Abstract
The potential health risks associated with sludge cake application to agricultural land are managed by controlling the levels of Escherichia coli (E. coli) bacteria which indicate the risk of pathogen transfer. Analyses undertaken following post-digestion sludge dewatering have shown unpredictable levels of E. coli increase in stored sludge cake. Presently there is limited understanding on environmental parameters controlling the indicator bacteria density in storage and the contributory effects dewatering may have. This review aims to establish the state of current knowledge on innate and environmental factors influencing E. coli dynamics and survival in biosolids. A key factor identified is the effect of mechanical dewatering processes, which transform the sludge matrix environmental conditions through the increased availability of growth factors (e.g. nutrient and oxygen). Examples of storage practices from the agricultural and food industries are also discussed as successful methods to inhibit bacterial growth and survival, which could be extrapolated to the biosolids sector to regulate E. coli concentrations.
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Statement of Novelty
Increases in levels of pathogenic indicator bacteria are frequently observed in stored biosolids. However, little information is known of the environmental conditions prevailing in stored sludge cake and limited advice exists for the reduction of pathogenic indicators in biosolids during storage. This review aimed to establish the current state of knowledge on factors influencing E. coli growth and survival in stored biosolids. A key factor identified is the effect of mechanical dewatering processes, which transform the sludge matrix environmental conditions through the increased availability of growth factors (e.g. nutrient and oxygen). Examples of storage practices from the agricultural and food industries, such as ensiling and modified atmosphere packaging, are also discussed and might give indication of methods to inhibit bacterial growth and survival in biosolids.
Introduction
The markets for sludge application to agricultural land are changing. Regulatory reform by the UK’s Water Industry Regulator on sludge production and trade will encourage the delivery of efficiencies and higher product quality standards by 2020 [1]. Sludge has value in biogas energy production and the sale of biosolids to farmers as an alternative to manufactured fertiliser [1]. The improvement of product quality standards in association with market competition will drive tighter restrictions on acceptable levels of nitrogen, phosphorous, heavy metals and bacterial indicator densities present in biosolids products destined for agricultural application. The risk of pathogen transfer to agricultural land is reduced through anaerobic digestion (AD) treatment and articles demonstrating the successful decline in faecal indicator concentrations and biological stability from digestion processes are extensive [2]. Advice on temperature optimisation, retention time, suspended solids concentrations and links with methane production have enabled sludge producers to optimise digester outputs for maximum biogas yield with secondary advantages of pathogen indicator reductions, measured upon E. coli bacterial concentrations in the final end product. The international guidelines of acceptable bacterial concentrations in the sludge product are summarised in Table 1 and demonstrate the categories of product quality associated with the achieved indicator bacterial reductions.
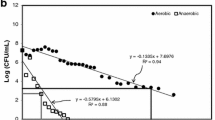
Challenges in meeting these required standards surface post mechanical dewatering, which aims to increase the economical transport value of the product by limiting the volume of water associated with the solid material. Studies on dewatering operations have highlighted a significant increase in pathogenic indicator bacteria [2,3,4,5,6,7,8]. Examples of indicator increases range from − 0.4 log to + 6.4 log units after centrifuge dewatering [9] emphasising the highly variable effects amongst treatment plants. Dewatering and digestion treatment type appear to have a significant impact on E. coli growth [5, 7]. A study comparing single phase thermophilic digestion with mesophilic anaerobic digestion (MAD) processes showed that although effluent from thermophilic digesters had < 102 CFU/g DS (a 6 log reduction from the raw influent), immediately after centrifuge dewatering the density of faecal coliforms was 106 CFU/g DS, an increase of four orders of magnitude. For MAD plants, E. coli increased by approximately one order of magnitude in the dewatered cake using standard culturing methods [5]. A continuation of this study also looked at E. coli densities in the stored biosolids produced after thermophilic and mesophilic digested sludge was centrifuge dewatered and stored at 35 °C. For both treatments E. coli concentrations increased within the first three days to between 108 and 109 cells/g DS. Higgins et al. [5] argues that these results suggest the conditions after dewatering are favourable for growth. Centrifuge dewatering may result in the destabilisation of the microbial ecology and provide conditions within the cake that encourage growth of faecal coliforms and E. coli. Higgins et al. [5] highlights that continued storage of the biosolids is a method that may allow the desired goals for indicator organism compliance to be met (Table 1). Limited research has been completed, however, on how the biosolids storage environment may influence the control of indicator behaviour. The key factors responsible for indicator growth and survival in stored dewatered biosolids and their relative importance have not yet been clearly identified [3]. The immediate concentration elevation post-dewatering and the prolonged survival during subsequent biosolids storage, suggests characteristics of the storage environment may be an underlying cause [5, 6].
