Abstract
Fast pyrolysis bio-oil (FPBO) is a liquid biofuel obtained from lignocellulosic residues. Moreover, biomass fly ashes (FAs) containing many minerals and micronutrients are obtained in the production process. Biomass ashes can be used as a lime substitute for amelioration of acid soils by increasing pH, providing nutrients for crop development and stimulating microbial activity. However, ash application might increase N-mineralization and induce nitrate losses via leaching. The main objective of this study was to investigate the applicability of FPBO-recovered FAs as soil amendment and their effects on soil microbial processes, plant development, and to evaluate the effects on soil leaching. In a greenhouse experiment, an acidic soil was amended with 2% of FAs and sown with a regional wheat variety. After 100 days, wheat was harvested and red clover was sown to simulate crop rotation. After 250 days, the soils were analysed microbiologically and physico-chemically. While no differences in plant yields were observed, FAs addition increased several soil chemical pools as well as certain microbiological parameters. Soil pH increased from 4.8 to 7.2, electrical conductivity from 89 to 407 µS cm−1, and the soil available P pool from 13.6 to 81.3 µg g−1 soil. Further, the nitrification rate, nitrate content in the soil leachates increased upon ash addition, in particular during the clover stage of the experiment. Summarized, despite not measurable effects on the plant growth, fly ash appears to enhance chemical and biological properties of soil cropped with wheat and clover without hinting towards negative environmental side-effects.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
Developing techniques to boost renewable energy is of utmost importance. Among them, Fast Pyrolysis Bio- Oil (FPBO) is a new technology for biofuel production from lignocellulosic waste streams. The potential use of the resulting FPBO biomass ashes as lime replacement and their inherent risks have never been studied. A responsible management of residual streams is necessary to assure sustainability of FPBO technology that could pave the way for replacing fossil fuels.
Introduction
The increasing need to replace fossil fuels with renewable energy sources and the global concern about CO2 emission has generated a growing interest for using biomass for energy production [1]. There are several ways in which biomass such as wood, straw and energy crops can be transformed for energy production including combustion, gasification or a more recent technique known as fast pyrolysis. In this process organic material is rapidly heated under anaerobic conditions to 400–600 °C, a temperature sufficient to breakdown the biomass structure devoid of melting of the inorganic elements [2]. The vapours produced during this process are cooled and condensed into a brown liquid called Fast Pyrolysis Bio-Oil (FPBO) that can be used for heating, power generation, and as substitute in conventional diesel engines [3,4,5]. Besides FPBO, other streams like charcoal and low calorific gases are produced during the process and further combusted to generate energy resulting in the production of biomass fly ashes (FAs).
Biomass ashes are rich in macronutrients like Ca, Mg, K, and P, and also contain micronutrients such as Fe, Mn, Zn, and Cu. However, they are usually deficient in C and N, which are lost during the combustion of the biomass and emitted in the form of gaseous oxides [6]. The hydroxides and carbonates contained in the ashes exert an acid-neutralising effect that induce a rise in soil pH [7, 8], which may affect the solubility and availability of different soil elements [9, 10]. Indeed, wood ash can act as a soil amendment and lime substitute in agricultural [11,12,13,14] and forest soils [15,16,17], especially when these are acidic.
Although FA is considered a waste according to the actual European regulations (Directive 2008/98/EC on waste and Commission Decision 2000/532/EC on the list of waste [18]), it has been shown that when properly blended in soil it can represent a boon to agriculture and forestry both improving soil properties and providing a solution for safe disposal [11, 19, 20]. Biomass FAs may contain some heavy metals that serve as microelements and are essential for plant development; however, prior studies have shown that most of heavy metals provided by the ash input are bounded to insoluble forms of soil organic matter [7, 21, 22], and thus not available for plants. Concomitantly, the pH rise induced by ash addition to soil generates a decrease in the solubility of these metals [23, 24]. Moreover, it is known that the application of biomass ashes to soil might enhance soil organic matter mineralization and nitrification [25, 26], and thus influence nutrient loss to soil eluates [7, 14, 27].
Biomass ashes also represent a viable alternative to mineral P fertilisers that often pose eutrophication risks [28,29,30] and risks associated with other elements like Cu or U [31, 32]. Since N content in ashes can be neglected, an interesting option is to combine ashes with compost or nitrogenous fertilisers. In line with this, Kuba et al. [33] found that wood ash-amended compost increased plant cover, soil microbial biomass and respiration to a larger extent than mineral and organic fertilisers. Similar trends have been observed when combining biomass ashes with manure or slurries from anaerobic digestion [12, 14]. Nevertheless, it is still necessary to evaluate the benefits and potential drawbacks of FAs on soil properties and microorganisms to fill the gap concerning their effects on soil and plant growth. To date, there is still scarce information about the properties of biomass FAs when compared to those ashes obtained from coal [11, 34, 35] and no information on FPBO-FAs.
The main objective of the present study was to determine, at a mesocosm level, the effects of FAs derived from a fast pyrolysis process on several soil physico-chemical parameters including pH, electrical conductivity (EC) and nutrient content (C, N and P) over time in the presence and absence of Trifolium pratense (L.). We also determined soil organic matter (SOM) and particulate organic matter (POM) to assess if FAs promoted the degradation and turnover of OM in soil. The impact of this type of ashes was also assessed on microbial activity and microbial biomass, both indicators of soil fertility [36,37,38], as well as on the metabolic quotient (qCO2), which has been used as a proxy of microbial efficiency and environmental stress [14, 39, 40]. In addition, real-time PCR was used to estimate the abundance of key microorganisms involved in N and P cycles including ammonia oxidizing archaea (AOA), ammonia oxidizing bacteria (AOB), and bacteria containing phoD genes encoding for alkaline phosphatases (ALPs) [41]. We hypothesize that the application of ashes will promote: (i) higher plant yields due to an improved nutrient status of the soil (ii) changes in SOM composition and stability over time (iii) favour the abundance of AOB rather than AOA due to an increased pH, and (iv) higher abundance of ALP bacteria owing to an increase in the different soil P-fractions over time following ash addition.
