Abstract
In this study, the effect of moisture contents [2.69 wt% (bone-dry), 5 wt% and 10 wt%] on product yields and process conversion efficiency during fast pyrolysis of a pre-treated trommel fines feedstock was investigated at 500 °C. Experiments were carried out using a 300 g h−1 bubbling fluidised bed rig. Yields of organic liquids ranged from 15.2 to 19.6 wt% of feedstock, which decreased with increasing moisture content. Hence, the bone-dry feedstock gave the maximum yield and consequently the highest process conversion efficiency of 43%. Increased moisture content also led to increase formation of unidentified gas products, indicating increased conversion of organic liquids. Due to the high ash content of the feedstocks, about 52 wt% solid residues, containing around 82% ash was recovered in the char pot in each case. Hence, to maximize oil yields during fast pyrolysis, trommel fines would require extensive drying to remove the original 46 wt% moisture as well as reducing the ash content considerably. XRF analysis of the ash in the feedstock and solid residues showed that the main elements present included Ca, Si, Fe, Pb, K, Cl and Al. Apart from the presence of Pb (which may be from the glass contents of the feedstock), the solid residues could be used for land reclamation or co-incinerated at cement kilns for cement manufacture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Statement of Novelty
Efforts are needed to find solutions to the handling of trommel fines (FT), due to recent (2016) changes in UK Landfill Regulations, increasing landfill rates for FT with over 10% loss-on-ignition. To the best of our knowledge, there are not reports or publications yet on the fast pyrolysis of trommel fines. In the same vein, we do not know anyone who has carried out and reported any work on the influence of moisture content on fast pyrolysis of trommel fines. It is hoped that this work will encourage interests from waste management industry to look at using pyrolysis as an option for the treatment of this problem wastes—trommel fines.
Introduction
Trommel fines (< 25 mm particle size) from mechanical recycling of municipal solid waste (MSW) come as a complex heterogeneous waste, with high moisture contents and a near-equal high proportions of inorganic and organic materials. The inorganic materials include aggregates, glass, ceramics, stones, bones, concrete and sand. In general, the organic components of trommel fines include fibre, plastics, wood, food waste and textiles. Traditionally, this waste had been disposed in landfills; but the amendment of the Landfill Tax Regulation has led to a dramatic increase in the gate fee paid for trommel fines [1]. This has arisen from the recent introduction of the Loss on Ignition (LOI) test in April 2016, which stipulates that only wastes with LOI less than 10% will be considered eligible for the lower rate of tax. This measure has caused anxiety among waste processors, who have resorted to finding alternative solutions. In theory, LOI comprises of losses due to combustion of the organic materials in the wastes as well as the thermal decomposition of some inorganic materials such as carbonates. In reality, it would appear that the target of regulators is to eliminate the organic load in trommel fines, in order to improve the level of recycling of wastes. This regulation also has the potential to contribute to the EU Zero Waste Programme [2].
For such a heterogeneous waste stream, thermochemical processing including incineration, pyrolysis and gasification may be options due to their ability to convert both biodegradable and non-biodegradable organic materials. Compared to biological processes such as anaerobic digestion (AD), fermentation and compositing, thermochemical methods can handle the different synthetic organic polymers in trommel fines. In addition, these thermal recovery methods can achieve high conversion efficiencies in relatively short processing times compared to biological processes [3]. However, the thermochemical methods can be differentiated on this basis of their process conditions and products. Incineration leads to complete combustion of organic materials, producing heat energy for electricity and heating. Gasification converts carbonaceous materials into a syngas product, comprising mainly of carbon monoxide gas and hydrogen gas, which can be burnt in a gas engine for electricity and heat or processed via Fischer–Tropsch synthesis to make liquid hydrocarbons. Pyrolysis can be tuned to give a wider range of products including a solid char, liquid (oil) product and gas [4]. One of the major advantages of pyrolysis over the two other thermochemical technologies, is the variety of product and their potentially wider range of applications [5, 6]. Pyrolysis is also considered to be a cleaner energy recovery process than conventional MSW incineration due to lower amounts of nitrogen oxides (NOx) and sulphur oxides (SO2) produced, resulting from the inert atmosphere in the pyrolysis processes. In addition, pyrolysis can be a more direct source of chemicals than syngas. Fast pyrolysis, is particularly designed to produce enhanced yields of liquid product, which tend to have more robust handling incentive than syngas during downstream processing.
All the thermochemical technologies discussed above are only effective in handling wastes streams with certain calorific values and moisture contents. In general, thermochemical technologies require feedstock with less than 15 wt% moisture. Therefore, waste streams with high moisture contents would require a drying stage; however the energy for this process could be derived from the waste heat sources from thermochemical processing. For instance, the gas products and chars from pyrolysis and gasification could be burnt to provide energy for feedstock drying. Incineration flue gases could also be used. The research challenge is therefore about finding the optimum moisture contents of specific feedstock for a given thermochemical processing technology. Trommel fines have relatively high moisture contents of over 40 wt% [7] and would require drying to make them suitable for thermal recovery.