The unpredictable rise in indicator bacteria post-dewatering highlights a need for further understanding of the parameters affecting the growth and death of E. coli bacteria. With the opportunity for sludge markets to increase competition amongst sludge producers and waste retailers, the need for improved biosolids quality and assurance of risk is fundamental for a successful sludge market to establish.
Dewatering Shear Effects and Chemical Modification
Following digestion, liquid sludge is combined with polyelectrolyte (polymer) as a flocculation aid before mechanical dewatering. Monteleone et al. [7] suggests that polyelectrolyte effects are an important consideration when explaining E. coli increase in biosolids products. Mechanical dewatering relies upon polymers to condition the sludge, forming flocs and inevitably aggregating bacteria and sludge organic matter. Research has shown that large amounts of bioavailable protein and polysaccharide exist in centrifuge dewatered cakes [4]. These elements will be incorporated within floc matrices and could provide a substrate source promoting bacterial growth. The formation of a floc may also provide protection for bacteria cells from environmental stressors, prolonging cell survival [10, 11]. Arguments against this, however, are the effects of centrifuge shearing which is thought to cause floc disruption and bacterial dispersal, elevating indicator concentrations in biosolids [2, 7,8,9]. Chen et al. [2] conducted a laboratory scale simulation of both belt filter press and centrifuge dewatering. Results indicated that with more shear during dewatering, a greater increase in faecal coliform density occurred during storage. Increases from original values to 5 log units after 2 days of storage were observed in centrifuged samples [2]. To explain this phenomenon Chen et al. [2] suggests that soluble proteins and other organics are released during shearing and serve as substrates for microbial growth. Evidence for this argument was observed in cake samples spiked with 1 mL of a glucose/bacto-peptone mixture where, after 24 h, coliforms had increased by 2 orders of magnitude compared to control samples. These results indicate that substrate is a limiting factor in biosolids and that the provision of substrates stimulates microbial growth [2].
As a counter argument to this Higgins et al. [5] highlights that for regrowth to occur a significant time is needed to increase counts by several orders of magnitude. E. coli doubling time is 20 min under optimal conditions, which are unlikely to be found in sludge treatment environments. The typical centrifuge retention time for a unit of sludge is 20 min. Therefore the large increase in bacteria density immediately after centrifuge dewatering cannot be explained by regrowth alone. In addition, the release and floc break up in a centrifuge seems unlikely as the process is conditioned with polyelectrolyte flocculation aids to support aggregation and the formation of the cake product [5]. Instead, Higgins et al. [5] suggest that bacteria enter a viable but non culturable (VBNC) state during digestion. The VBNC state prevents bacteria enumeration using standard plating techniques, and therefore a value of only culturable bacteria after digestion can be detected with these methods. During dewatering the bacteria may be reactivated, entering a culturable state and giving a greater number of counts in standard enumeration methods. Bacteria will enter a VBNC state after exposure to environmental stress such as nutrient deprivation or high temperature which are conditions that can be present in digestion [5]. Research methods compared (competitive) polymerase chain reaction (cPCR) (targeting E. coli) and standard culturing methods (SCM). For both thermophilic and mesophilic samples, results support the non-culturable theory. The E. coli densities measured before and after dewatering with SCM indicated fewer E. coli bacteria were present after digestion, suggesting that a proportion of the bacteria measured were in a non-culturable state [5]. The cPCR results from MAD samples were at least one order of magnitude higher in E. coli density for digester effluent than SCM results. Following dewatering, cPCR and SCM E. coli results were equivalent. Higgins et al. [5] attributes this difference to a reactivation of bacteria during dewatering. Although Higgins et al. [5] dispute the argument of shear force effects in the centrifuge dispersing bacterial flocs they do argue that one of the reactivation mechanisms may be due to the release of growth factors as a result of shear.
The levels of shear experienced in centrifuge dewatering may disrupt cells; Chen et al. [12] identified two factors that negatively impacted methanogenic activity when studying volatile organic sulphur compound (VOSC) production in anaerobically digested biosolids. A comparison between high and medium dry solids (DS) producing centrifuges found a 3.7 times reduction in methane production rate from high DS centrifuge samples. This difference was attributed to the possible effect of shearing on methanogens. Researchers suggested that a higher level of shearing would lead to greater cell lysis inhibiting methanogenesis [12]. The shear and destruction of bacterial cells may provide additional bioavailable protein which supports the theory of nutrient release in centrifuge dewatering operations to support bacterial populations in the fresh biosolids. Sun et al. [13] investigated dewatering processes on cyanobacteria-containing sludge and attributed cell lysis to flocculation turbulence and pressure from mechanical operations on flocs. Recent research has suggested cellular excretion materials, as a consequence of cell lysis, may be used as nutrients for the remaining microbial population. Although Higgins et al. [5] suggests it is the reactivation of VBNC cells that causes indicator increase it may be feasible that a proportion of the VBNC cells lyse and provide cellular nutrients for the remaining culturable and reactivated bacteria in the dewatered biosolids. Confirmation of this is evident in work completed by Murata et al. [14] who examined the release of cytoplasmic materials into culture medium by studying the activity of β-galactosidase (cytoplasmic enzyme). Results from culture medium fractions, after incubation, suggested that cytoplasmic materials are released as a consequence of cell lysis and may act as a nutrient supplement for the survival of the remaining population. Although stored biosolids are considered nutrient limiting with regards to readily bioavailable nutrients, studies above suggest the sludge material after mechanical dewatering processes may have an increased amount of bioavailable nutrients able to support cell growth. As E. coli are able to replicate rapidly under favourable growth conditions [15] the indicator bacteria are likely to have a competitive advantage, utilising available nutrients and proliferating within the post-dewatering biosolids environment. Further study is required to clarify whether nutrients from lysed cells support remaining cell population survival [14] particularly in wastewater sludges and biosolids storage environments.