Material and Methods
Soil Sampling and Experimental Set-Up
To test the fertilizing effect of the FAs, a greenhouse trial was carried out from May 2016 to February 2017. Soil was sampled in May 2016 in the village of Trins (Tirol, Austria). The soil of the grassland sampling site was classified as eutric Cambisol [42], a lime-free sandy loam (sand 59.6%, clay 7.5%, silt 34.8%) that did not receive any type of amendment seven years prior to sampling. It is N-limited soil characterised by a pH value of 6.2, and total C and N contents of 7.2% and 0.71%, respectively. Schönegger et al. [29] described the soil physico-chemical properties in detail.
The biomass ashes used for this study were derived from the production of FPBO by BTG company (Biomass Technology Group & BlueBear, Enschede, The Netherlands) using untreated pine wood chips as feedstock [29]. A detailed description of the physico-chemical and heavy metal content of the FPBO ashes used in the present study was given in Schönegger et al. [29], and can be also found in the Deliverable “Ash Composition” from the Residue2Heat project [43]. Briefly, the ash had a pH value of 12.5, and total C and N contents were 4.4% and 0.13%, respectively. Prior to the experiment, soil was sieved (Ø < 4 mm) and homogenously mixed with ashes at a rate of 2% (w/w; fresh weight (fw) basis) according to the highest dose allowed in the Austrian Ash Use Guideline [44]. Unamended soil was used as a control. Polymethyl methacrylate (Perspex®) columns, darkened an the sides to limit light supply to the top of the column, were filled with 2000 g soil each (fresh weight, fw). After an equilibration period of 24 h at 4 °C [45, 46], columns were placed in a greenhouse and the experiment was set up. All the columns with and without ashes were set up in triplicate in a randomised block fashion, and were sampled in the beginning (T0) and after 250 days. The 0-day samples (T0) refers to the starting point of the experiment (after the equilibration period). The greenhouse temperature ranged from 10 to 35 °C with an average temperature of 20 °C with a light/dark cycle of 16/8. Soil columns were watered twice per week with 50 mL distilled water. Triticum aestivum subsp. spelta, a Tyrolean wheat variety, was sown in half of the columns that received FAs and half of the columns without ash addition (10 seeds per column, leaving three plants to develop) to evaluate the FAs effects on plant growth. In the columns sampled after 250 days (T250), wheat was harvested after 100 days, the soil was allowed to rest for 13 days and 15 seeds of red clover (Trifolium pratense L.) were sown in each column to simulate crop rotation. After 23 days the five strongest seedlings were left to develop during another growth period of 115 days. These columns were then destructively sampled and referred as “250-day” treatment (T250). Red clover was chosen because of its wide distribution as forage crop and its role as nitrogen-fixer in the crop rotation system. Soil samples were sieved (Ø < 2 mm) and each sample was divided in two subsamples, one was kept at 4 °C for physico-chemical and microbiological analyses, and another one at − 20 °C for molecular analyses.
Physico-Chemical Analyses
Soil samples (10 g, fw) were oven-dried at 105 °C for 24 h, and reweighed to evaluate dry matter (DM) content. Total carbon (TC) and total nitrogen (TN) were assessed in oven-dried samples using a CN analyser (TrueSpec CHN; LECO, St. Joseph, MI, USA). Soil water holding capacity (WHC) was quantified according to Öhlinger [47]. The pH and electrical conductivity (EC) were determined as described by Fernández-Delgado Juárez et al. [14]. To estimate dissolved organic carbon (DOC) 10 g of soil were shaken in 40 mL distilled water and the extracts were measured using a TOC-L analyser (Shimadzu, Kyoto, Japan). Inorganic N (NH4+ and NO3−), was determined in KCL extracts following the method of Kandeler [48, 49]. Total (Ptot), inorganic (Pinorg), and plant available phosphorus (Pav) were determined according to the method described by Illmer [50]. The combination of sieving and sedimentation steps according to Kettler et al. [51] was used to determine the soil texture in conjunction with particulate organic matter (POM) and soil organic matter (SOM).
Microbiological Analyses
Microbial P was determined by using the fumigation extraction method as described by Schönegger et al. [29]. Potential nitrification and nitrogen mineralization were assessed following the methods described by Kandeler [52, 53]. Soil basal respiration (BR) was measured as CO2 evolution from moist soil samples [54], and microbial biomass (Cmic) was measured by substrate-induced respiration (SIR) [55]. The metabolic quotient (qCO2) was calculated as described by Insam and Haselwandter [56].
Plant Yield
Twenty-three days after seeding, the five strongest seedlings of red clover Trifolium pratense (L.) were selected from each column, and left to develop and harvested after 115 days. The aboveground and the root biomass were weighed and oven-dried at 60 °C for 48 h to determine the total dry weight.
DNA Extraction and Real-Time PCR
Whole community DNA from soil samples was extracted and quantified as described by Schönegger et al. [29]. To quantify the bacterial phoD gene, and the abundance of ammonia-oxidizing bacteria and archaea (AOB and AOA, respectively), quantitative real-time PCR (qPCR) was conducted. For the quantification of the phoD gene the method by Schönegger et al. [29] was followed. For the quantification of AOA and AOB the primers and cycling conditions described in Bardelli et al. [57] were applied.
Leaching
Leachates were collected in 150-mL polyethylene-flasks, placed at the bottom of each column, every second week during the first 100-day period, and every third week during the second period until the end of the experiment (250 days) by watering the columns with 100 mL of distilled water. A 2-week break in the column watering, and consequently in the leaching collection was done between wheat sampling and red clover seedling to simulate the soil resting period between crops in a rotation system. EC and pH values, together with the nitrate content (NO3−) were measured in soil leachates as described in “Physico-Chemical Analyses” section.
