Abstract
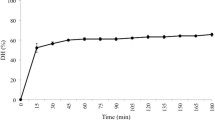
In the actual context of food safety and circular economy, the separation of the α137–141 antimicrobial peptide (TSKYR, 653 Da) coming from the hydrolysis of bovine hemoglobin, a non-valorized slaughterhouse by-product, and its reuse as a preservative agent for food products during storage would be of major interest. However, depending on the degree of hydrolysis (DH) of the bovine hemoglobin, the TSKYR peptide environment into the hydrolysate will be different and would impact its recovery yield by electrodialysis with ultrafiltration membranes (EDUF), a hybrid and eco-friendly technology. In this context, five DHs (3, 5, 10, 13 and 18%) were investigated to study the influence of the peptide populations on the α137–141 selective separation by EDUF. It appeared from these results that the most appropriate DH was of 5% since a lower population of peptides between 500 and 1000 Da was present and the subunits of hemoglobin were already digested. Hence, an enrichment factor of about 13 folds for the α137–141 was obtained in comparison with the initial hydrolysate. At our knowledge, it was the first time that the effect of the hydrolysis degree of a protein is demonstrated to impact preferentially the separation of a specific peptide by EDUF.
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Statement of Novelty
This work shows a new opportunity of a circular economy for meat industries. Indeed, high environmental impacts of slaughterhouse wastes could decrease by converting blood into natural preservatives. These bioproducts could be a great alternative for chemical additives largely used to protect food. Nevertheless, the separation of know and natural preservatives from slaughterhouse blood is not easy. This study shows how to maximize the recovery (and the efficiency) of the natural preservatives by using a new, eco-friendly and sustainable technology. Moreover, the obtained preservatives are safe and previously described as powerful antimicrobials. This work fits perfectly with the concept of waste bioconversion and shows a great proof-concept of circular economy for production of preservatives from slaughterhouse waste.
Introduction
Recently, due to the increasing pressure of the governments to decrease the wastes produced by the agri-food sector, the United Nations created a new program named “Transforming our world: 2030 for sustainable development”. Therefore, the food industries had an urgent need for potential technological ways to answer this problematic [1,2,3]. In meat industry, a new global pressure is growing to minimize the environmental impact and to decrease the unutilized parts of animals not directly involved in meat processing. Indeed, this kind of by-products can be defined as substances or objects resulting from a production process not primarily aimed at producing. Nevertheless, slaughterhouse by-products are various like bones, skins, wool or blood and are rich in bioactive compounds [4, 5]. Bovine blood is a by-product from slaughterhouse produced in large quantity all around the world since each slaughtered beef produces about 12 L of blood and considering that more than 4.3 million of beefs, only in France, were slaughtered in 2017 [4]. Blood contains large amount of hemoglobin, a protein not valorized since the main use of blood is focused on its colorless part, the plasma, for pharmaceutical and food industry applications [6, 7]. Indeed, after centrifugation of the raw bovine blood, the recovered red fraction contains mainly hemoglobin. This protein is broadly described as a rich source of bioactive peptides after enzymatic hydrolysis [8,9,10]. In addition, the antimicrobial activity of peptides generated from hemoglobin is particularly well-documented [11,12,13,14]. Antimicrobial peptides are particularly interesting due to their various structures and activities against pathogenic strains [15]. Their relative innocuity is due to their mechanism of action which is different from classical antibiotic [16]. Moreover, antimicrobial peptides are multifunctional and have generally another simultaneous bioactivity such as antioxidant [17]. Hence, antimicrobial peptides could be potential candidate for the substitution of various synthetic additive [18, 19]. One of these peptides from hemoglobin hydrolysis, the α137–141 fragment (Thr-Ser-Lys-Tyr-Arg), has interesting and particular characteristics. It contains only 5 amino-acids (for a molecular weight of 653 Da and a pI of 10.5) while the classical antimicrobial peptides are composed of 20 amino-acids or more. Moreover, this peptide is hydrophilic and without any secondary structure in contrary of classical antimicrobial peptides having hydrophobic residues and an α-helix in their structures [15]. At last, the α137–141 peptide has a large antibacterial spectrum, especially against pathogenic bacteria commonly responsible for food biological alteration [20]. Two recent studies showed that this peptide has also an antioxidant activity delaying the rancidity after addition into meat [21, 22]. Consequently, this antimicrobial and antioxidant peptide is a natural promising preservative for the substitution of synthetic additives widely used to protect food during its storage and distribution [2, 21, 22].
However, the main challenge in the production of such a bioactive peptide derived from proteins is its isolation from the complex feed hydrolysate. Indeed, the variety in sizes and charges of the peptides present after hydrolysis makes its separation difficult. Nevertheless, its separation, enrichment or purification, is a necessary step to obtain a significant bioactivity [23, 24]. Using solvent for peptide extraction is one possible approach [25,26,27]. Nevertheless, solvents or toxic chemicals are discouraged for food application and could cause environmental problem for their recycling or the process scale-up [28]. Consequently, the methodologies involved chromatography process or liquid–liquid separation are not appropriate due to their expensive costs for production of peptide fractions, generally with no-circular economy approach. Other technics as pressure-driven processes (like ultrafiltration) are more economic and sustainable but they are not able to separate compounds with similar molecular weights due to their weak selectivity [29, 30]. Recently, a hybrid technology, called electrodialysis with ultrafiltration membrane (EDUF), was developed to make headway in terms of high selectivity and eco-friendly separation of compounds from complex mixtures [30]. Indeed, EDUF allows the separation of molecules according to their charges (application of an electric field as driving force) and their molecular weights (cut-off of the filtration membrane). This technology showed successful results on the fractionation of hydrolysates from different sources, such as alfalfa white protein [31], soy [32] and snow crab [33]. Nonetheless, at our knowledge, no study demonstrates the influence of peptide population on the selective separation of one peptide from a complex hydrolysate during EDUF treatment. In this context, the aim of the present work was to study the effect of hydrolysis degree of bovine hemoglobin derived from slaughterhouse by-product on the EDUF process selectivity with a particular focus on the α137–141 antibacterial peptide recovery.