Escherichia coli Death Rate and Temperature
Temperature has been identified as a critical parameter controlling cell death rates and determining the biochemical conditions of AD [16]. Table 2 shows the concentration of indicator bacteria at different temperatures of digestion, treated biosolids storage and in agricultural manure. Lang and Smith [17] highlight that the optimum temperature for growth and survival of enteric organisms is within the range of 30 to 40 °C [18] and therefore mesophilic temperatures during MAD do not exert a critical thermal stress on the decay of E. coli or Salmonella. Temperature regulates processes including substrate limitation and microbial competition which, as shown in Table 2, have an influence on pathogen reduction and can indirectly cause pathogen inactivation [19, 20]. Post-digestion, the temperature of sludge will gradually reduce particularly after dewatering and during the biosolids storage phase which normally occurs in uncontrolled, open bays. The reoccurrence of faecal coliforms in post-digestion biosolids was attributed to this temperature reduction by Iranpour et al. [21] and researchers argue that maintaining a minimum temperature of 50 °C (representative of the up-stream thermophilic digestion process) may prevent growth of faecal coliforms. Sprigings and Le [22] showed that biosolids retained a higher temperature after centrifuge dewatering for approximately 12 h, possibly an effect of residual heat from the digestion process. Sprigings and Le [22] suggest that the role of residual heat in the first 12 to 24 h is an important factor in aiding the proliferation of E. coli. It may be that cooler temperatures in the following days of storage are preserving the growth and survival of bacteria within the biosolids storage environment. A long term monitoring programme of biosolids storage environments with temperature recordings has not been undertaken in previous research.
Studies on microbial dynamics in livestock manure and soils, which have greater associated datasets and form an analogous environment to biosolids may enhance understanding on the key parameters influencing indicator behaviour in biosolids. Semenov et al. [23] tested the effects of temperatures ranging from 7 to 33 °C on Salmonella and E. coli 0157:H7 survival in cow manure microcosms, and observed survival of both pathogens declined significantly with increasing mean temperatures. The authors hypothesised that the reduced survival at higher temperatures may be a consequence of greater stress and energy expenditure for a particular organism [23]. Therefore, the reducing storage temperatures identified by Sprigings and Le [22] and Iranpour et al. [21] in biosolids storage are likely to lessen the stress on cell functions and limit excessive energy expenditure for indicator bacteria held within the sludge matrix. Consequently, the steady growth and prolonged survival of E. coli bacteria is likely in uncontrolled biosolids storage environments. In addition, microorganisms antagonistic to enteropathogens in manure are more competitive at temperatures between 16 and 33 °C, possibly due to increased temperature initially causing faster growth [23]. Reduced antagonistic activity and competition from indigenous microorganisms is likely at lower temperatures [24]. The argument for increasing biosolids storage temperature is supported by evidence from Plachá et al. [25] who showed that lower temperatures during winter months cause prolonged cell survival in pig slurry. Temperature appears to have a significant influence on the growth and survival of indicators within biosolids and analogous environments. Further work to understand the temperature conditions favouring E. coli die-off will contribute to the control of biosolids quality and support the predictability in achieving microbial compliance targets.