Statistical Analyses
The impact of ashes on the different soil properties was evaluated over time and in the presence and absence of plants by factorial analysis of variance (ANOVA) with Statistica v12. Prior to analysis data were transformed to meet the normality assumption whenever it was necessary, after have being subjected to a Shapiro–Wilk test. Non-normal data were subjected to non-parametric tests for several independent samples (Kruskal–Wallis test). Microbiological parameters and gene abundance were additionally analysed by one-way ANOVA. Significant differences were analysed by paired comparisons with the Tukey’s HSD test. Non-normal data were subjected to non-parametric tests for several independent samples (Kruskal–Wallis test) and pairwise comparisons were performed using the Mann–Whitney test. To assess the effect of the ash addition on leaching, data were analysed by repeated measures analysis of variance (ANOVAR). When variables did not meet the sphericity condition (Mauchly’s test), the assumption violation was corrected with the Geisser–Greenhouse (G–G) procedure [58].
Results
Effects of FAs on Soil Physico-Chemical Properties
Tables 1 and 2 show an overview of the physico-chemical and microbiological properties, and the statistical output, respectively. The pH levels in the ash-amended soils were close to neutrality, being approximately two units higher than the control at the beginning of the experiment and after 250 days regardless of the plant presence (Tables 1, 2). The same trend following ash addition was observed for EC (Table 1). The ash-treated soils had higher DOC values (two-times) than those in the control soil after 250 days of incubation irrespective of the plant presence (Table 1). Moreover, this increase was also observed at T0, when the ash addition induced 60% higher DOC values than those in the controls soils (Table 1). At T0, SOM was approximately 10% higher in the ash-amended soils than in the control ones, while at T250, 10% less SOM was observed in soils amended with FAs than in the control soils (Tables 1, 2). The ash presence also induced a significant increase in POM relative to the control, at T0 and T250, whereas neither the time nor the plant presence had a significant effect on this parameter (Table 1). Ashes did not affect the C/N-ratio irrespective of the sampling time and plant presence (Table 2). At the beginning of the trial, the NH4+ content was significantly higher (seven-times) in the ash-amended soils than in the control ones. However, after 250 days of incubation the ash addition resulted in a lower NH4+ content compared to the control in the presence and the absence of plants (Table 1). Higher NH4+ levels were detected at the end of the trial in the control and the ash-amended soils when compared to T0 (Table 1.). The ash addition led to a higher content of NO3− relative to the control at both sampling times. An increase in this parameter was observed over time in the control and the ash-treated soils (Tables 1, 2). The presence of plants induced a decrease in NO3− values, and such effect was more discernible at T250 in the ash-treated soils, with values ranging from 200 to 370 µg NO3− g−1 dw (Tables 1, 2). Inorganic and total P contents were about two-times higher in the ash-treated soils than in the control treatment regardless of plant presence and time (Tables 1, 2). A significant increase in Pav was also detected with ash addition, with values rising from 5 to 55 µg g−1 at the beginning of the trial. Likewise, its content was about fivefold higher in the ash-treated soils after 250 days in the presence and absence of plants (Table 1). Available P increased over time and reached values of 80 µg g−1 at the end of the experiment in the ash-treated soils. Such an increase over time was also observed in the control soils reached values of 12–14 µg g−1 (Tables 1, 2).
Effects of FAs on Soil Microbiological Properties and Plant Yields
Microbial activity measured as basal respiration (BR) was significantly higher in the ash-amended soils than in the control ones at the beginning of the trial and after 250 days (Fig. 1a; Table 2). However, neither time nor crop presence had a significant effect on BR (Fig. 1a). Higher levels of microbial biomass (Cmic) were observed following ash addition at the beginning of the trial, and such increase was less pronounced after 250 days of incubation (Fig. 1b). At the beginning and at the end of the trial, Cmic decreased in the control and the ash-amended soils regardless of the plant presence (Fig. 1b; Table 2). Ash addition enhanced the metabolic quotient (Mann–Whitney, p ≤ 0.05) irrespective of time and presence of plants (Fig. 1c, Table 2).
a Basal respiration, b microbial biomass, and c metabolic quotient in the control (white) and the ash-treated (grey) soils in the presence (P) and the absence (NP) of plants at the beginning (T0) and at the end (T250) of the experiment. Bars indicate standard errors (n = 3). Different lower-case letters indicate significant differences between treatments (p ≤ 0.05) according to the Tukey’s HSD test. Different capital letters indicate significant differences between treatments (p ≤ 0.05) according to the Mann–Whitney test
After 250 days of incubation microbial P was lower in the presence of ashes (22–19 μg g−1) than in the control treatment (70–45 μg g−1), both in absence and presence of plants (Tables 1, 2). Neither the ash treatment nor the plant presence had a significant impact on nitrogen mineralization (Tables 1, 2); however, a reduction in this parameter was observed over time in both the control and the ash-treated soils (Tables 1, 2). Ash addition increased the soil potential nitrification (Table 2) that was 11- and 18-times higher at T0 and T250, respectively when compared to the control (Table 1). The potential nitrification also increased over time; Further, the presence of the crop did not show any influence on this parameter (Tables 1, 2).
The application of FAs did not have a significant impact on bacterial phoD gene abundance. An increase in the gene copy number of bacterial phoD gene was recorded in both the ash-amended and the control soils over time (Table 3). Nonetheless, amending soil with ashes led to a decrease in AOA gene copy number relative to the control at the end of the trial and regardless of the plant presence (Mann–Whitney, p < 0.001; Table 3). There were no significant changes in the AOB gene copy number following ash amendment (Table 3). The highest copy number was observed at T250 for the ash-amended soils in the presence of plants (Table 3). Regarding the AOA/AOB ratio, the ash-amended soils had a much lower ratio than the control ones at the end of the trial in the presence and absence of plants (Table 3).
Amending soil with ashes had no effect on the aboveground and root biomass compared to the control (data not shown).