Materials and Methods
Chemicals
All chemicals and solvents were of analytical grade from commercial suppliers: Sigma-Aldrich (Saint-Quentin Fallavier, France) or Flandres Chimie (Villeneuve d’Ascq, France). The purified bovine hemoglobin (BH) was also supplied by Sigma-Aldrich. The ultrapure water was prepared using a Milli-Q system. The standard α137–141 was provided by Genecust (Luxembourg).
Hydrolysate Preparation
A stock solution was prepared by adding 15 g of BH into 100 mL of ultrapure water. The real BH concentration (CBH) was dosed with the Drabkin’s method [34], after 30 min of centrifugation at 4000 min−1. After that, the stock solution was diluted to obtain a final CBH of 1% (w/v).
The hydrolysis was carried out at pH 3.5 and under a constant temperature of 23 °C, using the ratio enzyme/substrate = 1/11 (mole/mole) [35]. The BH was digested by pepsin from porcine gastric mucosa (EC 3.4.23.1, 3200–4500 units mg−1 protein), broadly described to generate bioactive peptides from BH [8,9,10]. The peptic hydrolysis was stopped by addition of sodium hydroxide (5 M) up to a final pH of 9 after 2.5 and 30 min, 3, 10 and 24 h corresponding to respective degrees of hydrolysis (DH) of 3, 5, 10, 13 and 18%. The DH was assessed by the ortho-phthaldialdehyde method [36]. After hydrolysis, the hydrolysate was decolorized in order to eliminate the remaining haem since it was shown in a previous study that the presence of haem has a negative impact on the migration transfer during EDUF [37]. Removing the haem consisted to decrease slowly the pH at a value of 4.7 with HCl (2 M). The haem was totally precipitated and peptides were maintained in the solution after 24 h at room temperature. Both phases were separated by a 30 min-centrifugation (4000 min−1). After this step, the hydrolysate was ready for EDUF electroseparation.
Electroseparation
Electrodialysis with Ultrafiltration Membrane Equipment
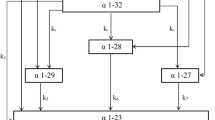
The electrodialysis cell was a MicroFlow type cell (Electrocell AB, Täby, Sweden) with an effective area of 10 cm2. The cell configuration is displayed in Fig. 1. The use of a cationic configuration allowed the recovery of the positive charged compounds. Four closed compartments were delimited by the different membranes. In this configuration, the anionic and cationic membranes separated the Na2SO4 (20 g L−1) electrodes rinsing solution. On both sides of the ultrafiltration membrane, the cationic peptides recovery (a 2 g L−1 KCl solution) and the feed compartments (a freshly prepared hydrolysate at 1% w/v) were circulated. Each compartment was connected to a separate external reservoir to allow the recirculation of the solution by using centrifugal pumps (Iwaki, Marcoussis, France). The flow rate of each solution was controlled by a flowmeter (Kobold Instrumentation, Cergy-Pontoise, France). Finally, the anode/cathode voltage difference was supplied by a variable 0–30 V power source HQ Power PS3003 (Xantrex, Burnaby, Canada).
The ultrafiltration membrane (UF) used was a polyethersulfone (PES) with a molecular weight cut-off of 10 kDa (HFK-131, Koch, France). The ion-exchange membranes were Neosepta cationic (CMX-SB) and anionic membrane (AMX-SB) from Astom (Tokyo, Japan).
Experimental and Cleaning Protocols
The EDUF treatment was performed during 4 h. All the compartments contained 500 mL of solution (Na2SO4, KCl or hydrolysate). The voltage was maintained constant (20 V), corresponding to an electric field of 9 V cm−1. The circulation flows were of 18 L h−1 for the electrode solution (Na2SO4) and 12 L h−1 for the cationic peptides recovery (KCl) and the feed compartments (hydrolysate). Every 30 min, samples were recovered for further RP-HPLC and LC–MS analyses. Moreover, the pH and the conductivity (mS.cm−1) were measured within the recovery and hydrolysate compartments. The demineralization rate (DR, in %) was calculated by the following Eq. (1):
where Conductivityt=0 (mS cm−1) is the conductivity measured at the beginning of the experiment before current application and Conductivityt (mS cm−1) is the conductivity at a given time t.
The current intensity (A) and the voltage (V) were also recorded. For the five selected hydrolysates (DH of 3, 5, 10, 13 and 18%), each electroseparation was carried out in triplicate. All the membranes in the EDUF cell were changed for a new tested DH.