Moisture Content of Dewatered Biosolids
An interesting phenomenon noted in Sprigings and Le [22] is the effect moisture may have on retaining heat in the biosolids matrix. A comparison of sites showed differences in the DS percentage of the dewatered sludge cake (Site A: 25% DS, Site B: 31% DS) and corresponded with site A maintaining a higher sludge cake temperature. A lower DS% will help the fresh cake retain heat from the AD process for a longer period, delaying cake cooling to ambient temperatures that are likely to support prolonged cell survival [23,24,25]. Currently biosolids storage post-dewatering, is conducted in open bays exposed to fluctuating weather conditions. Controlling the water content of sludge not only has operational benefits but may also be an influential factor for the control of indicator organisms [26,27,28]. In a study on cattle feedlot soil moisture content impacts on E. coli 0157, researchers found that at the lowest water contents [0.11 g H2O g−1 Dry Feed Surface Material (DFSM)] microbial activity was not detectable and E. coli 0157:H7 viability was lost [26]. Where feedlot soils had higher water contents, between 0.43/0.67 g H2O g−1 DFSM, E. coli 0157:H7 populations persisted at high levels. These data confirm that moisture content can improve survival and allow the growth of pathogenic bacteria. In agreement with these findings is work by Zaleski et al. [27] who studied the effects of solar drying in concrete lined drying beds on anaerobically digested biosolids. Over a period of 22 weeks the relationship between the number of faecal coliforms and percent DS was tracked. Although often above the DS range typically observed in more temperate climates (25 to 30% DS [22]) the study did show the change in percent DS fluctuating in response to rainfall events. Between weeks 15 and 19 of the study the DS fell to approximately 20% and with this an increase of 1.5 orders of magnitude in faecal coliform levels was observed. Subsequently this higher concentration decreased as the biosolids dried to 80% DS in the final study weeks [27]. Re-wetting of biosolids by rainfall events was examined by Rouch et al. [28] in air-dry storage of anaerobically digested biosolids. Rouch et al. [28] found an inverse relationship to the DS contents of the biosolids with regards to the survival of E. coli, enterococci and coliphages. Due to the growth of dormant or small residual populations of bacterial in the biosolids, bacterial regrowth could occur as cells become active upon rewetting [28].
In contrast to this, laboratory-scale research conducted by Lang and Smith [29] suggests that in dry, biosolids-amended soil, bacteria are protected within particles of sludge cake as drying restricts predatory activity. Results showed a marked increase in E. coli survival in air-dried samples of sludge-amended soil. The results highlight that ecological processes contributing to the decay of E. coli in sludge-amended soil are active under moist conditions but suppressed in dry soil [29]. Although this is contradictory to views of other researchers [26,27,28, 30, 31] the research provides indication that the survival times of enteric bacteria in biosolids amended soil may be shorted in moist, and extended in dry conditions [29]. Further evidence supporting this argument is highlighted by Jiang et al. [32] who studied E. coli 0157:H7 cell survival in manure-amended autoclaved soil. The study revealed that E. coli 0157:H7 could survive for extended periods of time in manure-amended soil even under very dry conditions of < 1% moisture content.
The effect of moisture content on bacterial behaviour in biosolids and analogous environments is inconsistent. It has been highlighted that bacterial survival is possible across a range of moist and dry conditions [27, 28, 29, 32]. The sludge environment is a challenging matrix, likely to be influenced by a great number of factors, which may impede experiments studying the effects of environmental parameters on bacterial growth. For example, the source material of sludge will be influential with regards to nutrient availability and the indigenous organisms present in the sampled material, which will influence the dynamics of the studied microorganisms. In addition the experimental design, particularly in up-scaled trials will be affected by ambient conditions such as seasonality and temperature. Nevertheless, as indicator survival could be sufficient for stored biosolids to fail microbial compliance assessments understanding of bacterial dynamics within operational DS ranges observed on treatment sites is necessary, particularly in temperate environments.
Modified Atmosphere in Sludge and Analogous Environments
Oxygen availability has a substantial effect on cell respiration and consequential energy production, regulating most cell activities [33]. Although E. coli bacteria are facultative anaerobes, and therefore resilient to oxygen deprivation [34, 35], the anaerobic conditions coupled with additional inimical factors present in AD may cause growth inhibition, and death. Mechanical dewatering causes substantial change of environmental conditions in the sludge product [2, 6]. In particular, the effects of oxygen availability in the process need to be explored. Qi et al. [8] investigated the impact of total solids on faecal coliform growth in centrifuged biosolids and found that the liquid to solid ratio governs regrowth. This finding is in contrast to other authors [2, 7, 9] who attribute regrowth to effects of centrifuge shearing. In agreement with Qi et al. [8] is a study conducted by Erdal et al. [36] who found more reactivation and regrowth in high solids cakes when compared to low solids cakes. Drier cake is likely to contain fewer water filled pores and therefore allow better air convection and diffusion. The increased exposure to oxygen is probable in centrifuge dewatering as this form of mechanical dewatering produces smaller flocs with an overall larger surface area [37]. Often continuous flow decanter centrifuges are employed in sludge treatment and contain an internal scroll conveyor that can amplify oxygen exposure [2]. As anaerobic digestion creates an environment dominated by obligate anaerobes the oxygen introduced during dewatering could have lethal effects on these populations of bacteria. E. coli are fast growing, facultative anaerobes that are likely to experience a selective advantage with increased oxygen availability [37]. This may contribute to the higher level of indicator growth observed in the initial phase of storage. Oxygen can disrupt methanogens which form a large fraction of the anaerobically digested sludge [2, 37]. A reduction in the methanogen population may reduce substrate competition and, if lysed, methanogen cells may provide additional nutrients for the surviving bacteria [14]. Chen et al. [2] suggests that this selective advantage may quickly disappear as the storage conditions of biosolids return to an anaerobic state. It may be possible that the increased growth resulting from factors associated with oxygen exposure may enable previously non-viable populations of E. coli bacteria to establish [5] and dominate, preserving elevated indicator concentrations. Controlling oxygen exposure during dewatering and subsequent storage may present a strategy to stabilise E. coli concentrations at post-digestion levels, ensuring compliance targets are predictably met. There are industrial sectors where controlled storage conditions are routinely applied to inhibit pathogen growth and that could constitute a base for knowledge transfer into biosolids storage. Ensiling and food preservation are two clear examples where oxygen depletion is utilised to artificially regulate microbial changes.