Effect of FAs on Soil Leachate Properties
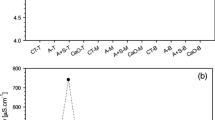
Ash addition increased the pH of soil leachates (ANOVAR p < 0.001) by about 0.5–1.0 U, and this effect was time-dependent (ANOVAR treatment × time; p < 0.001). Less pronounced pH differences were observed at the beginning and at the end of the trial (Fig. 2a). Moreover, from July onwards a higher pH value was recorded in the leachates from ash-treated soils in the presence of plants (Fig. 2a). Ash treatment also affected EC of soil leachates throughout the monitoring period and EC also increased over time (ANOVAR Time p < 0.001). The highest EC was found in the leachates from the ash-amended soils in the absence of plants (ANOVAR ash treatment × plant presence, p < 0.001; Fig. 2b). During the last 4 months of incubation, the differences in the ash-amended soils in the presence and the absence of plants became smaller until they ceased (ANOVAR time x plant x ash, p < 0.001; Fig. 2b). A similar trend as for EC was observed for NO3, registering a higher content in the leachates from the ash-treated soils when plants were absent (ANOVAR plant × time p < 0.001). The initial effects of the plant presence in the ash-amended soils diminished over time (ANOVAR Time × plant × ash, p < 0.001; Fig. 2c).
Values of pH (a), electrical conductivity (EC) (b), and nitrate concentration (c) of the leachates obtained from the control and the ash-treated soils over a period of 250 days in the presence and the absence of plants (P and NP respectively). Average values are given (n = 3); bars indicate standard errors
Discussion
This study reveals that FAs resulting from the production of FPBO have a potential use as a soil amendment since, in general, no harmful effects were observed from a chemical and microbiological viewpoint. Biomass ashes serve as a source of major elements and are thus able to alleviate nutrient deficiencies and neutralize soil acidification due to the formation of hydroxides and carbonates. Accordingly, in this study we observed an increase in soil pH towards neutrality following the addition of FPBO fly ashes. Soil pH is considered a determinant factor driving the composition, diversity and activity of microbial communities, since the pH affects nutrient availability and the synthesis and activity of soil enzymes [59]. These latter authors stated that crops responded better to organic amendments when soil pH ranges from weak-acidic to weak-alkaline levels, as those obtained in the current study. As expected, the application of FAs led to an increase in soil conductivity owing to the release of soluble salts, even though the EC values in the ash-amended soils did not exceed the threshold value of 4 mS cm−1 for plant health [60].
In the early phase of our study, SOM was higher in the ash-treated soils followed by a decrease after 250 days in the presence of ashes. However, POM was higher in case of ash amended soils at the beginning and the end of the trial. This trend suggests that the liming effect and the input of nutrients derived from ash application promoted SOM turnover and its conversion in more labile C forms, while the POM, that might be sourced in the FAs remained stable over time. In agreement with previous studies [61, 62], higher DOC values were recorded in the ash-amended soils which suggests that the rate of SOM that is mineralized over time contributes to the supply of more available forms of OM, which can potentially be absorbed to soil minerals, precipitate or be immobilized by the formation of POM [63]. Such an increase in DOC has been attributed to a fertilization effect favouring plant nutrient availability, and ultimately the biological activity and the turnover time for the different carbon compounds in the soil [61, 62].
Previous findings have shown that biomass ash addition induces soil mineralization processes [26, 64], and ultimately results in an increase of organic and inorganic N forms [17, 65]. However, as also occurred in our study, N mineralization may not always be affected by ash addition [66, 67] which we attribute to the C/N-ratio of the soil, and consequently to the N-limitation in the experimental site. In soils with a high C/N-ratio and low N availability, N mineralization does not seem to be affected by ash addition [68].
Ammonia-oxidizing bacteria and archaea are responsible for the first and limiting step of nitrification by converting ammonia to nitrite. Some authors have suggested a niche separation between both groups [69, 70] being AOB rather than AOA favoured by nutrient-rich environments characterised by a pH value close to neutrality [71,72,73]. Although AOA abundance was lower after 250 days of incubation following ash amendment, AOB abundance did not change, albeit, the ash-amended soils were characterised by an improved nutrient status (i.e., higher DOC and Ptot content) and a higher pH. Despite the lack of differences in AOB abundance the presence of ash remarkably stimulated the potential nitrification rate which is in line with the higher NO3− levels found in the ash-treated soils in comparison with the control ones at the end of the trial (Table 1). Nonetheless, such positive effects on nitrification after FA addition were not accompanied by an increase in plant yields. This might indicate that the mineralized N following ash amendment was not taken up by the plants, but rather lost via leaching. More specifically, the nutrients applied with the ash and/or released as a result of accelerated mineralization might have been taken up by the wheat crop at the beginning of the trial [29] when a significant increase in wheat biomass in the ash-treated soils was observed, but not later on by the red clover since as mentioned above no clear effects on plant root and aboveground biomass were observed. It is known that ash addition to soils may boost inorganic N losses via leaching due to an enhancement of mineralization [7, 15], similar to priming effects. This could explain the increased NO3− concentration in the leachates from the ash-amended soils compared to those from the control ones in the presence and the absence of plants.
Contrary to our hypothesis, we did not observe an increase in the abundance of phoD harbouring bacteria following ash addition, despite the increase in the different soil P-fractions over time. Therefore, the rise in P availability in the ash-treated soils did not immediately affect the functional genes encoding for alkaline phosphatase. An explanation could be that an application rate of 2% was not high enough to boosting the abundance of phoD harbouring bacterial populations. Moreover, the higher content of available P in the ash-amended soils could have inhibited the transcription by the Pho regulon, indeed suppressing ALP activity by bacteria. An increase in P-acquiring enzyme activities would be expected in case of P deficiency [74]. Once microorganisms mineralize the organic P, the P dissolved in the soil solution is available for plant uptake. The plant available P in our trial increased with the ash treatment, while the microbial P decreased. This suggests that this disequilibrium is probably due to the turnover of P immobilized in microbial biomass to inorganic forms.
Microbial biomass and activity have been suggested as indicators of the stability of the soil amendment, as they provide rapid information on soil quality owing to their sensitivity to change [38, 41]. In the present study, amending soil with FPBO FAs led to an increase in microbial biomass (measured as Cmic) and activity (assessed as basal respiration) relative to the control after 250 days of incubation. These positive effects may be explained by the increased nutrient availability. Additionally, the FAs used in our study were characterised by a low content in heavy metals as shown by Schönegger et al. [29]. Nonetheless, Sharma and Kalra [75] observed that the application of FAs resulted in a reduction in soil respiration, microbial biomass and enzymatic activities; and such effects were proportional to the amount of ash. Other authors did not detect any changes in soil microbial biomass C [76] and in microbial biomass measured as the total amount of phospholipid fatty acids [19] following FA addition. Despite the overall stimulation of microbial activity, the increased metabolic quotient in the ash-amended soils suggest that microbial communities may have been under a higher stress than those in the control soil specially towards the end of the study. This may have been caused by the lack of N in the FAs albeit the ash provided other nutrients and micronutrients, that allowed a boost of microbial activity [77, 78].