After each repetition, the EDUF was cleaned to remove a potential deposit in the apparatus, as previously described [31]: water (5 min), a NaOH solution (0.1 M, 30 min), water (5 min) and then a HCl solution (0.1 M, 20 min) were circulated in the system. Finally, the neutral pH was recovered in all the compartments by rinsing with ultrapure water.
Analyses
pH
The pH was measured in the feed and KCl solution every 30 min during the EDUF treatment with a pH-meter Model SP20 provided by Thermo Fisher Scientific (Villebon-sur-Yvette, France).
Conductivity
The conductivity was measured in the feed and KCl solution every 30 min during the EDUF treatment with a conductimeter 4520 (Jenway, France).
Current Intensity
The current intensity was directly read on the power supply and recorded every 30 min during the EDUF treatment.
Apparent System Resistance
The apparent system resistance was determined using the Ohm’s law. The current intensity and the voltage were monitored during the EDUF treatment as above-mentioned. The apparent system resistance was calculated according to the following Eq. (2):
where R is the global system resistance (Ω), I the current intensity (A) and U the voltage applied between the electrodes of the EDUF cell (V).
RP-HPLC analyses, Total Peptide Concentration and α137–141 Quantification
The liquid chromatographic system consisted of a waters 600E automated gradient controller pump module, a Waters Wisp 717 automatic sampling device and a Waters 996 photodiode array detector. Spectral and chromatographic data were stored on an NEC image 466 computer. Millennium software was used to plot, acquire and analyze chromatographic data. All the chromatographic processes were performed with a Vydac C4 column (250 mm × 4.6 mm, internal diameter). The mobile phases were ultrapure water/trifluoroacetic acid (1000:1, v/v) as eluent A, and acetonitrile/ultrapure water/trifluoroacetic acid (600:400:1, v/v/v) as eluent B. Samples were filtered through 0.20 µm filters and then injected. Online UV absorbance scans were performed between 200 and 390 nm at a rate of one spectrum per second with a resolution of 1.2 nm. Chromatographic analyses were completed with Millenium software [11, 38]. The volume of injection was 10 µL for the total hydrolysate and 100 µL for the samples from the recovery compartment. The flow rate was 0.6 mL min−1. The gradient applied was 100% (v/v) A over 5 min, 0–67% (v/v) B over 30 min, then 67–87% (v/v) B over 35 min.
The total peptide concentration of each sample from bovine hemoglobin hydrolysate was evaluated by measuring the total area corresponding to the peptides (Atot) using the Millennium software and correlated with the initial CBH measured by using the Drabkin’s method [34].
To identify and quantify rapidly the α137–141 peptide within the hydrolysate, the spectral comparison and assessment of peak purity were carried out by UV-spectral comparison as described previously [11, 38]. The α137–141 retention time was about 4 min. A standard curve was established by injections of standard α137–141 at concentrations ranging between 0 and 1 mg mL−1. The areas under the peak were taken from a chromatographic profile at 215 nm and allowed to quantify the α137–141 peptide present in sample by using the following Eq. (3) (R2 = 0.999):
where Cα137–141 being the α137–141 concentration (mg L−1) and Aα137–141 being the area peak (µV s).
The α137–141 purity (%) was assessed by (4):
where Aα137–141 being the area peak (µV s) and Atotal (µV s) being the total peptide area of chromatogram.
Transport Rate and Enrichment Factor
The transport rate was expressed in g m−2 h−1 from the previous total peptide concentration determined by HPLC considering the membrane surface area (m2) and the EDUF treatment duration (h).
The peptide enrichment factor (EF) was calculated by Eq. (5):
Mass Spectrometry Analyses
The LC–MS analysis were performed on a UFLC-XR device (Shimadzu, Japan) coupled to a QTRAP® 5500 MS/MS hybrid system triple quadrupole/linear ion trap mass spectrometer (AB Sciex, Foster City, CA, USA) equipped with a Turbo VTM ion source. Instrument control and data acquisition were performed using the Analyst 1.5.2 software. The RPLC separation was carried out on the same column used for the RP-HPLC analyses (§ 2.4.5). The elution was performed with the same gradient, previously described, using formic acid (0.1%) instead of trifluoroacetic acid (0.1%) for the RP-HPLC analyses. MS analysis was carried out in positive ionization mode using an ion spray voltage of 5500 V. The nebulizer gas (air) and the curtain gas (nitrogen) flows were set at 30 psi. The Turbo VTM ion source was set at 550 °C with the auxiliary gas flow (air) set at 50 psi. The MS/MS analyses were completed with the BioAnalyst 1.5.1 and Peaks 7 softwares.
Statistical Analyses
Mean values for various parameters (α137–141 concentration, α137–141 purity, α137–141 transport rate, α137–141 enrichment factor) were subjected to one-way analyses of variance. Statistical significance was declared at the p value ≤ 0.05.