A common agricultural practice in which oxygen exclusion restricts bacterial growth is agricultural silage production. Poor silage management has been shown to be a factor in E. coli 0157:H7 survival [38]. Studies on the growth of E. coli 0157 in poorly fermented laboratory silage showed an increase from initial numbers of 103 E. coli 0157 CFU g−1 to numbers in excess of 106 CFU g−1 reflecting the ability of this organism to multiply rapidly in air spoiled silage [38]. Similarly, a ‘controlled atmosphere’ process that is directly linked to microbial inhibition is Modified Atmosphere Packaging (MAP) in food preservation. The principle of MAP is the replacement of air in the package with a different fixed gas mixture [39]. An additional innovation in MAP food packaging has been the use of vacuum treatment [40]. The main target of vacuum packing is to reduce the residual oxygen in the package which will reduce oxidative chemical reactions and aerobic growth [40]. As oxygen exposure to the dewatered sludge matrix may be a precursor to E. coli growth, understanding principles and successes of MAP may be beneficial for sludge management applications. Table 3 shows examples of analogous environments that demonstrate microbial growth control.
Previous literature (Table 3) shows that reduced concentrations of oxygen exposure through ensiling, modified atmosphere and vacuum packaging appear to be most influential in preserving agricultural crops and the shelf life of food products by diminishing the growth of bacteria. It may therefore be possible that similar practices are able to reduce indicator growth or enhance die-off rates in biosolids storage environments where oxygen availability in the initial phases of storage has been attributed to higher concentrations of pathogenic indicator bacteria.
Summary
Research has identified high concentrations of indicator bacteria in anaerobically digested biosolids, immediately after mechanical dewatering [2,3,4, 6, 7]. This review aimed to establish the current state of knowledge on factors influencing E. coli growth and survival in stored biosolids, drawing on understanding from up-stream treatment processes and analogue storage environments that successfully limit the proliferation of E. coli bacteria. Key factors that have been identified from the scientific literature are the effects of mechanical dewatering processes which may release growth inducers and transform the environmental conditions of the sludge matrix. Nutrient availability for cell survival and growth is a significant factor to be considered alongside the physical environmental conditions that will be acting on cells held within stored biosolids. The rigorous level of treatment control that precedes biosolids storage is arguably superseded by a highly uncontrolled and poorly understood storage treatment in which external factors including temperature and moisture content may preserve elevated indicator concentrations. Examples of modified storage practices such as ensiling and MAP in the agricultural and food industries might give indication of methods to inhibit bacterial growth and survival, particularly when considered with external environmental parameter modifications. As assurance of biosolids quality is increasingly sought by agriculturalists, retailers and the public, regulations and safety assurance schemes are becoming more stringent. At a time when the water industry regulator of England and Wales in the UK, Ofwat, sets forward a new direction towards the opening of sludge markets in 2020 it is critical that the ambiguity concerning pathogen indicator concentrations is dispelled and sludge-to-land disposal routes are safeguarded.