Conclusions
This study evaluated the ecological impact of FAs resulting from Fast Pyrolysis Bio-Oil production as soil amendment. Our findings show the promising use of this “waste-resource” as soil amendment and as a recycling alternative to landfill disposal. The increased pH and EC, together with the input of nutrients from this type of ashes enhanced SOM turnover and increased the labile organic fraction. There was also a boost in the activity of the soil microbiota following ash amendment, even though the lack of C and N over time limited microbial growth. As hypothesized, the addition of FPBO-FAs to soil favoured the abundance of AOB rather than AOA; however, effects on the abundance of ALP bacteria following ash application were insignificant. Despite the positive effects on soil properties, and in contrast to our hypothesis, we did not observe higher plant yields for red clover after ash addition. Moreover, the potential nutrient losses via leaching might be a concern for large-scale application. Thus, it would be of future interest to conduct additional in situ trials to determine the maximum rate at which FPBO ashes can be safety applied and the relationship between land management, soil characteristics and microbial community function. Within this context, estimating the bioavailability of potential pollutants present in the ashes, e.g. heavy metals, is crucial for coming to a decision about their further suitability as soil amendment. Furthermore, their long-term effects on a crop rotation system, and if leguminous plants could alleviate N-deficiency, should be assessed. All in all, the results indicate that biomass FAs derived from FPBO production may be considered as an environmentally friendly lime substitute for improvement of acid soil.
References
AEBIOM, European Biomass Association.: European Bioengery Outlook Annual statistical report 2017. In: European Biomass Association (ed.), Brussels, Belgium, p. 264 (2017)
Leijenhorst, E.J., Wolters, W., van de Beld, L., Prins, W.: Inorganic element transfer from biomass to fast pyrolysis oil: review and experiments. Fuel Process. Technol. 149, 96–111 (2016). https://doi.org/10.1016/j.fuproc.2016.03.026
Bridgwater, A.V.: Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy. 38, 68–94 (2012). https://doi.org/10.1016/j.biombioe.2011.01.048
Lehto, J., Oasmaa, A., Solantausta, Y., Kytö, M., Chiaramonti, D.: Review of fuel oil quality and combustion of fast pyrolysis bio-oils from lignocellulosic biomass. Appl. Energy. 116, 178–190 (2014). https://doi.org/10.1016/j.apenergy.2013.11.040
Van de Beld, B., Holle, E., Florijn, J.: The use of pyrolysis oil and pyrolysis oil derived fuels in diesel engines for CHP applications. Appl. Energy 102, 190–197 (2013). https://doi.org/10.1016/j.apenergy.2012.05.047
Knapp, B.A., Insam, H.: Recycling of biomass ashes: current technologies and future research needs. In: Insam, H., Knapp, B.A. (eds.) Recycling of Biomass Ashes, pp. 1–16. Springer, Berlin (2011)
Ludwig, B., Rumpf, S., Mindrup, M., Meiwes, K.-J., Khanna, P.K.: Effects of lime and wood ash on soil-solution chemistry, soil chemistry and nutritional status of a pine stand in Northern Germany. Scand. J. For. Res. 17, 225–237 (2002). https://doi.org/10.1080/028275802753742891
Steenari, B.M., Karlsson, L.G., Lindqvist, O.: Evaluation of the leaching characteristics of wood ash and the influence of ash agglomeration. Biomass Bioenergy. 16, 119–136 (1999). https://doi.org/10.1016/s0961-9534(98)00070-1
Maresca, A., Hansen, M., Ingerslev, M., Astrup, T.F.: Column leaching from a Danish forest soil amended with wood ashes: fate of major and trace elements. Biomass Bioenergy. 109, 91–99 (2018). https://doi.org/10.1016/j.biombioe.2017.12.014
Augusto, L., Bakker, M.R., Meredieu, C.: Wood ash applications to temperate forest ecosystems—potential benefits and drawbacks. Plant Soil. 306, 181–198 (2008). https://doi.org/10.1007/s11104-008-9570-z
Basu, M., Pande, M., Bhadoria, P.B.S., Mahapatra, S.C.: Potential fly-ash utilization in agriculture: a global review. Prog. Nat. Sci. 19, 1173–1186 (2009). https://doi.org/10.1016/j.pnsc.2008.12.006
Bougnom, B.P., Niederkofler, C., Knapp, B.A., Stimpfl, E., Insam, H.: Residues from renewable energy production: their value for fertilizing pastures. Biomass Bioenergy. 39, 290–295 (2012). https://doi.org/10.1016/j.biombioe.2012.01.017
Bougnom, B.P., Mair, J., Etoa, F.X., Insam, H.: Composts with wood ash addition: a risk or a chance for ameliorating acid tropical soils? Geoderma 153, 402–407 (2009). https://doi.org/10.1016/j.geoderma.2009.09.003
Fernández-Delgado Juárez, M., Waldhuber, S., Knapp, A., Partl, C., Gómez-Brandón, M., Insam, H.: Wood ash effects on chemical and microbiological properties of digestate- and manure-amended soils. Biol. Fertil. Soils. 49, 575–585 (2013). https://doi.org/10.1007/s00374-012-0747-5
Huotari, N., Tillman-Sutela, E., Moilanen, M., Laiho, R.: Recycling of ash—for the good of the environment? For. Ecol. Manag. 348, 226–240 (2015). https://doi.org/10.1016/j.foreco.2015.03.008
Pitman, R.M.: Wood ash use in forestry—a review of the environmental impacts. Forestry 79, 563–588 (2006). https://doi.org/10.1093/forestry/cpl041
Fernández-Delgado Juárez, M., Gómez-Brandón, M., Knapp, A., Stöhr, D., Insam, H.: Chemical and microbiological properties of alpine forest soils: effects of pelletized ashes in a short-term trial. For. Ecol. Manag. 357, 42–49 (2015). https://doi.org/10.1016/j.foreco.2015.08.014
European Commission.: Commission notice on technical guidance on the classification of waste C/2018/1447. ISSN 1977–091X. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=OJ:C:2018:124:TOC. (2018) Accessed 4 Feb 2019
García-Sánchez, M., Siles, J.A., Cajthaml, T., García-Romera, I., Tlustoš, P., Száková, J.: Effect of digestate and fly ash applications on soil functional properties and microbial communities. Eur. J. Soil Biol. 71, 1–12 (2015). https://doi.org/10.1016/j.ejsobi.2015.08.004