Results
Characterization of the Whole Initial Hydrolysates
The RP-HPLC profiles of the five initial hydrolysates were presented in Fig. 2. It appeared from the chromatograms that at DH 3%, the hemoglobin protein was not totally hydrolyzed by the pepsin and the α- and β-chains were still present in the hydrolysate (retention time from 37 to 42 min). At this DH, a first peptide population was mainly present with a retention time from 22 to 36 min. This peptide population was composed of intermediate peptides with high molecular weights, formed of 20 or more amino-acids. At DH 5%, the α- and β-chains were totally digested (no peak present between 37 and 42 min) and the intermediate peptide population decreased in intensity (from 31 to 36 min) to lead to smaller peptides (from 20 to 30 min). The population between 5 to 8 min and 15 to 20 min of retention time increased in intensity. This population was essentially composed by “final peptides” which had low molecular weights and are generally composed by 5 to 10 amino-acids. This population increased with the DH evolution and was more present at high DH, as observed for DHs of 10, 13 and 18%. In the opposite, the intermediate peptide population (from 31 to 36 min) decreased when the DH increased. At the DH of 18%, this population was totally cleaved in smaller peptides. Therefore, in the conditions of hydrolysis carried out (pH 3.5, 23 °C, ratio enzyme/substrate = 1/11), the enzymatic mechanism was of “zipper” type [35], as described for the first time in 1952 [39]. It allowed the generation of different peptide populations from a substrate according to the hydrolysis duration. Briefly, the enzyme catalytic speed is high at the proteolysis beginning creating peptides with high molecular weights. During hydrolysis, these generated peptides become new substrates for the enzyme and can be hydrolyzed into smaller peptides. When the hydrolysis time is high, peptide population is composed by peptides with small molecular weights.
RP-HPLC profiles of whole hydrolysates at five different hydrolysis degrees. Peptide population were essentially composed by peptides with high molecular weights (high retention times) at short hydrolysis times (weak DH). In the opposite, peptide population were essentially composed by peptides with small molecular weights (low retention times) at high hydrolysis times (high DH)
The identification of all peptides present was performed by LC–MS/MS. The number and the repartition by mass-ranges of the peptides are displayed in Table 1. So, the number of peptides during the hydrolysis varied from 78 to 162, with a progressive accumulation of low molecular weights (< 1000 Da) from 29 (at a DH of 3%) to 121 (at a DH of 18%).
Recently, the hydrolysis kinetics of bovine or porcine hemoglobin by pepsin have been studied for hydrolysis times from 0 min to 24 h [40]. Each time corresponded to a different DH with a new and different peptide population. These peptide populations were various in terms of present peptides. After a hydrolysis of 2.5 min, the α and β chains of hemoglobin disappeared in favor of a large mass of peptides eluted before the hem. Moreover, when the hydrolysis degree increased, the peptide molecular weights decreased corresponding to the cleavage from hydrophobic high molecular weight peptides to hydrophilic low molecular weight peptides.
Electrodialytic Paramaters Evolution
Conductivity, Demineralization Rate and pH
For the hydrolysate compartment, the conductivity as well as the pH did not evolve significantly during the EDUF treatment whatever the tested DH (data not shown) with an averaged value of 9 mS cm−1 and a pH of 4.7 which was the initial pH of the decolorized hydrolysate. On the opposite, the conductivity of the cationic peptides recovery compartment decreased throughout the EDUF treatment and that whatever the tested DH (Fig. 3). This conductivity decrease was in accordance with the electrodialytic cell configuration used. Indeed, the K+ migrated from the KCl solution to the electrode solution through the cation-exchange membrane and the Cl− migrated from the recovery compartment to the feed through the UF membrane. However, if the initial conductivity was the same for all DHs (about 3 mS cm−1), the final conductivity and its behavior during the experiment were not similar: the conductivity decreased, or demineralization rate increased as a function of DH decrease. Consequently, the highest final demineralization rates were observed for hydrolysates produced at DHs of 3 and 5% (89.40 ± 0.83 and 87.25 ± 4.09%, respectively), while at a DH of 18% the resulting hydrolysate presented the lowest demineralization rate after 4 h of treatment: 18% DH demineralization rate was of 49.00 ± 2.01%, close to DH of 13% (54.32 ± 1.68%). Moreover, for all tested DHs, a two-steps demineralization was observed: a first rapid demineralization period followed by a second slower demineralization step. This two-steps demineralization did not appear at the same time according to the DH. Hence, for DHs of 10, 13 and 18%, the conductivity decreased quickly during the first 30 min with a mean slope of − 1.48, to thereafter slowed down with a mean demineralization slope of − 0.26. For DHs of 3% and 5%, the second step began only after respectively about 1 h and 0.5 h of experiment with respective slopes of − 0.48 and − 0.56. This two-steps conductivity decrease was due to the fact that the limiting current density was reached and overpassed during the process. This phenomenon was broadly described in previous papers [31, 41, 42]. Overpassing the limiting current density induced the phenomenon of water-splitting which could occur on the feed-side of the anion-exchange membrane and/or the recovery-side of the cation-exchange membrane [42]. In our case, water splitting occurred mainly at the interface of the cation-exchange membrane since the pH in the feed compartment was stable all along the treatment, which was not the case for the recovery compartment. Indeed, the release of OH− at the interface of the CEM into the peptide recovery compartment induced a fast increase of its pH: after about 30 min of treatment, for DHs higher or equal to 5%, the pH of the recovery compartment was up to 10 for an initial value of about 5 while at 3% DH, the pH increased to 10 after about 1 h (data not shown). These results are quite in accordance with the appearance of the second demineralization step. Indeed, by generating OH− species in the peptide recovery compartment, the pH of the solution will increase and also its conductivity. In fact, the generation of OH− entering the peptide recovery compartment has compensated for the migration of K+ and Cl− leaving this compartment: the electrical conductivity of OH− is largely higher than the ones of Cl− and K+ [42], so the generation of OH− in the solution has slowed down the decrease in conductivity due to the leaving of K+ and Cl−. This explained that the conductivity decrease of the 3% DH was more pronounced in comparison with other DHs since the limiting current density reaching and so the generation of OH− were delayed. This also applied for the 5% DH but at a lower extent.