References
OFWAT: Towards Water 2020—Policy Issues: Customer Engagement and Outcomes. OFWAT, London (2016)
Chen, Y.C., Higgins, M.J., Beightol, S.M., Murthy, S.N., Toffey, W.E.: Anaerobically digested biosolids odor generation and pathogen indicator regrowth after dewatering. Water Res. 45, 2616–2626 (2011). https://doi.org/10.1016/j.watres.2011.02.014
Dentel, S.K., Qi, Y., Herson, D.S.: Improving the assessment of risk from pathogens in biosolids: fecal coliform regrowth, survival, enumeration and assessment. Water Sci. Technol. 57, 189–193 (2008)
Higgins, M.J., Chen, Y., Murthy, S.N., Hendrickson, D., Schafer, P., Farrell, J.: WERF phase 2: the impact of digestion and dewatering on reactivation and regrowth of viable but non-culturable indicator bacteria. In: WEFTEC 06, pp. 3146–3161. Water Environment Foundation (2006)
Higgins, M.J., Chen, Y., Murthy, S.N., Hendrickson, D., Farrel, J., Schafer, P.: Reactivation and growth of non-culturable indicator bacteria in anaerobically digested biosolids after centrifuge dewatering. Water Res. 41, 665–673 (2007). https://doi.org/10.1016/j.watres.2006.09.017
Higgins, M.J., Chen, Y.C., Murthy, S.N., Hendrickson, D.: Latest developments on the emerging issue of E. coli and fecal coliform reactivation and regrowth after dewatering. In: Proceedings on Moving Forward Wastewater Biosolids Sustainability: Technical, pp. 204–419. International Water Association, Moncton, Canada (2007)
Monteleone, M.C., Furness, D., Jefferson, B., Cartmell, E.: Fate of E. coli across mechanical dewatering processes. Environ. Technol. 25, 825–831 (2004). https://doi.org/10.1080/09593330.2004.9619374
Qi, Y., Dentel, S.K., Herson, D.S.: Effect of total solids on fecal coliform regrowth in anaerobically digested biosolids. Water Res. 42, 3817–3825 (2008). https://doi.org/10.1016/j.watres.2008.06.001
Qi, Y., Dentel, S.K., Herson, D.S.: Increases in fecal coliform bacteria resulting from centrifugal dewatering of digested biosolids. Water Res. 41, 571–580 (2007). https://doi.org/10.1016/j.watres.2006.11.004
Mahendran, B., Lishman, L., Liss, S.N.: Structural, physicochemical and microbial properties of flocs and biofilms in integrated fixed-film activated sludge (IFFAS) systems. Water Res. 46, 5085–5101 (2012). https://doi.org/10.1016/j.watres.2012.05.058
Lin, H., Zhang, M., Wang, F., Meng, F., Liao, B.Q., Hong, H., Chen, J., Gao, W.: A critical review of extracellular polymeric substances (EPSs) in membrane bioreactors: characteristics, roles in membrane fouling and control strategies. J. Memb. Sci. 460, 110–125 (2014). https://doi.org/10.1016/j.memsci.2014.02.034
Chen, Y., Higgins, M.J., Maas, N.A., Murthy, S.N., Toffey, W.E., Foster, D.J.: Roles of methanogens on volatile organic sulfur compound production in anaerobically digested wastewater biosolids. Water Sci. Technol. 52, 67–72 (2005)
Sun, F., Hu, W., Pei, H., Li, X., Xu, X., Ma, C.: Evaluation on the dewatering process of cyanobacteria-containing AlCl3 and PACl drinking water sludge. Sep. Purif. Technol. 150, 52–62 (2015). https://doi.org/10.1016/j.seppur.2015.06.030
Murata, M., Noor, R., Nagamitsu, H., Tanaka, S., Yamada, M.: Novel pathway directed by σE to cause cell lysis in Escherichia coli. Genes Cells 17, 234–247 (2012). https://doi.org/10.1111/j.1365-2443.2012.01585.x
Todar, K.: Online Textbook of Bacteriology: Nutrition and Growth of Bacteria
Scaglia, B., D’Imporzano, G., Garuti, G., Negri, M., Adani, F.: Sanitation ability of anaerobic digestion performed at different temperature on sewage sludge. Sci. Total Environ. 466–467, 888–897 (2014). https://doi.org/10.1016/j.scitotenv.2013.07.114
Lang, N.L., Smith, S.R.: Time and temperature inactivation kinetics of enteric bacteria relevant to sewage sludge treatment processes for agricultural use. Water Res. 42, 2229–2241 (2008). https://doi.org/10.1016/j.watres.2007.12.001
International Comission on Microbiological Specifications for Food (ICMSF): Microbial Ecology of Foods, vol. 1: Factors Affecting Life and Death of Microorganisms, ICMSF, New York (1980)
Smith, S.R., Lang, N.L., Cheung, K.H.M., Spanoudaki, K.: Factors controlling pathogen destruction during anaerobic digestion of biowastes. Waste Manage. 25, 417–425 (2005). https://doi.org/10.1016/j.wasman.2005.02.010