Perucci, P., Monaci, E., Onofri, A., Vischetti, C., Casucci, C.: Changes in physico-chemical and biochemical parameters of soil following addition of wood ash: a field experiment. Eur. J. Agron. 28, 155–161 (2008). https://doi.org/10.1016/j.eja.2007.06.005
Eriksson, J.: Dissolution of hardened wood ashes in forest soils: studies in a column experiment. Scand. J. For. Res. 2, 23–32 (1998)
Nieminen, M., Piirainen, S., Moilanen, M.: Release of mineral nutrients and heavy metals from wood and peat ash fertilizers: field studies in finnish forest soils. Scand. J. For. Res. 20, 146–153 (2005). https://doi.org/10.1080/02827580510008293
Chirenje, T., Ma, L.Q.: Effects of acidification on metal mobility in a papermill-ash amended soil. J. Environ. Qual. 28, 760–766 (1999). https://doi.org/10.2134/jeq1999.00472425002800030005x
Dimitriou, I., Eriksson, J., Adler, A., Aronsson, P., Verwijst, T.: Fate of heavy metals after application of sewage sludge and wood–ash mixtures to short-rotation willow coppice. Environ. Pollut. 142, 160–169 (2006). https://doi.org/10.1016/j.envpol.2005.09.001
Hansen, M., Saarsalmi, A., Peltre, C.: Changes in SOM composition and stability to microbial degradation over time in response to wood chip ash fertilisation. Soil Biol. Biochem. 99, 179–186 (2016). https://doi.org/10.1016/j.soilbio.2016.05.012
Odlare, M., Pell, M.: Effect of wood fly ash and compost on nitrification and denitrification in agricultural soil. Appl. Energy. 86, 74–80 (2009). https://doi.org/10.1016/j.apenergy.2008.04.004
Maresca, A., Hyks, J., Astrup, T.F.: Long-term leaching of nutrients and contaminants from wood combustion ashes. Waste Manag. 74, 373–383 (2018). https://doi.org/10.1016/j.wasman.2017.11.056
Bachmann, S., Eichler-Lobermann, B.: Soil phosphorus pools as affected by application of poultry litter ash in combination with catch crop cultivation. Commun. Soil Sci. Plant Anal. 41, 1098–1111 (2010). https://doi.org/10.1080/00103621003687182
Schönegger, D., Gómez-Brandón, M., Mazzier, T., Insam, H., Hermanns, R., Leijenhorst, E., Bardelli, T., Fernández-Delgado Juárez, M.: Phosphorus fertilising potential of fly ash and effects on soil microbiota and crop. Resour. Conserv. Recycl. 134, 262–270 (2018). https://doi.org/10.1016/j.resconrec.2018.03.018
Cruz-Paredes, C., López-García, Á., Rubæk, G.H., Hovmand, M.F., Sørensen, P., Kjøller, R.: Risk assessment of replacing conventional P fertilizers with biomass ash: residual effects on plant yield, nutrition, cadmium accumulation and mycorrhizal status. Sci. Total Environ. 575, 1168–1176 (2017). https://doi.org/10.1016/j.scitotenv.2016.09.194
Moilanen, M., Fritze, H., Nieminen, M., Piirainen, S., Issakainen, J., Piispanen, J.: Does wood ash application increase heavy metal accumulation in forest berries and mushrooms? For. Ecol. Manag. 226, 153–160 (2006)
Tilman, D., Cassman, K.G., Matson, P.A., Naylor, R., Polasky, S.: Agricultural sustainability and intensive production practices. Nature 418, 671 (2002)
Kuba, T., Tscholl, A., Partl, C., Meyer, K., Insam, H.: Wood ash admixture to organic wastes improves compost and its performance. Agric. Ecosyst. Environ. 127, 43–49 (2008). https://doi.org/10.1016/j.agee.2008.02.012
Taylor, E.M., Schuman, G.E.: Fly ash and lime amendment of acidic coal spoil to aid revegetation. J. Environ. Qual. 17, 120–124 (1988). https://doi.org/10.2134/jeq1988.00472425001700010018x
Ukwattage, N.L., Ranjith, P.G., Bouazza, M.: The use of coal combustion fly ash as a soil amendment in agricultural lands (with comments on its potential to improve food security and sequester carbon). Fuel 109, 400–408 (2013). https://doi.org/10.1016/j.fuel.2013.02.016
Insam, H., Parkinson, D., Domsch, K.H.: Influence of macroclimate on soil microbial biomass. Soil Biol. Biochem. 21, 211–221 (1989). https://doi.org/10.1016/0038-0717(89)90097-7
Yao, H., He, Z., Wilson, M.J., Campbell, C.D.: Microbial biomass and community structure in a sequence of soils with increasing fertility and changing land use. Microb. Ecol. 40, 223–237 (2000). https://doi.org/10.1007/s002480000053
Schloter, M., Nannipieri, P., Sørensen, S.J., van Elsas, J.D.: Microbial indicators for soil quality. Biol. Fertil. Soils. 54, 1–10 (2018). https://doi.org/10.1007/s00374-017-1248-3
Anderson, T.H., Domsch, K.H.: The metabolic quotient for CO2 (qCO2) as a specific activity parameter to assess the effects of environmental conditions, such as pH, on the microbial biomass of forest soils. Soil Biol. Biochem. 25, 393–395 (1993). https://doi.org/10.1016/0038-0717(93)90140-7
Wardle, D.A., Ghani, A.: A critique of the microbial metabolic quotient (qCO(2)) as a bioindicator of disturbance and ecosystem development. Soil Biol. Biochem. 27, 1601–1610 (1995). https://doi.org/10.1016/0038-0717(95)00093-T
Nannipieri, P., Ascher, J., Ceccherini, M.T., Landi, L., Pietramellara, G., Renella, G.: The links between microbial diversity and soil functions. In: Innovative Soil-Plant Systems for Sustainable Agricultural Practices, pp. 120–131 (2003)
IUSS Working Group WRB: World Reference Base for Soil Resources 2014, update 2015 International soil classification system for naming soils and creating legends for soil maps (2015)
Gómez-Brandón, M., Fernández-Delgado Juárez, M., Insam, H.: Ash characterization. D6.3. Ref. Ares(2017)6328165—21/12/2017. https://cordis.europa.eu/project/rcn/199298/reporting/en (2019)
Bundesministerium für Land- und Forstwirtschaf, Umwelt und Wasserwirtschaft.: Richtlinie für den sachgerechten Einsatz von Pflanzenaschen zur Verwertung auf Land- und Forstwirtschaftlich genutzten Flächen. p. 74 (Guideline of the Austrian Agriculture Ministry for the adequate use of Biomass ashes in agricultural and forestry areas). https://www.bmnt.gv.at/land/produktion-maerkte/pflanzliche-produktion/boden-duengung/Bodenschutz.html (2011). Accessed 4 Feb 2019.