There are a large number of studies on the effect of moisture on the pyrolysis of different mixed waste streams including MSW [8,9,10], sewage sludge [11, 12], organic fines from compost [12] and refuse derived fuel (RDF) [13, 14]. Literature shows that organic wastes and other biosolids can be effectively pyrolyzed at moisture contents of below 10 wt% [11, 12]. However, there is no information in literature on the effect of moisture content on fast pyrolysis of trommel fines.
In this work, the effect of feedstock moisture content on the fast pyrolysis of trommel fines has been investigated at a temperature of 500 °C, using a 300 g h−1 bench-scale bubbling fluidised-bed reactor. Earlier research work showed that a temperature around 500 °C [7] gave the maximum yield of liquid products from trommel fines, which agreed with the optimum temperature reported for fast pyrolysis [15]. In these experiments, the moisture content of the trommel fines was nominally varied from bone-dry to 10 wt%. Due to the heterogeneous nature of the feedstock, pyrolysis experiments were carried out in triplicates, followed by detailed analysis of the reaction products, using a number of analytical equipment.
Materials and Methods
Trommel Fines Feedstock
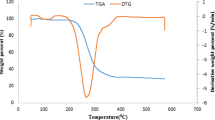
Trommel fines feedstock was supplied by Biffa Ltd, a UK commercial waste management company based in Leicester. The samples originated from the recycling of household wastes, following a mechanical processing step to remove majority of the metals, glass, and plastics material. In a typical process, the remaining materials are crushed or shredded and passed from trommel screens for size classification. The ‘unders’ which represent particle sizes less than 25 mm are then classed as trommel fines [16, 17]. At Biffa Ltd, the ‘unders’ are further passed through a 10 mm screen to reduce the heterogeneity of the sample, which was used in this present study. Further pre-treatment of the ‘as-received’ feedstock was carried out to ensure that the sample met the particle size requirements of the bubbling fluidised bed reactor used in this study according to the procedure described in an earlier paper [7]. Briefly, the feedstock was bone-dried to 2.69 wt% moisture content in an oven at 60 °C for 24 h, sieved and physically separated by manual removal of large visible stones, glasses, concrete and bones. The remaining material was then ground using a Retsch Ltd., Germany, Heavy-Duty Cutting Mill, Knife Mill Type SM2000. The ground sample was again sieved to obtain a final prepared feedstock, with particle size range of 0.5–2 mm. This fraction accounted for approximately 70 wt% and more than 80% of the energy content (calorific value) of the original feedstock [7]. Table 1 summarises the main characteristics of this fraction. Thermogravimetric analysis (TGA) of the feedstock is shown in Fig. 1 [7]. In order to investigate the effect of moisture content on the fast pyrolysis of the feedstock, two additional samples with nominal moisture contents of 5 wt% and 10 wt% were prepared, respectively. In the preparation, the bone-dry feedstock (2.69 wt%, moisture) was wetted with the required amount of water and left in a cool, dark place for 72 h before use. Just before pyrolysis, the actual moisture contents of the feedstocks were determined again and reported in Table 2.
Fast Pyrolysis Rig
Fast pyrolysis experiments were carried out in an existing 300 g h−1 bench-scale bubbling fluidized bed reactor unit, which is shown in Fig. 2. In a brief description, it consists mainly of a dome-bottomed cylindrical hopper-feeder system, a reaction (fast pyrolysis) chamber and a product collection system. The feeder uses a dual screw gravimetric feeding system with variable speed motor for feeding, attached to a fast screw. The pyrolysis chamber is a tubular steel reactor having 41 mm internal diameter and a height of 320 mm. About 150 g of calcined quartz sand of particle size range of 500–600 µm was used as bed material, fluidized by a stream of nitrogen gas (99.9% purity) at an optimized flow rate of 6 L min−1 for heat transfer [18, 19]. The product collection system consisted of a water-cooled condenser and two dry ice/acetone-cooled condensers, followed by a cotton wool filter.