Evolution of conductivities into the recovery compartment during the EDUF treatment in the different conditions of bovine hemoglobin degree of hydrolysis. The KCl solution conductivities decreased all along the EDUF treatment with an important first-step decrease during the first 30 min, regardless the used DH
The difference in appearance times according to the DH could be explained by the peptide population diversity and/or the membrane fouling. Indeed, the elevation of DH increased the number of peptides able to migrate as well as decreased their size facilitating their potential migration. So, the ionic migration from the recovery compartment could be slowed down more rapidly, because the peptides in the feed-side of the UF membrane were in competition with the ions in counter-current flow (i.e. from recovery to feed). Concerning membrane fouling, the fact that small peptides were more numerous as seen on Fig. 2 and Table 1, these peptides would have probably interacted or increased the possibilities of interactions with the membranes, probably the UF membrane. These results showed that the most important ionic migration was observed at the low DHs. However, it is suggested that for the peptide migration, the most appropriate DH would be the hydrolysate at the DH of 5%, i.e. the lowest DH without the undigested subunits of hemoglobin.
Apparent System Resistance
With a 20 V constant voltage applied (U) during the EDUF treatment, the intensity (I) decreased from 0 to 4 h. Hence, according to the Ohm’s law, the initial resistances of the system (R) were similar for all tested DHs and increased all along the process duration (data not shown), from around 64.5 Ω at the beginning to 250 Ω at the end, as presented on Fig. 4. However, the apparent system resistance increased more rapidly at higher DHs and resistances of 142.86 ± 10.20 Ω were observed for DH of 13 and 18% after 1 h of treatment versus 80.00 ± 3.20 Ω at a DH of 3% and 86.96 ± 3.78 Ω at 5%. These results confirmed the previous results obtained for conductivity and concluding to the appearance of water splitting. Due to the resistance increase in the EDUF cell, the limiting current density was reached in advance at high DHs, generating OH− at the CEM interface in contact with the recovery compartment. These results would be probably due to the formation of a fouling on the ultrafiltration membrane side in contact with the hydrolysate. As mentioned previously, the peptide population diversity as well as their higher number and lower sizes at higher DHs would have probably interacted or increased the possibilities of interactions with the membranes, increasing consequently the global resistance of the cell and the generation of OH− at the CEM. It was reported in the literature that the interaction peptide–peptide and peptide-membrane can affect the transfer ability of the membrane [43]. Moreover, according to previous works [32], the polyethersulfone (PES) ultrafiltration membrane acquired more negative charges at pH measured during the EDUF treatment (about 10) and with this EDUF configuration. This would explain the increase in membrane fouling with a DH elevation by electrostatic interactions of the peptides charged positively [32, 44, 45].
Effect of Hydrolysis Degree on the Total Peptide Migration
Figure 5 shows the RP-HPLC profiles of the recovery compartment after 4 h of EDUF treatment for the hydrolysates at five different DHs. At DH 3%, few peptide peaks were clearly identified according to their low intensities on the chromatogram. At DH 5%, a first population appeared from 14 to 17 min and a second one between 22 and 30 min, mainly corresponding to the retention time of the intermediate peptides. From DH 5% to 18%, the intensities of peptide peaks between 15 to 30 min increased and the number of peptide peaks increased too.
Moreover, Fig. 6 presents the evolution of recovered total peptide concentration during the 4 h of experiment. The recovered concentration for DH 3% showed a linear trend with a plateau reached rapidly (30 min) at a total peptide concentration of 0.97 ± 0.14 mg L−1. The evolution of recovered concentrations for DH 5 and 10% were quite similar with total peptide concentrations of 17.01 ± 2.41 and 13.09 ± 4.75 mg L−1, respectively. The DHs of 13 and 18% showed a trend similar with a final peptide concentration of 68.48 ± 1.62 and 67.43 ± 5.36 mg L−1, respectively. For the hydrolysate with a DH of 3%, the peptide migration was the least effective. The peptide transfer towards the KCl solution was slowed down by the small quantity of peptides able to migrate due to the low hydrolysis degree of the protein. Indeed, the identification of peptides by LC–MS/MS (Table 2) showed that only 4 peptides out of 78 in the whole hydrolysate are recovered in the KCl solution and 3 of these 4 peptides had a mass ranging from 501 to 1000 Da. Hence, when the DH increased, the recovered peptide population increased too. The maximal number of recovered peptides was recorded for the hydrolysate at a DH of 18%. In this condition, 29 peptides migrated through the ultrafiltration membrane with masses ranging from 501 to 1000 Da. In addition, the highest concentrations of peptides in the recovery compartment were observed for hydrolysates at DH of 13 and 18%, with respective peptide concentrations of 68.48 ± 1.62 and 67.43 ± 5.36 mg L−1. From Table 2, it also appeared that peptides with a mass between 501 and 1000 Da migrated more than the other peptides. According to Table 1, the first reason was that the peptides in this mass-range were the more numerous and their number increased with an increase in DH. Secondly, the digestion of the peptides with high molecular weights (> 2000 Da) induced their progressive disappearance during the hydrolysis. These results were in accordance to a recent study which showed that when the DH increased, the peptide number increased too [40]. So, the small peptides could interact less with the high peptides and then migrate more through the ultrafiltration membrane at an advanced DH. On the other hand, the number of peptides able to migrate were higher than at low DH. So, the migration of a given small peptide (< 1000 Da) would be more competitive at a high DH due to the number of peptides generated and present in the mixture.