Gómez-Brandón, M., Fernández-Delgado Juárez, M., Zangerle, M., Insam, H.: Effects of digestate on soil chemical and microbiological properties: a comparative study with compost and vermicompost. J. Hazard. Mater. 302, 267–274 (2016). https://doi.org/10.1016/j.jhazmat.2015.09.067
Goberna, M., Podmirseg, S.M., Waldhuber, S., Knapp, B.A., Garcia, C., Insam, H.: Pathogenic bacteria and mineral N in soils following the land spreading of biogas digestates and fresh manure. Appl. Soil Ecol. 49, 18–25 (2011). https://doi.org/10.1016/j.apsoil.2011.07.007
Öhlinger, R.: Maximum water-holding capacity. In: Schinner, F., Öhlinger, R., Kandeler, E., Margesin, R. (eds.) Methods in Soil Biology. Springer, Berlin, p. 385 (1996).
Kandeler, E.: Ammonium. In: Schinner, F., Öhlinger, R., Kandeler, E., Margesin, R. (eds.) Methods in Soil Biology, pp. 406–408. Springer, Berlin (1996)
Kandeler, E.: Nitrate. In: Schinner, F., Öhlinger, R., Kandeler, E., Margesin, R. (eds.) Mthods in Soil Biology, pp. 408–410. Springer, Berlin (1996)
Illmer, P.: Total, organic, inorganic and plant available phosphorus. In: Schinner, F., Öhlinger, R., Kalender, E., Margesin, R. (eds.) Methods in Soil Biology, pp. 412–416. Springer, Berlin (1996)
Kettler, T.A., Doran, J.W., Gilbert, T.L.: Simplified method for soil particle-size determination to accompany soil-quality analyses. Soil Sci. Soc. Am. J. 65, 849–852 (2001). https://doi.org/10.2136/sssaj2001.653849x
Kandeler, E.: N-mineralization under watterlogged conditions. In: Schinner, F., Öhlinger, R., Kandeler, E., Margesin, R. (eds.) Bodenbiologische Arbeitsmethoden, pp. 141–143. Springer, Berlin (1996)
Kandeler, E.: Potential nitrification. In: Schinner, F., Öhlinger, R., Kandeler, E., Margesin, R. (eds.) Methods in Soil Biology, pp. 146–419. Springer, Berlin (1996)
Heinemeyer, O., Insam, H., Kaiser, E., Walenzik, G.: Soil microbial biomass and respiration measurements - an automated technique based on infra-red gas analysis. Plant Soil. 116, 191–195 (1989). https://doi.org/10.1007/BF02214547
Anderson, J.P.E., Domsch, K.H.: A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol. Biochem. 10, 215–221 (1978). https://doi.org/10.1016/0038-0717(78)90099-8
Insam, H., Haselwandter, K.: Metabolic quotient of the soil microflora in relation to plant succession. Oecologia 79, 174–178 (1989). https://doi.org/10.1007/bf00388474
Bardelli, T., Ascher-Jenull, J., Burkia Stocker, E., Fornasier, F., Arfaioli, P., Fravolini, G., Alves Medeiros, L.R., Egli, M., Pietramellara, G., Insam, H., Gómez-Brandón, M.: Impact of slope exposure on chemical and microbiological properties of Norway spruce deadwood and underlying soil during early stages of decomposition in the Italian Alps. CATENA 167, 100–115 (2018). https://doi.org/10.1016/j.catena.2018.04.031
Potvin, C., Lechowicz, M.J., Tardif, S.: The statistical analysis of ecophysiological response curves obtained from experiments involving repeated measures. Ecology 71, 1389–1400 (1990). https://doi.org/10.2307/1938276
Luo, G., Li, L., Friman, V.-P., Guo, J., Guo, S., Shen, Q., Ling, N.: Organic amendments increase crop yields by improving microbe-mediated soil functioning of agroecosystems: a meta-analysis. Soil Biol. Biochem. 124, 105–115 (2018). https://doi.org/10.1016/j.soilbio.2018.06.002
Haynes, R.J.: Reclamation and revegetation of fly ash disposal sites—Challenges and research needs. J. Environ. Manage. 90, 43–53 (2009). https://doi.org/10.1016/j.jenvman.2008.07.003
Holmström, S.J., Riise, G., Strand, L.T., Geibe, C., Van Hees, P.A., Wu, Q., Lundström, U.S.: Effects of lime and ash treatments on DOC fractions and low molecular weight organic acids in soil solutions of acidified podzolic soils. Water Air Soil Pollut. 3(4), 97–120 (2003).
