10.1016/j.biortech.2007.01.051
Shahidi, F., Synowiecki, J.: Isolation and characterization of nutrients and value-added products from snow crab (Chionoecetes opilio) and shrimp (Pandalus borealis) processing discards. J. Agric. Food Chem. 39, 1527–1532 (1991)
Shirai, K., Guerrero, I., Huerta, S., Saucedo, G., Castillo, A., Gonzalez, O., Hall, G.M.: Effect of initial glucose concentration and inoculation level of lactic acid bacteria in shrimp waste ensilation. Enzyme Microb. Technol. 28, 446–452 (2001). https://doi.org/10.1016/s0141-0229(00)00338-0
Beaulieu, L., Thibodeau, J., Bryl, P., Carbonneau, M.E.: Characterization of enzymatic hydrolyzed snow crab (Chionoecetes opilio) by-product fractions: a source of high-valued biomolecules. Bioresour. technol. 100, 3332–3342 (2009). https://doi.org/10.1016/j.biortech.2009.01.073
Chen, Y.C., Chiang, T.J., Liang, T.W., Wang, I.L., Wang, S.L.: Reclamation of squid pen by Bacillus licheniformis TKU004 for the production of thermally stable and antimicrobial biosurfactant. Biocatal. Agric. Biotechnol. 1, 62–69 (2012)
Wang, S.L., Liu, K.C., Liang, T.W., Kuo, Y.H., Wang, C.Y.: In vitro antioxidant activity of liquor and semi-purified fractions from fermented squid pen biowaste by Serratia ureilytica TKU013. Food Chem. 119, 1380–1385 (2010). https://doi.org/10.1016/j.foodchem.2009.09.017
Wang, S.L., Yen, Y.H., Tsiao, W.J., Chang, W.T., Wang, C.L.: Production of antimicrobial compounds by Monascus purpureus CCRC31499 using shrimp and crab shell powder as a carbon source. Enzyme Microb. Technol. 31, 337–344 (2002). https://doi.org/10.1016/S0141-0229(02)00135-7
ISO: Determination of nitrogen content, ISO 937:1978 standard. In: International Standards Meat and Meat Products. International Organization for Standadization, Genève (1978)
ISO: Determination of moisture content, ISO 937:1997 standard. In: International Standards Meat and Meat Products. International Organization for Standadization, Genève (1997)
ISO: Determination of crude ash content, ISO 5984:2002 standard. In: Animal feeding stuffs. International Organization for Standadization, Genève (2002)
ISO: Determination of total fat content, ISO 1443:1973 standard. In: International Standards Meat and Meat Products. International Organization for Standadization, Genève (1973)
Adler-Nissen, J.: Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. J. Agric. Food Chem. 27, 1256–1262 (1979). https://doi.org/10.1021/jf60226a042
Sharma, O.P., Bhat, T.K.: DPPH antioxidant assay revisited. Food Chem. 113, 1202–1205 (2009). https://doi.org/10.1016/j.foodchem.2008.08.008
Arnao, M.B., Cano, A., Acosta, M.: The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 73, 239–244 (2001). https://doi.org/10.1016/S0308-8146(00)00324-1
Sandasi, M., Leonard, C.M., Viljoen, A.M.: The in vitro antibiofilm activity of selected culinary herbs and medicinal plants against Listeria monocytogenes. Lett. Appl. Microbiol. 50, 30–35 (2010). https://doi.org/10.1111/j.1472-765X.2009.02747.x
Niu, C., Gilbert, E.S.: Colorimetric method for identifying plant essential oil components that affect biofilm formation and structure. Appl. Environ. Microbiol. 70, 6951–6956 (2004). https://doi.org/10.1128/Aem.70.12.6951-6956.2004
Brenner, S.: The genetics of Caenorhabditis elegans. Genetics 77, 71–94 (1974)
Stiernagle, T.: Maintenance of C. elegans. In: Hobert, O. (Ed.) WormBook: The Online Review of C. elegans Biology. The C. elegans research community: (2006)
Beaulieu, L., Thibodeau, J., Bonnet, C., Bryl, P., Carbonneau, M.: Detection of antibacterial activity in an enzymatic hydrolysate fraction obtained from processing of Atlantic rock crab (Cancer irroratus) by-products. PharmaNutrition. 1, 149–157 (2013)