Rosenblum, J., Bisesi, M., Castano, J., Tamkin, A., Ciotola, R., Lee, J., Martin, J.: Influence of seasonal fluctuation and loading rates on microbial and chemical indicators during semi-continuous anaerobic digestion. Environ. Technol. 36, 1308–1318 (2014). https://doi.org/10.1080/09593330.2014.986537
Iranpour, R., Palacios, R., Cox, H.H.J., Abkian, V.: Solving fecal coliform growth/reactivation in biosolids during full-scale post-digestion processes. Water Sci. Technol. 52, 283–288 (2005)
Sprigings A.E., Le M.: Growth potential—a new method for estimation of the risk of E. coli regrowth in digested sludge cake. In: 16th European Biosolids and Organic Resources Conference, Leeds (2011)
Semenov, A.V., Van Bruggen, A.H.C., Van Overbeek, L., Termorshuizen, A.J., Semenov, A.M.: Influence of temperature fluctuations on Escherichia coli O157:H7 and Salmonella enterica serovar Typhimurium in cow manure. FEMS Microbiol. Ecol. 60, 419–428 (2007). https://doi.org/10.1111/j.1574-6941.2007.00306.x
Cools, D., Merckx, R., Vlassak, K., Verhaegen, J.: Survival of E. coli and Enterococcus spp. derived from pig slurry in soils of different texture. Appl. Soil Ecol. 17, 53–62 (2001)
Plachá, I., Venglovský, J., Sasáková, N., Svoboda, I.F.: The effect of summer and winter seasons on the survival of salmonella typhimurium and indicator micro-organisms during the storage of solid fraction of pig slurry. J. Appl. Microbiol. 91, 1036–1043 (2001). https://doi.org/10.1046/j.1365-2672.2001.01471.x
Berry, E.D., Miller, D.N.: Cattle feedlot soil moisture and manure content: II. Impact on Escherichia coli O157. J. Environ. Qual. 34, 656–663 (2005). https://doi.org/10.2134/jeq2005.0656
Zaleski, K.J., Josephson, K.L., Gerba, C.P., Pepper, I.N.L.: Survival, growth, and regrowth of enteric indicator and pathogenic bacteria in biosolids, compost, soil, and land applied biosolids. J. Residuals Sci. Technol. 2, 49–63 (2005)
Rouch, D.A., Mondal, T., Pai, S., Glauche, F., Fleming, V.A., Thurbon, N., Blackbeard, J., Smith, S.R., Deighton, M.: Microbial safety of air-dried and rewetted biosolids. J. Water Health 9, 403–414 (2011). https://doi.org/10.2166/wh.2011.134
Lang, N.L., Smith, S.R.: Influence of soil type, moisture content and biosolids application on the fate of Escherichia coli in agricultural soil under controlled laboratory conditions. J. Appl. Microbiol. 103, 2122–2131 (2007). https://doi.org/10.1111/j.1365-2672.2007.03490.x
Pietronave, S., Fracchia, L., Martinotti, M.G.: Researchers analyze how microorganisms suppress pathogen regrowth. Biocycle 43, 57–60 (2002)
Wang, L., Mankin, K.R., Marchin, G.L.: Survival of fecal bacteria in dairy cow manure. Am. Soc. Agric. Eng. 47, 1239–1246 (2004)
Jiang, X., Morgan, J., Doyle, M.P.: Fate of Escherichia coli O157: H7 in manure-amended soil. Appl. Environ. Microbiol. 68, 2605–2609 (2002). https://doi.org/10.1128/AEM.68.5.2605
Alberts, B., Bray, D., Hopkin, K., Johnson, A., Lewis, J., Raff, M., Roberts, K., Walter, P.: Essential Cell Biology. Garland Science. Taylor and Francis Group, London (2010)
Hutchison, M.L., Nicholson, F.A., Smith, K.A., Keevil, C.W., Chambers, B.J., Moore, A.: Study on farm manure applications to agricultural land and an assessment of the risks of pathogen transfer into the food chain. FS2526 (2000)
Henkel, S.G., Ter Beek, A., Steinsiek, S., Stagge, S., Bettenbrock, K., Teixeira de Mattos, M.J., Sauter, T., Sawodny, O., Ederer, M.: Basic regulatory principles of Escherichia coli’s electron transport chain for varying oxygen conditions. PLoS ONE (2014). https://doi.org/10.1371/journal.pone.0107640
Erdal, Z.K., Mendenhall, T.C., Neely, S., Wagoner, D., Quigley, C.: Implementing improvements in a North Carolina Residuals Management Program. In: WEF/AWWA/CWEA Joint Residuals and Biosolids Management. Alexandria, VA (2003)
Chen, Y., Higgins, M., Beightol, S., Araujo, G., Murthy, S., Barben, E., Toffey, W.: Odour generation and pathogen indicator regrowth after dewatering: are they related. In: Proceedings of the Mid-Atlantic Biosolids Association Conference, pp. 787–793 (2007)
Fenlon, D.R., Wilson, J.: Growth of Escherichia coli O157 in poorly fermented laboratory silage: a possible environmental dimension in the epidemiology of E. coli O157. Lett. Appl. Microbiol. 30, 118–121 (2000). https://doi.org/10.1046/j.1472-765X.2000.00679.x