Effect of the Hydrolysis Degree on the Antibacterial α137–141 Peptide Recovery
As observed on Fig. 5 for the peptide recovery chromatograms, at a DH of 3%, the α137–141 peptide was not present (retention time of 5 min). At DH 5%, the α137–141 peptide started to be present (5 min). From DH 5% to 18%, the α137–141 peptide peak showed a decrease in the recovery compartment when the hydrolysate DH increased.
Table 3 details the concentration, purity and transport rate for the α137–141 peptide in the different conditions of hydrolysate DH. Due to the “zipper” mechanism and the fact that the α137–141 (retention time of about 4 min on Fig. 2) was a final peptide in these hydrolysis conditions [35], its concentration in the hydrolysate increased over the hydrolysis time. The final α137–141 concentration in the peptide recovery compartment for the hydrolysate with a DH of 3% was the poorest: no significant α137–141 concentration was measured at the end of the EDUF treatment. For DHs higher that 3%, the α137–141 transfer through the ultrafiltration membrane was the least effective with the hydrolysate at a DH of 18% (0.17 ± 0.04 g m−2 h−1). Consequently, no significant enrichment factor was observed at a DH of 18% although the α137–141 concentration was higher in the whole hydrolysate. The hydrolysate with a DH of 5% presented the higher transport rate of α137–141 peptide during the 4 h of EDUF treatment with a value of 0.67 ± 0.04 g m−2 h−1, which corresponded to a concentration factor of fourfold higher than at DH of 18%. Hence, the observed enrichment factor decreased with an increase in DH.
Concerning the purity of the α137–141 peptide into the recovery compartment, it was up to 10.27 ± 0.04% at a DH of 5%. This value of α137–141 purity was eightfold higher than the value at a DH of 18% (1.20 ± 0.04%): an enrichment factor of about 13 was obtained at a DH of 5%. To explain the best migration of α137–141 at a DH of 5%, the evolution of its concentration into the recovery compartment was followed during the 4 h of experiment (Fig. 7). At the end of EDUF treatment, the highest α137–141 concentration into the recovery compartment was obtained with the hydrolysate at a DH of 5% (5.33 ± 0.34 mg L−1). Moreover, its evolution was the most linear compared to the recoveries with the other DH. This behavior was reflected by the competition with other peptides with similar mass-range. Indeed, the more the DH increased, the more the peptides from 500 to 1000 Da increased in the whole hydrolysate.
Evolution of the α137–141 peptide concentration in the KCl solution during the EDUF treatment in the different conditions of bovine hemoglobin degree of hydrolysis. The α137–141 concentration increased with the DH increase with an optimum at the DH of 5%. After this DH, the α137–141 concentration decreased
So, these data demonstrated that the peptide population had a strong effect on the α137–141 selective separation. Indeed, the α137–141 transfer through the ultrafiltration membrane was less effective in the presence of numerous small peptides (from 0 to 1000 Da). According to these results, the most appropriate DH is of 5% to separate selectively the antibacterial α137–141 peptide. Indeed, this hydrolysate has the smaller population of small peptides (< 1000 Da), after the hydrolysate at DH of 3% but this last one contains undigested sub-units of hemoglobin.
Conclusion
This study demonstrated that the degree of hydrolysis of a protein modulates the process selectivity and the peptide migration rate. With an advanced degree of hydrolysis, the peptides migrated at a higher concentration into the cationic peptide recovery compartment. Likewise, the number of recovered total peptides increased into the recovery compartment. Consequently, the most appropriate degree of hydrolysis was 5% to separate preferentially the α137–141 and to increase its purity up to 13-fold. With higher hydrolysis degree, the α137–141 antibacterial peptide was in competition with the other peptides which had the same mass range. So, the α137–141 recovery decreased as the hydrolysis progressed. To the best of our knowledge, it was the first time that the effect of the hydrolysis degree is demonstrated to impact preferentially the separation of a specific peptide by electrodialysis with ultrafiltration membranes.