Bench scale 300 g h−1 fluidized bed fast pyrolysis rig set-up. Key: 1—feed hopper with twin screw feeder, 2—fast screw, 3—nitrogen preheater, 4—fluidised bed reactor, 5—cyclone, 6—charpot, 7—metal transition pipe, 8—glass transition pipe, 9—water cooled condenser, 10—dry ice acetone condenser, 11—ruber tansition pipe, 12—cotton wool filter, 13—gas meter, 14—micro-gc, 15—gas vent
Fast Pyrolysis Experiments
Each experiment was initiated by starting the inert nitrogen flow for 10 min to remove the air within the system, followed by preheating the reactor using an electrical furnace. The fast pyrolysis temperature was maintained at approximately 500 °C, by setting the heater temperature at 50 °C above the reactor temperature throughout the duration of each run. Once the temperatures of the fluidising medium reached a steady state, the prepared trommel fines feedstock was continuously fed into the reactor at the middle of the fluidized bed by nitrogen entrained flow via the air-cooled feeding tube (Fig. 2). The feeding rate was set at an average of 170 g h−1 and each experiment lasted for 1 h. After pyrolysis in the reactor, the pyrolysis vapours including aerosols, water and non-condensable gases, were carried by the nitrogen gas stream through a cyclone to remove entrained solids, which were collected in the char pot. The ensuing vapour stream then passed through the system of condensers, where the condensable components were collected as liquids. Two sets of liquid products were collected each time; the first from the water-cooled condenser and the second from the two dry ice/acetone condensers, respectively. Any escaping vapour was then mostly captured in the cotton wool filter, before the non-condensable gases directed into a gas meter, where the volume of the exit gas was recorded. A portion of the exit gases was taken by an automatic sampling system into an automated online gas chromatograph (GC) for gas composition analysis, while the remainder was vented through an installed ventilation system.
Characterization of Fast Pyrolysis Products
For each moisture content variable, fast pyrolysis experiment was conducted five times and the resulting mass balances were compared to select the three tests with the closest yields of pyrolysis products, i.e. within standard deviations of < 5% of each other. These were then used to compute the average product mass balance closures reported in this work [18, 19]. Detailed characterisations of the gas products and solid residues obtained from the three selected experiments were carried out in triplicates and average results reported with the standard deviations. However, GC/MS analyses were carried out only the liquid products from the experiment with the highest mass balance closure for each set of moisture content experiments. Similarly, XRF elemental analyses (in triplicates) were carried out only on the bone-dried trommel fines feedstock and its solid residue from the test with the highest mass balance closure.
Analysis of Gas Products
During a pyrolysis run, the non-condensable gases were sampled every 3 min into a Micro-gas chromatograph with a thermal conductivity detector (TCD) from Varian Chromatography System Inc. The gas components were separated on two columns (Varian CP-5A Molsieve and CP-PortaPLOT) prior to detection. Quantitation was achieved by external standard method by calibrating the detector response using a standard gas mix containing hydrogen, oxygen, carbon monoxide, carbon dioxide and C1–C4 hydrocarbon gases at 3 vol% concentrations in nitrogen. The mass yields of the gas components calculated using the general gas equation, based on the gas volume composition obtained from GC analysis, total gas volume and the exit gas temperature and recorded pressure.
Characterization of Liquid Products
Each liquid product was found to be composed of both an aqueous and an organic fraction. The first-condensate liquid products also contained some solids. Further analysis of the organic fraction was carried out using GC–MS to determine the main organic compounds present. Elemental analysis and heating values of the liquid products were also determined.
Volumetric Karl-Fischer (KF) titration was used to determine the water content of all the fast pyrolysis primary and secondary condensates [20]. The primary and secondary condensates were dissolved in a known amount of acetone (1:6) prior to analyses. The dilution with acetone served two purposes; (a) to form a miscible solution of pyrolysis oil and water in acetone; (b) to dilute the samples and adjust the pH of the solution and water contents to the optimum ranges for Karl-Fischer titration of between 5 and 7 and < 50 wt%, respectively [21, 22]. The water content obtained automatically from the KF titrator. A blank determination using the same amount of acetone was used to correct the final water contents [19].
Solids content in the primary condensates were determined using the vacuum filtration technique suggested by Oasmaa and Peacocke [23]. The sample was filtered through a pre-dried and pre-weighed Whatman No. 2 qualitative filter paper with mean pore size of 8 µm. The filter paper with the retentate was then washed with excess amount of acetone until the filtrate became clear. The filter paper with the residue was air-dried for approximately 15 min and placed in an oven at 105 °C for 1 h, cooled in a desiccator and weighed. The drying, cooling and weighing steps were repeated until a constant weight was obtained.
CHNS analyses of the primary and secondary condensates were carried out using a CE-440 Carlo Erba Elemental Analyzer with ± 0.3% absolute accuracy [19]. In the procedure, the liquid samples were mixed with a known amount of acetone (1:6) to obtain the carbon, hydrogen and nitrogen (CHNS) contents. The CHNS composition of the organic fraction of the liquid product was calculated by subtracting the carbon, hydrogen and oxygen contents of the product water and the added acetone. Hence, the CHN data were obtained on dry, solvent-free basis. Oxygen content was determined by difference, using the percentage composition of CHN [24].