Rebeca, B.D., Penavera, M.T., Diazcastaneda, M.: Production of fish-protein hydrolysates with bacterial proteases—yield and nutritional-value. J Food Sci. 56, 309–314 (1991). https://doi.org/10.1111/j.1365-2621.1991.tb05268.x
Fling, S.P., Gregerson, D.S.: Peptide and protein molecular-weight determination by electrophoresis using a high-molarity tris buffer system without urea. Anal. Biochem. 155, 83–88 (1986). https://doi.org/10.1016/0003-2697(86)90228-9
Manni, L., Ghorbel-Bellaaj, O., Jellouli, K., Younes, I., Nasri, M.: Extraction and characterization of chitin, chitosan, and protein hydrolysates prepared from shrimp waste by treatment with crude protease from Bacillus cereus SV1. Appl. Biochem. Biotechnol. 162, 345–357 (2010). https://doi.org/10.1007/s12010-009-8846-y
Sila, A., Sayari, N., Balti, R., Martinez-Alvarez, O., Nedjar-Arroume, N., Moncef, N., Bougatef, A.: Biochemical and antioxidant properties of peptidic fraction of carotenoproteins generated from shrimp by-products by enzymatic hydrolysis. Food Chem. 148, 445–452 (2014). https://doi.org/10.1016/j.foodchem.2013.05.146
Jiang, W., Hu, S.W., Li, S.J., Liu, Y.: Biochemical and antioxidant properties of peptidic fraction generated from crab (Portunus trituberculatus) shells by enzymatic hydrolysis. Int. J. Food Sci. Tech. 52, 2479–2488 (2017). https://doi.org/10.1111/ijfs.13533
Sachindra, N.M., Bhaskar, N.: In vitro antioxidant activity of liquor from fermented shrimp biowaste. Bioresour. technol. 99, 9013–9016 (2008). https://doi.org/10.1016/j.biortech.2008.04.036
Feher, D., Barlow, R.S., Lorenzo, P.S., Hemscheidt, T.K.: A 2-substituted prodiginine, 2-(p-Hydroxybenzyl)prodigiosin, from Pseudoalteromonas rubra. J. Nat. Prod. 71, 1970–1972 (2008). https://doi.org/10.1021/np800493p
Vitale, G.A., Sciarretta, M., Palma Esposito, F., January, G.G., Giaccio, M., Bunk, B., et al.: Genomics–metabolomics profiling disclosed marine vibrio spartinae 3.6 as a producer of a new branched side chain prodigiosin. J. Nat. Prod. 83, 1495–1504 (2020)
Juang, R.S., Yeh, C.L.: Adsorptive recovery and purification of prodigiosin from methanol/water solutions of Serratia marcescens fermentation broth. Biotechnol. Bioproc. E. 19, 159–168 (2014). https://doi.org/10.1007/s12257-013-0547-2
Arivizhivendhan, K.V., Mahesh, M., Boopathy, R., Swarnalatha, S., Mary, R.R., Sekaran, G.: Antioxidant and antimicrobial activity of bioactive prodigiosin produces from Serratia marcescens using agricultural waste as a substrate. J. Food Sci. Technol. 55, 2661–2670 (2018). https://doi.org/10.1007/s13197-018-3188-9
Flemming, H.C., Wingender, J.: The biofilm matrix. Nat. Rev. Microbiol. 8, 623–633 (2010). https://doi.org/10.1038/nrmicro2415
Aslam, S.: Effect of antibacterials on biofilms. Am. J. Infect. Control 36, e9–e11 (2008). https://doi.org/10.1016/j.ajic.2008.10.002
Donlan, R.M.: Preventing biofilms of clinically relevant organisms using bacteriophage. Trends Microbiol. 17, 66–72 (2009). https://doi.org/10.1016/j.tim.2008.11.002
Van Houdt, R., Michiels, C.W.: Biofilm formation and the food industry, a focus on the bacterial outer surface. J. Appl. Microbiol. 109, 1117–1131 (2010). https://doi.org/10.1111/j.1365-2672.2010.04756.x
Estrela, A.B., Heck, M.G., Abraham, W.R.: Novel approaches to control biofilm infections. Curr. Med. Chem. 16, 1512–1530 (2009). https://doi.org/10.2174/092986709787909640
Naves, P., Del Prado, G., Huelves, L., Gracia, M., Ruiz, V., Blanco, J., Rodríguez-Cerrato, V., Ponte, M., Soriano, F.: Measurement of biofilm formation by clinical isolates of Escherichia coli is method‐dependent. J. Appl. Microbiol. 105, 585–590 (2008)
Dheilly, A., Soum-Soutera, E., Klein, G.L., Bazire, A., Compere, C., Haras, D., Dufour, A.: Antibiofilm activity of the marine bacterium Pseudoalteromonas sp. strain 3J6. Appl. Environ. Microbiol. 76, 3452–3461 (2010). https://doi.org/10.1128/AEM.02632-09