References
Goot, A.J.V.D., Pelgrom, P.J.M., Berghout, J.A.M., Geerts, M.E.J., Jankowiak, L., Hardt, N.A., Keijer, J., Schutyser, M.A.I., Nikiforidis, C., Boom, R.M.: Concepts for further sustainable production of foods. J. Food Eng. 168, 42–51 (2016)
Lafarga, T., Hayes, M.: Bioactive peptides from meat muscle and by-products: generation, functionality and application as functional ingredients. Meat Sci. 98, 227–239 (2014)
Pfaltzgraff, L.A., Bruyn, M.D., Cooper, E.C., Budarin, V., Clark, J.H.: Food waste biomass: a resource for high-value chemicals. Green Chem. 15, 307–314 (2013)
Lynch, S.A., Mullen, A.M., O’Neill, E.E., Garcia, C.A.: Harnessing the potential of blood proteins as functional ingredients: a review of the state of the art in blood processing. Compr. Rev. Food Sci. Food Saf. 16, 330–344 (2017)
Bechaux, J., Gatellier, P., Page, J.-F.L., Drillet, Y., Sante-Lhoutellier, V.: A comprehensive review of bioactive peptides obtained from animal byproducts and their applications. Food Funct. 10(10), 6244–6266 (2019)
Bah, C.S.F., Bekhit, A.E.-D.A., Carne, A., McConnell, M.A.: Slaughterhouse blood: an emerging source of bioactive compounds. Compr. Rev. Food Sci. Food Saf. 12, 314–331 (2013)
Hyun, C.-K., Shin, H.-K.: Utilization of bovine blood plasma obtained from a slaughterhouse for economic production of probiotics. J. Ferment. Bioeng. 86, 34–37 (1998)
Adje, E.Y., Balti, R., Kouach, M., Dhulster, P., Guillochon, D., Nedjar-Arroume, N.: Obtaining antimicrobial peptides by controlled peptic hydrolysis of bovine hemoglobin. Int. J. Biol. Macromol. 49, 143–153 (2011)
Gomes, I., Dale, C.S., Casten, K., Geigner, M.A., Gozzo, F.C., Ferro, E.S., Heimann, A.S., Lakshmi, A.D.: Hemoglobin-derived peptides as novel type of bioactive signaling molecules. AAPS J. 12, 658–668 (2010)
Nedjar-Arroume, N., Dubois-Delval, V., Adje, E.Y., Traisnel, J., Krier, F., Mary, P., Kouach, M., Briand, G., Guillochon, D.: Bovine hemoglobin: an attractive source of antibacterial peptides. Peptides 29, 969–977 (2008)
Choisnard, L., Froidevaux, R., Nedjar-Arroume, N., Lignot, B., Vercaigne-Marko, D., Krier, F., Dhulster, P., Guillochon, D.: Kinetic study of the appearance of an anti-bacterial peptide in the course of bovine haemoglobin peptic hydrolysis. Biotechnol. Appl. Biochem. 36, 187–194 (2002)
Froidevaux, R., Krier, F., Nedjar-Arroume, N., Vercaigne-Marko, D., Kosciarz, E., Ruckebusch, C., Dhulster, P., Guillochon, D.: Antibacterial activity of a pepsin-derived bovine hemoglobin fragment. FEBS Lett. 491, 159–163 (2001)
Daoud, R., Dubois, V., Bors-Dodita, L., Nedjar-Arroume, N., Krier, F., Chihib, N.-E., Mary, P., Kouach, M., Briand, G., Guillochon, D.: New antibacterial peptide derived from bovine hemoglobin. Peptides 26, 713–719 (2005)
Nedjar-Arroume, N., Dubois-Delval, V., Miloudi, K., Daoud, R., Krier, F., Kouach, M., Briand, G., Guillochon, D.: Isolation and characterization of four antibacterial peptides from bovine hemoglobin. Peptides 27, 2082–2089 (2006)
Powers, J.-P.S., Hancock, R.E.W.: The relationship between peptide structure and antibacterial activity. Peptides 24, 1681–1691 (2003)
Li, Y., Xiang, Q., Zhang, Q., Huang, Y., Su, Z.: Overview on the recent study of antimicrobial peptides: origins, functions, relative mechanisms and application. Peptides 37, 207–215 (2012)
Pushpanathan, M., Gunasekaran, P., Rajendhran, J.: Antimicrobial peptides: versatile biological properties. Int. J. Pept. 2013, 675931 (2013)
Stewart, K.M., Horton, K.L., Kelley, S.O.: Cell-penetrating peptides as delivery vehicles for biology and medicine. Org. Biomol. Chem. 6, 2242–2255 (2008)
Gontard, N.: Les Emballages Actifs. Editions TEC & DOC, Paris (2000)
Catiau, L., Traisnel, J., Delval-Dubois, V., Chihib, N.-E., Guillochon, D., Nedjar-Arroume, N.: Minimal antimicrobial peptide sequence from hemoglobin alpha-chain: KYR. Peptides 32, 633–638 (2011)
Przybylski, R., Firdaous, L., Châtaigné, G., Dhulster, P., Nedjar, N.: Production of an antimicrobial peptide derived from slaughterhouse by-product and its potential application as meat preservative. Food Chem. 211, 306–313 (2016)
Przybylski, R., Bazinet, L., Firdaous, L., Kouach, M., Goossens, J.-F., Dhulster, P., Nedjar, N.: Harnessing slaughterhouse by-products: from wastes to high-added value natural food preservative. Food Chem. 304, 125448 (2020)
Aluko, R.E.: Determination of nutritional and bioactive properties of peptides in enzymatic pea, chickpea and mung bean protein hydrolysates. J. AOAC Int. 91, 947–956 (2008)
Udenigwe, C.C., Aluko, R.E.: Food protein derived bioactive peptides: production, processing and potential health benefits. J. Food Sci. 71, 11–24 (2012)
Agyei, D., Ongkudon, C.M., Wei, C.Y., Chan, A.S., Danquah, M.K.: Bioprocess challenges to the isolation and purification of bioactive peptides. Food Bioprod. Process. 98, 244–256 (2016)
