Abstract
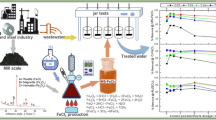
Coagulation is capable of treating metalworking fluid (MWF) wastewater after MWF oil recovery, as an alternative fuel by dissolved-air flotation, but it yields a substantial amount of oil-contaminated sludge. The oil-contaminated sludge is a resource of coagulant recovery, which reduces the sludge disposal and coagulant cost. This study examined the acid recovery and reusability of iron coagulant for MWF wastewater treatment. Ferric chloride (FeCl3) was used as a coagulant, while hydrochloric acid (HCl) was used in the solubilisation process. Without MWF contamination, the iron hydroxide (Fe(OH)3) to HCl ratio of 3:1 was required to completely dissolve the Fe(OH)3 sludge within 5 min. The presence of MWF oil on the sludge substantially decreased the acid recovery of Fe(OH)3. Even at 30 min, the Fe(OH)3 to HCl ratio of 3:1 could recover only 87.07% by mass of iron. The use of anionic polymer (polyacrylate) as a coagulant aid for MWF treatment made the acid recovery of iron coagulant less effective. Acid-recovered iron coagulant can be reused for four cycles to treat the MWF wastewater because increasing the cycles of reuse lowers the treatment efficiency. The use of HCl waste in this approach reduces both the operational cost and potential global warming effect.







Similar content being viewed by others
References
Demirbas, E., Kobya, M.: Operating cost and treatment of metalworking fluid wastewater by chemical coagulation and electrocoagulation processes. Process Saf. Environ. Prot. 105, 79–90 (2017)
Brinksmeier, E., Meyer, D., Huesmann-Cordes, A.G., Herrmann, C.: Metalworking fluids—mechanisms and performance. CIRP Ann. 64(2), 605–628 (2015)
Friesen, M.C., Costello, S., Thurston, S.W., Eisen, E.: Distinguishing the common components of oil- and water-based metalworking fluids for assessment of cancer incidence risk in autoworkers. Am. J. Ind. Med. 54(6), 450–460 (2011). https://doi.org/10.1002/ajim.20932
Gerulová, K., Tatarka, O., Štefko, T., Škulavík, T.: The study of metalworking fluids biodegradability by indirect measurement of bacterial inoculum respiration. J. Slovak Univ. Technol. 23(36), 65–75 (2015). https://doi.org/10.1515/rput-2015-0008
Rabenstein, A., Koch, T., Remesch, M., Brinksmeier, E., Kuever, J.: Microbial degradation of water miscible metal working fluids. Int. Biodeterior. Biodegrad. 63(8), 1023–1029 (2009). https://doi.org/10.1016/j.ibiod.2009.07.005
Yu, L., Han, M., He, F.: A review of treating oily wastewater. Arab. J. Chem. 10(2), S1913–S1922 (2017). https://doi.org/10.1016/j.arabjc.2013.07.020
Bensadok, K., Belkacem, M., Nezzal, G.: Treatment of cutting oil/water emulsion by coupling coagulation and dissolved air flotation. Desalination 206, 440–448 (2007)
Phenrat, T., Marhaba, T.F., Rachakornkij, M.: Leaching behaviors of arsenic from arsenic-iron hydroxide sludge during TCLP. J. Environ. Eng. 134(8), 671–682 (2008)
Karamalidis, A.K., Voudrias, E.A.: Application of stabilization/solidification technology on oil refinery sludge contaminated by heavy metals. J. Environ. Sci. Health. A Tox. Hazard. Subst. Environ. Eng. 39(4), 961–971 (2005)
Keeley, J., Jarvis, P., Judd, S.J.: Coagulant recovery from water treatment residuals: a review of applicable technologies. Crit. Rev. Environ. Sci. Technol. 44(24), 2675–2719 (2014). https://doi.org/10.1080/10643389.2013.829766
Xu, G.R., Yan, Z.C., Wang, Y.C., Wang, N.: Recycle of Alum recovered from water treatment sludge in chemically enhanced primary treatment. J. Hazard. Mater. 161, 663–669 (2009)
Keeley, J., Smith, A.D., Judd, S.J., Jarvis, P.: Acidified and ultrafiltered recovered coagulants from water treatment works sludge for removal of phosphorus from wastewater. Water Res. 88, 380–388 (2016). https://doi.org/10.1016/j.watres.2015.10.039
Keeley, J., Jarvis, P., Smith, A.D., Judd, S.J.: Coagulant recovery and reuse for drinking water treatment. Water Res. 88, 502–509 (2016)