Papa, R., Parrilli, E., Sannino, F., Barbato, G., Tutino, M.L., Artini, M., Selan, L.: Anti-biofilm activity of the Antarctic marine bacterium Pseudoalteromonas haloplanktis TAC125. Res. Microbiol. 164, 450–456 (2013). https://doi.org/10.1016/j.resmic.2013.01.010
Silipo, A., Leone, S., Lanzetta, R., Parrilli, M., Sturiale, L., Garozzo, D., et al.: The complete structure of the lipooligosaccharide from the halophilic bacterium Pseudoalteromonas issachenkonii KMM 3549T. Carbohydr. Res. 339, 1985–1993 (2004). https://doi.org/10.1016/j.carres.2004.05.008
Bandara, H.M., Lam, O.L., Watt, R.M., Jin, L.J., Samaranayake, L.P.: Bacterial lipopolysaccharides variably modulate in vitro biofilm formation of Candida species. J. Med. Microbiol. 59, 1225–1234 (2010). https://doi.org/10.1099/jmm.0.021832-0
Valle, J., Da Re, S., Henry, N., Fontaine, T., Balestrino, D., Latour-Lambert, P., Ghigo, J.M.: Broad-spectrum biofilm inhibition by a secreted bacterial polysaccharide. Proc. Natl. Acad. Sci. U. S. A. 103, 12558–12563 (2006). https://doi.org/10.1073/pnas.0605399103
Holden-Dye, L., Walker, R.J.: Anthelmintic drugs. In: WormBook: The Online Review of C. elegans Biology. 1–13 (2007). https://doi.org/10.1895/wormbook.1.143.1
Holden-Dye, L., Walker, R.: Anthelmintic drugs and nematocides: studies in Caenorhabditis elegans. In: WormBook: The Online Review of C. elegans Biology. 1–29 (2014). https://doi.org/10.1895/wormbook.1.143.2
Lustigman, S., Prichard, R.K., Gazzinelli, A., Grant, W.N., Boatin, B.A., McCarthy, J.S., Basanez, M.G.: A research agenda for helminth diseases of humans: the problem of helminthiases. PLoS Negl. Trop. Dis. 6, e1582 (2012). https://doi.org/10.1371/journal.pntd.0001582
Abad, P., Gouzy, J., Aury, J.M., Castagnone-Sereno, P., Danchin, E.G., Deleury, E., et al.: Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat. Biotechnol. 26, 909–915 (2008). https://doi.org/10.1038/nbt.1482
Coles, G.C., Jackson, F., Pomroy, W.E., Prichard, R.K., von Samson-Himmelstjerna, G., Silvestre, A., Taylor, M.A., Vercruysse, J.: The detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 136, 167–185 (2006). https://doi.org/10.1016/j.vetpar.2005.11.019
Bar-Eyal, M., Sharon, E., Spiegel, Y.: Nematicidal activity of Chrysanthemum coronarium. Eur. J. Plant Pathol. 114, 427–433 (2006). https://doi.org/10.1007/s10658-006-0011-7
Jiang, Z., Boyd, K.G., Mearns-Spragg, A., Adams, D.R., Wright, P.C., Burgess, J.G.: Two diketopiperazines and one halogenated phenol from cultures of the marine bacterium, Pseudoalteromonas luteoviolacea. Nat. Prod. Lett. 14, 435–440 (2000). https://doi.org/10.1080/10575630008043781
Colgrave, M.L., Kotze, A.C., Ireland, D.C., Wang, C.K., Craik, D.J.: The anthelmintic activity of the cyclotides: natural variants with enhanced activity. Chembiochem. 9, 1939–1945 (2008). https://doi.org/10.1002/cbic.200800174
Chatterjee, J., Laufer, B., Kessler, H.: Synthesis of N-methylated cyclic peptides. Nat. Protoc. 7, 432–444 (2012). https://doi.org/10.1038/nprot.2011.450
Gu, Y.Q., Mo, M.H., Zhou, J.P., Zou, C.S., Zhang, K.Q.: Evaluation and identification of potential organic nematicidal volatiles from soil bacteria. Soil Biol. Biochem. 39, 2567–2575 (2007). https://doi.org/10.1016/j.soilbio.2007.05.011
Acknowledgements
This study was performed in the framework of the BlueShell project (Exploring Shellfish By-Products as a source of Blue Bioactivies) supported by ERA-Marine Biotech (ERAMBT) and Fonds voor Wetenschappelijk Onderzoek— Vlaanderen (FWO).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zou, Y., Tortorella, E., Robbens, J. et al. Bioactivity Screening of Hydrolysates From Brown Crab Processing Side Streams Fermented by Marine Pseudoalteromonas Strains. Waste Biomass Valor 12, 2459–2468 (2021). https://doi.org/10.1007/s12649-020-01195-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-020-01195-y