Kapel, R., Froidevaux, R., Nedjar-Arroume, N., Fertin-Bazus, A., Dhulster, P., Guillochon, D.: Continuous production of a peptidic fraction containing the intermediate opioid peptide LVV-haemorphin-7 (LVVh-7) by peptic hydrolysis of bovine haemoglobin in a continuous membrane reactor. Biotechnol. Appl. Biochem. 37, 317–324 (2003)
Elagli, A., Belhacene, K., Dhulster, P., Froidevaux, R.: Sutainable efficient way for opioid peptide LVV-h7 preparation from enzymatic proteolysis in a microfluidic-based reaction-extraction process with solvent recycling. J. Chromatogr. B 1020, 24–28 (2016)
Agyei, D., Danquah, M.: Industrial-scale manufacturing of pharmaceutical-grade bioactive peptides. Biotechnol. Adv. 29, 272–277 (2011)
Saxena, A., Tripathi, B.P., Kumar, M., Shadi, V.K.: Membrane-based techniques for the separation and purification of proteins: an overview. Adv. Colloid Interface Sci. 145, 1–22 (2009)
Bazinet, L., Firdaous, L.: Separation of bioactive peptides by membrane processess: technologies and devices. Recent Pat. Biotechnol. 7, 9–27 (2013)
Firdaous, L., Dhulster, P., Amiot, J., Gaudreau, A., Lecouturier, D., Kapel, R., Lutin, F., Vézina, L.-P., Bazinet, L.: Concentration and selective separation of bioactive peptides from an alfalfa white protein hydrolysate by electrodialysis with ultrafiltration membranes. J. Membr. Sci. 329, 60–67 (2009)
Roblet, C., Doyen, A., Amiot, J., Bazinet, L.: Impact of pH on ultrafiltration membrane selectivity during EDUF purification of soy peptides from a complex matrix. J. Membr. Sci. 435, 207–217 (2013)
Suwal, S., Roblet, C., Doyen, A., Amiot, J., Beaulieu, L., Legault, J., Bazinet, L.: Electrodialytic separation of peptides from snow crab by-product hydrolysate: effect of cell configuration on peptide selectivity and local electric field. Sep. Purif. Technol. 127, 29–38 (2014)
Crosby, W.H., Munn, J.L., Furth, F.W.: Standardizing a method for clinical hemoglobimetry. US Armed Forces Med. J. 5, 693–703 (1954)
Dubois, V., Nedjar-Arroume, N., Guillochon, D.: Influence of pH on the appearance of active peptides in the course of peptic hydrolysis of bovine haemoglobin. Prep. Biochem. Biotechnol. 35, 85–102 (2005)
Church, F.C., Swaisgood, H.E., Porter, D.H., Catignani, L.: Spectrophotometric assay using o-phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins. J. Dairy Sci. 66, 1219–1227 (1983)
Vanhoute, M., Firdaous, L., Bazinet, L., Froidevaux, R., Lecouturier, D., Guillochon, D., Dhulster, P.: Effect of haem on the fractionation of bovine haemoglobin peptic hydrolysate by electrodialysis with ultrafiltration membranes. J. Membr. Sci. 365, 16–24 (2010)
Zhao, Q.Y., Piot, J.M., Gautier, V., Cottenceau, G.: Peptic peptide mapping by HPLC, on line with photodiode array detection, of a haemoglobin hydrolysate produced at pilot-plan scale from an ultrafiltration process. Appl. Microbiol. Biotechnol. 45, 778–784 (1996)
Linderstrom-Lang, K.: Proteins and enzymes III. Lane Med. Lect. 6, 53–72 (1952)
Zouari, O., Przybylski, R., Hannioui, M., Sion, L., Dhulster, P., Nedjar-Arroume, N.: High added-value co-product: the porcine cruor is an attractive source of active peptides. J. Nutr. Health Food Sci. 7, 1–9 (2020)
Doyen, A., Roblet, C., L’Archevêque-Gaudet, A., Bazinet, L.: Mathematical sigmoid-model approach for the determination of limiting and over-limiting current density values. J. Membr. Sci. 452, 453–459 (2014)
Labbé, D., Bazinet, L.: Effect of membrane type on cation migration during green tea electromigration and equivalent mass transported calculation. J. Membr. Sci. 275, 220–228 (2006)
Lebrun, F., Bazus, A., Dhulster, P., Guillochon, D.: Influence of molecular interaction of bovine haemoglobin hydrolysate with an organic membrane. J. Membr. Sci. 146, 113–124 (1998)
Gourley, L., Gauthier, S.F., Pouliot, Y.: Separation of casein hydrolysates using polysulfone ultrafiltration membranes with pH and EDTA treatments applied. Lait 78, 633–646 (1995)
Gourley, L., Gauthier, S.F., Pouliot, Y., Mollé, D., Léonil, J., Maubois, J.F.: Identification of casein peptides interacting with polysulfone ultrafiltration membranes. Lait 78, 633–646 (1998)
Acknowledgements
This work was realized within the French-Canadian International Associated Laboratory for “Bioproduction of natural antimicrobials” (LIAAN). These results are a part of a project that has received funding from the Bio Based Industries Joint Undertaking under the European Union’s Horizon 2020 research and innovation program under Grant Agreement No 745762. The authors thank Christine Vanuxem, English teacher from Lille I University, and Pamela Podkowycz for assisting in the review of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Przybylski, R., Bazinet, L., Kouach, M. et al. Slaughterhouse By-Product Valorization: Hydrolysis Degree Modification for Higher Antimicrobial Recovery by Electroseparation. Waste Biomass Valor 12, 1977–1989 (2021). https://doi.org/10.1007/s12649-020-01154-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-020-01154-7