Compositional analyses of organic fractions of the liquid products (bio-oil) were performed using a PerkinElmer Clarus 680 GC–MS system [19]. The samples diluted with acetone were used for GC–MS analysis after filtration through a 0.2 µm pore size Sartorius filter. A sample volume of 1 µL was injected into the GC column via an injection port maintained at 300 °C, with 1:50 split ratio. The GC oven programme was initially held at 50 °C for 2 min, then ramped at 5 °C min−1 to 275 °C, and finally held at 275 °C for 3 min, giving a 50-min analysis time. Helium was used as carrier gas at a constant flow rate of 15 mL min−1. A column splitter was used to enable simultaneous detection of compounds separated on the columns by MS and FID detectors. Mass spectra were obtained using 70 eV ionisation energy in the molecular mass range of m/z = 35–300, with a scan time of 0.35 s. Assignments of the main peaks were made from mass spectral detection (NIST05 MS library). The detector temperature was 250 °C.
Characterization of Solid Residues
Solid residues obtained from these experiments were distributed into the bed material, char pot and liquid product obtained as primary condensates. However, in fast pyrolysis, the solid residue of interest is usually those found in the char pot and in this study, they represent over 90 wt% of the solid residues. Hence, only the characterization of the solid residue from the char pot was carried out and reported in this present study.
The ash content of solid residues obtained from the char pot was determined according to the ASTM D1762-84 method [25]. Approximately 4–5 g of solid residue was weighed into each pre-calcined and pre-weighed crucible set (crucible and lid) and placed in a furnace. The samples were ashed 750 °C for 6 h, followed by cooling in a desiccator to room temperature. After weighing, the ash content was then obtained by the difference in mass between the crucible + ash and the empty crucible. The average of five samples was taken to further reduce the deviation.
The solid residues were further analysed for CHNS composition using the same elemental analyzers used for the liquid products as described in “Characterization of Liquid Products” section. In addition, both the ash content bone-dry feedstock (2.69 wt%) and the solid residue obtained from its fast pyrolysis were analysed for other elements. The simple scan analysis was carried out using a Bruker S8 Tiger X-ray Fluorescence (XRF) spectrometer (University of Birmingham), which is capable of quantifying elements from sodium to uranium. Firstly, the samples were separately pulverised with mortar and pestle to make fine powders and weighed into a sample cup with Mylar window. The volume of each sample was approximately 1 cm3. The results of the elements with concentrations ≥ 0.02 wt% are reported in this work.
Determination of Heating Values of Pyrolysis Products
In this study, the calorific value of the liquid product was carried out only on the primary condensates, which had more organic product and less water content than the secondary condensates (please see Fig. 3 below). Approximately 1 g of the solvent-free primary condensate was burnt completely in an excess oxygen environment in a steel bomb calorimeter (Parr 6100 calorimeter) at constant volume. The same procedure was repeated for the solid products based on the ASTM D2015 Method [26]. The calorific values of gas product were estimated from the volume percentage of gas component and their calorific values (as higher heating values, HHV), according to Eq. 1.
where i…n is the each combustible component in the gas product, x is mass fraction of combustible component in gas product, CV is the alorific value of combustible gas component in MJ kg−1.
Mass Balance Calculation
Mass balances (wt% on dry feed basis) were calculated by comparing the mass yields of final fast pyrolysis products (liquids, solid residues and non-condensable gases) with the mass of trommel fines feedstock processed. All metal, glassware items and transition pipes used in the bench-scale pyrolysis unit were weighed before and after each run, to calculate the yields of pyrolysis products. By difference in weight of trommel fines added to hopper before and trommel fines left in hopper after run, the amount of trommel fines fed can be calculated. The solid yield is a combination of the solid residue collected in the char pot (6), reactor (4) and metal transition pipe (7), and the solid fines or solids present in the tar derived liquid. The liquid product was fractionated into six fractions, which are (1) water condenser tar derived oil, (2) transition pipe 1, (3) dry ice acetone condenser 1, (4) transition pipe 2, (5) dry ice acetone condenser 2, and (6) cotton wool filter. 1 was taken as the primary condensate, while 2–6 was taken as the secondary condensate. In addition, the permanent gas yields were calculated based on the gas composition obtained from GC analysis, total gas volume and the exit gas temperature and the recorded pressure. The pyrolysis temperature was the average value of temperature data recorded by two K-type thermocouples at the fluidised-bed every 5 min throughout the run.
Results and Discussions
Effect of Feedstock Moisture Content on Overall Mass Balance and Product Yields
To calculate the yields of the different products obtained from the fast pyrolysis of the different trommel fines feedstock, the following equations were used;
Table 2 shows the process parameters, product yields, product distributions and mass balance closure results obtained from the fast pyrolysis of PT trommel fines at different moisture contents. The primary aim of these sets of experiments was to investigate the effect of moisture content on the fast pyrolysis of trommel fines at three moisture content levels; 2.69 wt% (bone dry), 5 wt% and 10 wt%. The product yields were determined on a dry basis, by considering the moisture content of each feedstock. Therefore, the water yields presented is the water generated (reaction water) by the fast pyrolysis reaction excluding the water input in the feed.