Norström, S.H., Bylund, D., Vestin, J.L.K., Lundström, U.S.: Initial effects of wood ash application to soil and soil solution chemistry in a small, boreal catchment. Geoderma 187, 85–93 (2012). https://doi.org/10.1016/j.geoderma.2012.04.011
Schwesig, D., Kalbitz, K., Matzner, E.: Mineralization of dissolved organic carbon in mineral soil solution of two forest soils. J. Plant Nutr. Soil Sci. 166, 585–593 (2003). https://doi.org/10.1002/jpln.200321103
Khanna, P.K., Raison, R.J., Falkiner, R.A.: Chemical properties of ash derived from Eucalyptus litter and its effects on forest soils. For. Ecol. Manag. 66, 107–125 (1994). https://doi.org/10.1016/0378-1127(94)90151-1
Saarsalmi, A., Smolander, A., Kukkola, M., Moilanen, M., Saramäki, J.: 30-Year effects of wood ash and nitrogen fertilization on soil chemical properties, soil microbial processes and stand growth in a Scots pine stand. For. Ecol. Manag. 278, 63–70 (2012). https://doi.org/10.1016/j.foreco.2012.05.006
Moilanen, M., Saarsalmi, A., Kukkola, M., Issakainen, J.: Effects of stabilized wood ash on nutrient status and growth of Scots pine—Comparison between uplands and peatlands. For. Ecol. Manag. 295, 136–144 (2013). https://doi.org/10.1016/j.foreco.2013.01.021
Rosenberg, O., Persson, T., Hogbom, L., Jacobson, S.: Effects of wood-ash application on potential carbon and nitrogen mineralisation at two forest sites with different tree species, climate and N status. For. Ecol. Manag. 260, 511–518 (2010). https://doi.org/10.1016/j.foreco.2010.05.006
Ring, E., Jacobson, S., Nohrstedt, H.-Ö.: Soil-solution chemistry in a coniferous stand after adding wood ash and nitrogen. Can. J. For. Res. 36, 153–163 (2006). https://doi.org/10.1139/x05-242
Schleper, C., Nicol, G.W.: Ammonia-oxidising archaea—physiology, ecology and evolution Adv. Microb. Physiol. 57(57), 1–41 (2010). https://doi.org/10.1016/B978-0-12-381045-8.00001-1
Wessen, E., Hallin, S.: Abundance of archaeal and bacterial ammonia oxidizers—possible bioindicator for soil monitoring. Ecol. Indic. 11, 1696–1698 (2011). https://doi.org/10.1016/j.ecolind.2011.04.018
Verhamme, D.T., Prosser, J.I., Nicol, G.W.: Ammonia concentration determines differential growth of ammonia-oxidising archaea and bacteria in soil microcosms. Isme J. 5, 1067–1071 (2011). https://doi.org/10.1038/ismej.2010.191
Glaser, K., Hackl, E., Inselsbacher, E., Strauss, J., Wanek, W., Zechmeister-Boltenstern, S., Sessitsch, A.: Dynamics of ammonia-oxidizing communities in barley-planted bulk soil and rhizosphere following nitrate and ammonium fertilizer amendment. FEMS Microbiol. Ecol. 74, 575–591 (2010). https://doi.org/10.1111/j.1574-6941.2010.00970.x
Di, H.J., Cameron, K.C., Shen, J.-P., Winefield, C.S., O’Callaghan, M., Bowatte, S., He, J.-Z.: Ammonia-oxidizing bacteria and archaea grow under contrasting soil nitrogen conditions. FEMS Microbiol. Ecol. 72, 386–394 (2010). https://doi.org/10.1111/j.1574-6941.2010.00861.x
Fraser, T.D., Lynch, D.H., Bent, E., Entz, M.H., Dunfield, K.E.: Soil bacterial phoD gene abundance and expression in response to applied phosphorus and long-term management. Soil Biol. Biochem. 88, 137–147 (2015). https://doi.org/10.1016/j.soilbio.2015.04.014
Sharma, S.K., Kalra, N.: Effect of fly ash incorporation on soil properties and plant productivity—a review. J. Sci. Ind. Res. 65, 383–390 (2006)
Nayak, A.K., Raja, R., Rao, K.S., Shukla, A.K., Mohanty, S., Shahid, M., Tripathi, R., Panda, B.B., Bhattacharyya, P., Kumar, A., Lal, B., Sethi, S.K., Puri, C., Nayak, D., Swain, C.K.: Effect of fly ash application on soil microbial response and heavy metal accumulation in soil and rice plant. Ecotoxicol. Environ. Saf. 114, 257–262 (2015). https://doi.org/10.1016/j.ecoenv.2014.03.033
Bang-Andreasen, T., Nielsen, J.T., Voriskova, J., Heise, J., Rønn, R., Kjøller, R., Hansen, H.C.B., Jacobsen, C.S.: Wood ash induced pH changes strongly affect soil bacterial numbers and community composition. Front. Microbiol. 8, 1400 (2017). https://doi.org/10.3389/fmicb.2017.01400
Perucci, P., Monaci, E., Casucci, C., Vischetti, C.: Effect of recycling wood ash on microbiological and biochemical properties of soils. Agron. Sustain. Dev. 26, 157–165 (2006). https://doi.org/10.1051/Agro:2006009
Acknowledgements
Open access funding provided by University of Innsbruck and Medical University of Innsbruck. The authors thank Monika Leitner, Daniela Pirkebner, Peter Schönswetter, and Evert Leijenhorst for kindly providing materials and greenhouse space to perform this study. G.F. acknowledges support of the Erasmus + Program, and M.G.-B. acknowledges support by the Programa Ramón y Cajal (RYC-2016–21231; Ministerio de Economía y Competitividad).
Funding
The Residue2Heat project has received funding from the European Union’s Horizon 2020 Research and Innovation program under Grant Agreement No. 654650.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Fernández-Delgado Juárez, M., Fabiani, G., Mazzier, T. et al. Reclamation of Acid Soils with Biomass Ashes from Pyrolytic Wood Liquefaction. Waste Biomass Valor 11, 5067–5078 (2020). https://doi.org/10.1007/s12649-019-00789-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-019-00789-5