Luthy, R., Selleck, R.E., Galloway, T.R.: Removal of emulsified oil with organic coagulants and dissolved air flotation. J. Water Pollut. Control Fed. 50(2), 331–346 (1978)
Louie, S.M., Tilton, R.D., Lowry, G.V.: Critical review: impacts of macromolecular coatings on critical physicochemical processes controlling environmental fate of nanomaterials. Environ. Sci. Nano 3, 283–310 (2016). https://doi.org/10.1039/c5en00104h
Phenrat, T., Saleh, N., Sirk, K., Kim, H.-J., Tilton, R.D., Lowry, G.V.: Stabilization of aqueous nanoscale zerovalent iron dispersions by anionic polyelectrolytes: adsorbed anionic polyelectrolyte layer properties and their effect on aggregation and sedimentation. J. Nanopart. Res. 10, 795–814 (2008)
Phenrat, T., Kumloet, I.: Electromagnetic induction of nanoscale zerovalent iron particles accelerates the degradation of chlorinated dense non-aqueous phase liquid: proof of concept. Water Res. 107, 19–28 (2016)
Phenrat, T., Fagerlund, F., Illangasekare, T., Lowry, G.V., Tilton, R.D.: Polymer-modified Fe0 nanoparticles target entrapped NAPL in two dimensional porous media: effect of particle concentration, NAPL saturation, and injection strategy. Environ. Sci. Technol. 45(14), 6102–6109 (2011)
Song, J.-E., Phenrat, T., Marinakos, S., Xiao, Y., Liu, J., Wiesner, M.R., Tilton, R.D., Lowry, G.V.: Hydrophobic interactions increase attachment of gum arabic and PVP coated Ag nanoparticles to hydrophobic surfaces. Environ. Sci. Technol. 45(14), 5988–5995 (2011)
Alam, M., Akarm, D., Sharmin, E., Zafar, F., Ahmad, S.: Vegetable oil based eco-friendly coating materials: a review article. Arab. J. Chem. 7(4), 469–479 (2014). https://doi.org/10.1016/j.arabjc.2013.12.023
Zhang, D., Wang, L., Qian, H., Li, X.: Superhydrophobic surfaces for corrosion protection: a review of recent progresses and future directions. J. Coat. Technol. Res. 13(1), 11–29 (2016). https://doi.org/10.1007/s11998-015-9744-6
Sadhukhan, J., Joshi, N., Shemfe, M., Lloyd, J.R.: Life cycle assessment of sustainable raw material acquisition for functional magnetite bionanoparticle production. J. Environ. Manage. 199, 116–125 (2017). https://doi.org/10.1016/j.jenvman.2017.05.048
Japan Environmental Management Association for Industry (JEMAI): Japanese carbon footprint program. Japan Environmental Management Association for Industry (JEMAI). http://www.cfp-japan.jp/english (2017). Accessed 1 May 2018
Energy Policy and Planning Office: CO2 emission per kWh. Ministry of Energy. http://www.eppo.go.th/index.php/en/en-energystatistics/co2-statistic?issearch=1&isc=1&ordering=order&category_id=858&xf_37=1 (2018). Accessed 12 Dec 2018
Intergovernmental Panel on Climate Change: 2006 IPCC Guidelines for National Greenhouse Gas Inventories Volume 5 Waste. In., p. 135. Hayama, Japan (2006)
Acknowledgements
This research was generously supported by the research and researcher for industries (RRi) programme, Thailand Research Fund (TRF) (Grant No. MSD58I0088). We gratefully appreciated the assistance of the staff at the Faculty of Engineering, Naresuan University and C.E.O. International Waste Company Limited who contributed to the success of this research. In addition, we appreciate some partial funding from the Office of Higher Education Commission (OHEC) and the S&T Postgraduate Education and Research Development Office (PERDO) for the additional financial support of the research program. Last but not least, PM appreciates the funding from the Graduate School of Naresuan University for her Master degree.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Special Issue: Waste Management & Valorization—ATHENS 2017 Conference.
Rights and permissions
About this article
Cite this article
Mooheng, P., Soratana, K. & Phenrat, T. Acid-Assisted Recycling of Iron Hydroxide Sludge as a Coagulant for Metalworking Fluid Wastewater Treatment. Waste Biomass Valor 10, 3635–3645 (2019). https://doi.org/10.1007/s12649-019-00696-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-019-00696-9