The overall mass balance closure for all investigated trommel fines moisture content where between 88 and 98 wt% on dry basis. The solid residues remained fairly identical from the pyrolysis of the three feedstock, indicating that the reduction in liquid yields would translate to increase in gas products. However, the mass balance closures decreased with increasing water content and this could be linked to the decrease in the total liquid yields as shown in Table 2. During the analysis of gas products, a number of unidentified components were detected by the micro-GC but could not be quantified. Hence, not accounting for these gases was likely the source of the lower mass balance obtained with increasing moisture contents in the feedstock.
In comparison, it is clear from Table 2 that the yield of primary condensate decreased with increasing moisture content and the secondary condensate organic yield increased slightly. This was not unexpected as a large majority of the aqueous phase was found in the colder dry-ice condensers. In addition, the reaction water yields were found to decrease with increasing moisture content, indicating that high water content may promote reforming reactions, leading to the reduction in reaction water and increase in gas yields. Overall, the liquid yields were much smaller than those reported in literature from the fast pyrolysis of RDF and MSW and its components due to the high ash content of trommel fines [24, 25, 27, 28].
The measured gas yield in this work increased only slightly with increasing moisture content, but with some noticeable changes in composition. According to Table 2, the gas products contain mainly of carbon dioxide and propylene with small quantities of C1–C4 hydrocarbon gases, which affected the HHV of the gases but not in a linear trend. Interestingly, there was a number of gaseous compounds detected by the GC but which could not be identified and their peak sizes increased with increased feed moisture contents. Hence, it would be acceptable to attribute this to the conversion of the fast pyrolysis vapour into gas, as evidenced by the reduction in yields of organic liquids. Increased moisture content has been reported to influence gas yields and compositions during high-temperature pyrolysis [29]. For instance, Liu et al. [29] reported that bound moisture was more effective than free moisture (by soaking) in producing hydrogen via steam gasification during high-temperature (600–1000 °C) pyrolysis of sewage sludge, leading to reduction of organic liquid yields. Although, a similar reduction in organic liquid yields was observed in this work, the pyrolysis temperature used here was not high enough to promote steam-reforming reactions; rather formation of other gaseous species (e.g. formaldehyde, ammonia, HCN) was more plausible [30, 31].
Large amounts of solid residues were obtained in the char pot after the fast pyrolysis runs with different moisture content in the feedstock. Approximately 52 wt% dry basis of the solid residues were recovered each time. This was not surprisingly as the feedstock contained very high ash content. Operating the fast pyrolysis as such high content of ash could not be sustainable and efficient. Therefore, further research will be required to reduce the ash content of the feedstock prior to or during the pyrolysis process.
Effect of Feedstock Moisture Content on Liquid Product Characteristics
The water in the liquid products originated from the moisture content of the feedstock and dehydration reactions occurring during the pyrolysis process [32]. The reaction water was obtained by deducting the moisture content of the feedstocks (reporting on dry basis); hence the water reported in Table 3 refers to reaction water. In this work, the results showed that the total reaction water in the liquid products (primary and secondary condensate) from these investigations were in the range of 33.5–39.9%, which were rather high when compared to literature values of 15–35 wt% for biomass-derived bio-oil [15, 33]. Figure 3 shows the photographs of the primary and secondary condensates produced from fast pyrolysis of PT trommel fines with different moisture content. The presence of polyesters and polyamides from textile wastes may be responsible for the high reaction water yields, arising from their degradation. Sarker et al. [34] reported that large quantities of water was produced from the pyrolysis of waste polyethylene terephthalate (PET)—a polyester-based plastic. The pH values of the liquid products ranged from 2.18 to 3.88, as expected from pyrolysis oils derived from biogenic-rich feedstocks [16, 35, 36]. In all cases, the primary condensates were more acidic (lower pH) than the secondary condensates, possibly due the presence of large amounts of water which diluted the latter liquid products.
Table 3 shows that the solid content in the primary condensates increased with increasing moisture content due to increased gas flow (additional steam) and solid entrainment. They fluctuated in the range of 3–29 wt% (dry basis) which was a higher range than of those of wood-derived bio-oils [36]. Solids in the liquid products were composed of entrained char and ash fines leaving the cyclone. In addition, they also contained inorganic materials (sand and glass fines) from the ash. These solid fines could have escaped the cyclone due to their particulate sizes (less than about 10 µm in diameter) and the high gas stream velocity in the cyclone.