Sivertsvik, M., Jeksrud, W.K., Rosnes, J.T.: A review of modified atmosphere packaging of fish and fishery products—significance of microbial growth, activities and safety. Int. J. Food Sci. Technol. 37, 107–127 (2002). https://doi.org/10.1046/j.1365-2621.2002.00548.x
Floros, J., Matsos, K.: Introduction to modified atmosphere packaging. In: Han, J. (ed.) Innovations in Food Packaging, pp. 159–184. Elsvier, London (2005)
Environment Agency: The Microbiology of Sewage Sludge-Part 1—An Overview of the Treatment and Use in Agriculature of Sewage Sludge in Relation to Its Impact on the Environment and Public Health, Bristol (2003)
ADAS: THE SAFE SLUDGE MATRIX: Guidelines for the Application of Sewage Sludge to Agricultural Land, Gleadthorpe Research Centre (2001)
European Union: Working Document on Sludge, 3rd Draft. ENV.E.3/LM, Brussels (2000)
US EPA (United States Environmental Protection Agency): A Plain English Guide to the EPA Part 503 Biosolids Rule. https://water.epa.gov/scitech/wastetech/biosolids/503pe_index.cfm
Le, M.S., Mayhew, M.E., Back, P.A.: Effectiveness of secondary digesters as a pathogen controller in winter. J. CIWEM. 16, 292–295 (2002)
Gollop, N., Zakin, V., Weinberg, Z.G.: Antibacterial activity of lactic acid bacteria included in inoculants for silage and in silages treated with these inoculants. J. Appl. Microbiol. 98, 662–666 (2005). https://doi.org/10.1111/j.1365-2672.2004.02504.x
Herrmann, C., Heiermann, M., Idler, C.: Effects of ensiling, silage additives and storage period on methane formation of biogas crops. Bioresour. Technol. 102, 5153–5161 (2011). https://doi.org/10.1016/j.biortech.2011.01.012
Dunière, L., Sindou, J., Chaucheyras-Durand, F., Chevallier, I., Thévenot-Sergentet, D.: Silage processing and strategies to prevent persistence of undesirable microorganisms. Anim. Feed Sci. Technol. 182, 1–15 (2013). https://doi.org/10.1016/j.anifeedsci.2013.04.006
Avery, L.M., Killham, K., Jones, D.L.: Survival of E. coli O157:H7 in organic wastes destined for land application. J. Appl. Microbiol. 98, 814–822 (2005). https://doi.org/10.1111/j.1365-2672.2004.02524.x
Bach, S.J., McAllister, T.A., Baah, J., Yanke, L.J., Veira, D.M., Gannon, V.P.J., Holley, R.A.: Persistence of Escherichia coli O157:H7 in barley silage: effect of a bacterial inoculant. J. Appl. Microbiol. 93, 288–294 (2002). https://doi.org/10.1046/j.1365-2672.2002.01695.x
Byrne, C., O’Kiely, P., Bolton, D., Sheridan, J., McDowell, D., Blair, I.: Fate of Escherichia coli 0157:H7 during silage fermentation. J. Food Prot. (2002). https://doi.org/10.4315/0362-028x-65.12.1854
Chen, Y., Sela, S., Gamburg, M., Pinto, R., Weinberg, Z.G.: Fate of Escherichia coli during ensiling of wheat and corn. Appl. Environ. Microbiol. 71, 5163–5170 (2005). https://doi.org/10.1128/AEM.71.9.5163-5170.2005
Dunière, L., Gleizal, A., Chaucheyras-Durand, F., Chevallier, I., Thévenot-Sergentet, D.: Fate of Escherichia coli O26 in corn silage experimentally contaminated at ensiling, at silo opening, or after aerobic exposure, and protective effect of various bacterial inoculants. Appl. Environ. Microbiol. 77, 8696–8704 (2011). https://doi.org/10.1128/AEM.06320-11
Heinrich, V., Zunabovic, M., Nehm, L., Bergmair, J., Kneifel, W.: Influence of argon modified atmosphere packaging on the growth potential of strains of Listeria monocytogenes and Escherichia coli. Food Control 59, 513–523 (2016). https://doi.org/10.1016/j.foodcont.2015.06.010
Garrido, M.D., Hernández, M.D., Espinosa, M.C., López, M.B.: Enhanced quality characteristics of refrigerated seabream (Sparus aurata) fillets packed under different systems (modified atmosphere vs. vacuum). J. Aquat. Food Prod. Technol. 25, 156–168 (2016). https://doi.org/10.1080/10498850.2013.838814
Acknowledgements
The authors would like to thank the Natural Environment Research Council (NERC) and Severn Trent Plc. for their financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Fane, S., Vale, P., Bajón-Fernández, Y. et al. Influence of Innate Sludge Factors and Ambient Environmental Parameters in Biosolids Storage on Indicator Bacteria Survival: A Review. Waste Biomass Valor 11, 6105–6114 (2020). https://doi.org/10.1007/s12649-019-00865-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-019-00865-w