The CHNS elemental compositions of both the primary and secondary condensate liquids at different moisture content are also listed in Table 3. The percentages of carbon for both primary and secondary condensate decreased with increasing moisture content. With regards to nitrogen contents, increase in moisture content led to a slight increase in nitrogen content for both the primary and secondary condensate and are higher than typical wood-derived bio-oil [33]. The higher nitrogen content may be due to the high nitrogen content in the original trommel fines feedstock, e.g. from the polyamide-type textiles. The presence of nitrogen compounds can be a drawback when burning the liquids because of the high potential for NOx emissions.
The higher heating values (HHV) of the primary and secondary condensate liquids are also shown in Table 3. The HHV decreased with increasing moisture contents for both the primary and secondary condensates. Hence, MCR-01 liquids gave the highest HHV for the primary condensate (32.4 MJ kg−1) and secondary condensate (17.4 MJ kg−1), respectively. This was due to the increased presence of water, which diluted the oils. The HHV of the primary condensate was found to be slightly higher when compared to HHV of bio-oils reported literature [36, 37], which would be due to the presence of plastics in the trommel fines feedstock, which are more energy dense than biomass.
Effect of Feedstock Moisture Content on the Composition of Organic Liquid Products
The organic liquid products obtained from these experiments were composed of different classes of compounds [38]. The chromatograms of primary and secondary condensate liquids from MCR-01 test and the retention times of the major compounds identified by GC/MS are shown in Supplementary Information (Fig. SI1). The main components were furans, aromatic hydrocarbons and phenols with various carbon numbers. The main phenolic compounds included phenol, methoxy phenols and dimethoxy phenols, which could be obtained from the pyrolysis of the lignin constituent of the feedstocks (Fig. 4). Increasing the moisture content increased the intensity of the phenol groups in the primary condensate but overall, the intensity of peaks in the chromatogram from the secondary condensates (Fig. 4) seemed to also increase with increasing moisture content. Essentially, the increase in moisture content led to further cracking of the compounds to produce lighter fractions. The secondary condensate liquid fraction collected from the dry ice condensers showed higher presence of nitrogen containing organic compounds (Fig. 4a). These compounds are in the form of amines, pyridines and their derivatives. This finding is in line with the result of the elemental analysis of the secondary condensate liquid which showed an increase in nitrogen content with increasing moisture content (Table 3).
Effect of Feedstock Moisture Content on Solid Product Characteristics
Ash content, elemental composition, and calorific values of the solid products obtained from the experiments are tabulated in Table 4. Table 2 above showed that more than 52 wt% of the feedstock was recovered as solid residues at the end of the pyrolysis process and moisture content of the feedstock had no effect amount of solids obtained. It was obvious that ash were the main components of the solids produced (Table 4). The ash content ranged for 82–87 wt% (dry basis).
The CHNS compositions and heating value of solid products are also listed in Table 4. The moisture content of the feedstock had no major effect on the percentage of carbon in the solid residues, so that the heating values of the solid products were similar (4–5.54 MJ kg−1). The low calorific values of the solid residues might not make them viable as a source of process heat, but the requirement on LOI of solid materials destined for landfill would require them to be burnt. In addition, presence of other elements in the ash fractions of bone-dry feedstock and the solid residues obtained from it are shown in Table 5. The outlines of the XRF analysis are given in the Supplementary Information (Fig. SI2). Results show that the ash fractions contained a variety of elements, however the most prominent elements were calcium, silicon, lead, potassium, chlorine, aluminium and iron. Of particular concern was the presence of lead in the feedstock (3.51 wt%) and char-pot solid residue (0.39 wt%), which are higher than the 0.09 wt% maximum allowable limit of the element in waste-derived materials for cement production [39]. The presence of lead in the feedstock could be from the glass contents, so that the reduction in the lead content of the char-port residue may indicate the retention of larger glass particles in the bed material (Fig. SI3 in Supplementary Information).
Effect of Feedstock Moisture Content on Trommel Fine Conversion Efficiency
To determine whether trommel fines samples can be used for energy recovery via fast pyrolysis, process conversion efficiency (ƞ) was calculated based on the ratio of the energy content in the product to that in the feedstock. This was obtained by using the higher heating values (HHV) and the mass of each component (m) as follows:
Figure 5 shows the effect of feedstock moisture content on the efficiency of fast pyrolysis process conversion of trommel fines feedstock to non-solid products. The fast pyrolysis process conversion efficiency range between 24–43%, with the highest energy yields obtained from the feedstock with the lowest (2.69 wt%) moisture content. Although, increasing moisture contents led to the formation of gas components that could not be accounted, it is still clear from Fig. 5 that, the trend energy yields corresponded to the decrease in the yields of organic liquid product. In fast pyrolysis, the liquid product is regarded as the main product. Therefore, to achieve high organic liquid yields and high energy yield, trommel fines would need to by pyrolyze at very low moisture contents, which would require an intensive drying step.
Conclusions
The influence of feed moisture content on the fast pyrolysis product yields from a pre-treated trommel fines feedstock has been experimentally investigated. Increasing the moisture contents of the feedstock led to a decrease in the yields of organic liquid products, so that the highest yield of 19.6 wt% (dry basis) was obtained from the bone-dry feedstock. However, increasing the trommel fines moisture content had no effect on recovered solid residues because of the high ash content of the feedstock (36.2 wt% dry basis). In line with decreased liquid yields, the energy yield from this work, decreased with increasing moisture content of the feedstock used.
Clearly, the ash content of the feedstock was a massive problem that affected the efficiency of fast pyrolysis of trommel fines in this work. XRF elemental analysis show that the feedstock contained 3.51 wt% of lead, which reduced to 0.39 wt% in the char-pot solid residues. This could indicate that the lead content came from the glass particles in the trommel fines, so that the lower lead concentration in the char-pot residue due to the retention of glass in the bed material. Apart from the presence of lead, the char-pot solid residue could be used for land reclamation or co-incinerated in cement kilns from cement manufacture. Therefore, ash removal (e.g. by an aqueous washing and sedimentation) and moisture reduction (by drying) are required to obtain a trommel fines feedstock that would give better conversion efficiency during fast pyrolysis process. These will require further research.
References
HMRC: Loss on ignition testing regime. Landfill tax briefing (2016). Available online at: https://www.entrust.org.uk/assets/uploads/documents/LFT_Tax_Briefing_January_2016.pdf. Accessed 01 August 2016
EU Commission: Towards a circular economy: a zero waste programme for Europe. Brussels, Belgium (2014)
McKendry, P.: Energy production from biomass (Part 2): conversion technologies. Biores. Technol. 8, 37–54 (2002)
Saffarzadeh, A., Shimaoka, T., Motomura, Y., Watanabe, K.: Chemical and mineralogical evaluation of slag products derived from the pyrolysis/melting treatment of MSW. Waste Manage. 26, 1443–1452 (2006)
Schaefer, W.D.: Disposing of solid wastes by pyrolysis. Environ. Sci. Technol. 9, 98–98 (1975)
Malkow, T.: Novel and innovative pyrolysis and gasification technologies for energy efficient and environmentally sound MSW disposal. Waste Manage. 24, 53–79 (2004)
Eke, J., Onwudili, J.A., Bridgwater, A.V.: Physical pre-treatment of biogenic-rich trommel fines for fast pyrolysis. Waste Manage. 70, 81–90 (2017)
Ates, F., Miskolczi, N., Borsodi, N.: Comparison of real waste (MSW and MPW) pyrolysis in batch reactor over different catalysts. Part I: product yields, gas and pyrolysis oil properties. Biores. Technol. 133, 443–454 (2013)
Islam, M.N., Beg, M.R.A., Islam, M.R.: Pyrolytic oil from fixed bed pyrolysis of municipal solid waste and its characterization. Renew. Energy 30, 413–420 (2005)
Miskolczi, N., Ates, F., Borsodi, N.: Comparison of real waste (MSW and MPW) pyrolysis in batch reactor over different catalysts. Part II: contaminants, char and pyrolysis oil properties. Biores. Technol. 144, 370–379 (2013)
Trinh, T.H., Jensen, P.A., Dam-Johansen, K., Knudsen, N.O., Sørensen, H.R.: Influence of the pyrolysis temperature on sewage sludge product distribution, bio-oil, and char properties. Energy Fuels 27, 1419–1427 (2013)
Agar, D.A., Kwapinska, M., Leahy, J.J.: Pyrolysis of wastewater sludge and composted organic fines from municipal solid waste: laboratory reactor characterisation and product distribution. Environ. Sci. Poll. Res. (2018) https://doi.org/10.1007/s11356-018-1463-y
Cozzani, V., Nicolella, C., Petarca, L., Rovatti, M., Tognotti, L.: A fundamental study on conventional pyrolysis of a refuse-derived fuel. Ind. Eng. Chem. Res. 34, 2006–2020 (1995)
Bosmans, A., Vanderreydt, I., Geysen, D., Helsen, L.: The crucial role of waste-to-energy technologies in enhanced landfill mining: a technology review. J. Cleaner Prod. 55, 10–23 (2013)
Bridgwater, A.V.: Biomass fast pyrolysis process for biomass. Therm. Sci. 8, 21–49 (2004)
Fitzgerald, G.C.: Pre-processing and treatment of municipal solid waste (MSW) prior to incineration. In: Klinghoffer, N.B., Castaldi, M.J. (eds). Waste to Energy Conversion Technology, pp. 55–71. Woodhead Publishing Limited, Cambridge (2013)
Pitchell, J.: Municipal solid waste processing: material recovery facilities. In: Waste Management Practices: Municipal, Hazardous and Industrial, 2nd edn., pp. 165–196. CRC Press, London (2014)
Kalgo, S.A.: The development and optimisation of a fast pyrolysis process for bio-oil production. PhD Thesis, submitted to Aston University, Birmingham (2011)
Banks, S.W., Nowakowski, D.J., Bridgwater, A.V.: Fast pyrolysis processing of surfactant washed Miscanthus fuel processing technology. Fuel Proc. Technol. 128, 94–103 (2014)
ASTM D240: Standard test method for heat of combustion of liquid hydrocarbon fuels by bomb calorimeter. ASTM International, West Conshohocken (2009)
ASTM E203: Standard test method for water using volumetric Karl Fischer titration. ASTM International, West Conshohocken (2001)
Mohammed, I.Y., Kazi, F.K., Abakr, Y.A., Yusuf, S., Razzaque, M.A.: Novel method for the determination of water content and higher heating value of pyrolysis oil. BioRes 10(2), 2681–2690 (2015)
Oasmaa, A., Peacocke, C.: A guide to physical property characterisation of biomass-derived fast pyrolysis liquids. Technical Research Centre of Finland, Espoo (2001)
Aiken, A.C., DeCarlo, P.F., Jimenez, J.L.: Elemental analysis of organic species with electron ionization high-resolution mass spectrometry. Anal. Chem. 79(21), 8350–8358 (2007)
ASTM D1762-84: Standard test method for chemical analysis of wood charcoal (2013)
ASTM D2015: Standard test method for gross calorific value of coal and coke by the adiabatic bomb calorimeter. American Society for Testing and Materials International, USA (2015)
Zhou, C., Yang, W., Blasiak, W.: Characteristics of waste printing paper and cardboard in a reactor pyrolyzed by preheated agents. Fuel Process. Technol. 116, 63–71 (2013)
Chen, S., Meng, A., Long, Y., Zhou, H., Li, Q., Zhang, Y.: TGA pyrolysis and gasification of combustible municipal solid waste. J. Energy Inst. 88(3), 332–343 (2015)
Liu, H., Zhang, Q., Hu, H., Li, A., Yao, H.: Influence of residual moisture on deep dewatered sludge pyrolysis. Int. J. Hydrogen Energy 39, 1253–1261 (2014)
Zhang, X., Li, J., Yang, W., Blasiak, W.: Formation mechanism of levoglucosan and formaldehyde during cellulose pyrolysis. Energy Fuels 25(8), 3739–3746 (2011)
Meesuk, S., Cao, J.-P., Sato, K., Hoshino, A., Utsumi, K., Takarada, T.: Nitrogen conversion of pig compost during pyrolysis. J. Chem. Eng. Jpn. 46(8), 556–561 (2013)
Balat, M.: Mechanism of thermochemical biomass conversion processes. Part 1: reactions of pyrolysis. Energy Sources 30, 620–635 (2008)
Elliott, D.: Water, alkali and char in flash pyrolysis oils. Biomass Bioenerg. 7, 179–185 (1994)
Sarker, M., Kabir, A., Rashid, M.M., Molla, M., Mohammad, D.: Waste polyethylene terephthalate (PETE-1) conversion into liquid fuel. J. Fund. Renew. Energy Appl. 1, 1–5 (2011)
Islam, M.S., Miah, M.Y., Ismail, M., Jamal, M.S., Banik, S.K., Saha, M.: Production of bio-oil from municipal solid waste by pyrolysis. Bangladesh J. Sci. Ind. Res. 45(2), 91–94 (2010)
Czernik, S., Bridgwater, A.V.: Overview of applications of biomass fast pyrolysis oil. Energy Fuels 18, 590–598 (2004)
Velghe, I., Carleer, R., Yperman, J., Schreurs, S.: Study of the pyrolysis of municipal solid waste for the production of valuable products. J. Anal. Appl. Pyrol. 92(2), 366–375 (2011)
Mohan, D., Pittman, C.U., Steele, P.H.: Pyrolysis of wood/biomass for bio-oil: a critical review. Energy Fuels 20, 848–889 (2006)
Achternboach, M., Brautigan, K.-R., Hartlied, N., Kupsch, C., Richers, U., Stemmermann, P.: Heavy metals in cement and concrete resulting from the co-incineration of wastes in cement kins with regard to the legitimacy of waste utilization. Project Report, Forschungszentrum Kalrlsruhe, GmBH: (2003). Available online at: http://www.itas.kit.edu/pub/v/2003/acua03b.pdf. Accessed 28 October 2018
Acknowledgements
The authors are grateful to the European Bioenergy Research Institute (EBRI) at Aston University for their continuous support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Eke, J., Onwudili, J.A. & Bridgwater, A.V. Influence of Moisture Contents on the Fast Pyrolysis of Trommel Fines in a Bubbling Fluidized Bed Reactor. Waste Biomass Valor 11, 3711–3722 (2020). https://doi.org/10.1007/s12649-018-00560-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-018-00560-2