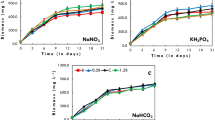
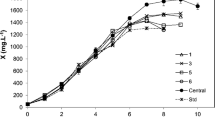
Abstract
In this study, heterotrophic growth conditions for Micractinium sp. ME05 cells were investigated for the improvement of biomass production. Plackett Burman (PB) method was used to screen process variables, namely, pH, carbon source and yeast extract concentrations, temperature and inoculum ratio, that affect the biomass production. The Box-Behnken (BB) design of response surface methodology (RSM) was applied to evaluate the interaction effect of process variables and to optimize them. The biomass obtained from PB design was 1.07 g/L and pH, temperature and carbon source concentration were selected based on their positive effect on biomass production. Applying response optimizer tool of RSM, the highest biomass obtained was 2.08 g/L. The results revealed that a 1.9-fold increase in biomass concentration was achieved by manipulating cultivation conditions which would be valuable for large scale cost efficient industrial applications of biomass production.
Graphical Abstract



Similar content being viewed by others
References
Brennan, L., Owende, P.: Biofuels from microalgae-A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 14, 557–577 (2010). doi:10.1016/j.rser.2009.10.009
Suali, E., Sarbatly, R.: Conversion of microalgae to biofuel., Renew. Sustain. Energy Rev. 16, 4316–4342 (2012). doi:10.1016/j.rser.2012.03.047
Perez-Garcia, O., Escalante, F.M.E., de-Bashan, L.E., Bashan, Y.: Heterotrophic cultures of microalgae: Metabolism and potential products. Water Res. 45, 11–36 (2011). doi:10.1016/j.watres.2010.08.037
Rattanapoltee, P., Kaewkannetra, P.: Utilization of agricultural residues of pineapple peels and sugarcane bagasse as cost-saving raw materials in Scenedesmus acutus for lipid accumulation and biodiesel production. Appl. Biochem. Biotechnol. 173, 1495–1510 (2014). doi:10.1007/s12010-014-0949-4
Agwa, O.K., Ibe S.N., Abu, G.O.: Heterotrophic cultivation of Chlorella sp. using different waste extracts. Int. J. Biochem. Biotechnol. 2, 289–297 (2013)
Sonmez, C., Elcin, E., Akın, D., Avni, H., Yucel, M.: Bioresource technology evaluation of novel thermo-resistant Micractinium and Scenedesmus sp. for efficient biomass and lipid production under different temperature and nutrient regimes. Bioresour. Technol. 211, 422–428 (2016). doi:10.1016/j.biortech.2016.03.125
Chojnacka, K., Marquez-Rocha, F.-J.: Kinetic and Stoichiometric Relationships of the Energy and Carbon Metabolism in the Culture of Microalgae. Biotechnology 3, 21–34 (2004). doi:10.3923/biotech.2004.21.34
Huang, G., Chen, F., Wei, D., Zhang, X., Chen, G.: Biodiesel production by microalgal biotechnology. Appl. Energy. 87, 38–46 (2010). doi:10.1016/j.apenergy.2009.06.016
Chen, C.-Y., Yeh, K.-L., Aisyah, R., Lee, D.-J., Chang, J.-S.: Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: A critical review. Bioresour. Technol. 102, 71–81 (2011). doi:10.1016/j.biortech.2010.06.159
Wen, Z.-Y., Chen, F.: Heterotrophic production of eicosapentaenoic acid by microalgae. Biotechnol. Adv. 21, 273–294 (2003). doi:10.1016/S0734-9750(03)00051-X
Chen, F.: High cell density culture of microalgae in heterotrophic growth. Trends Biotechnol. 14, 421–426 (1996). doi:10.1016/0167-7799(96)10060-3
Liu, J., Sun, Z., Zhong, Y., Gerken, H., Huang, J., Chen, F.: Utilization of cane molasses towards cost-saving astaxanthin production by a Chlorella zofingiensis mutant. J. Appl. Phycol. 25, 1447–1456 (2013). doi:10.1007/s10811-013-9974-x
Miao, X., Wu, Q.: Biodiesel production from heterotrophic microalgal oil. Bioresour. Technol. 97, 841–846 (2006). doi:10.1016/j.biortech.2005.04.008
Najafpour, G.D., Poi Shan, C.: Enzymatic hydrolysis of molasses. Bioresour. Technol. 86, 91–94 (2003). doi:10.1016/S0960-8524(02)00103-7
Gaurav, K., Srivastava, R., Sharma, J.G., Singh, R., Singh, V.: Molasses based growth and lipid production by Chlorella pyrenoidosa: A potential feedstock for biodiesel. Int. J. Green Energy. 5075, 150122092222001 (2015). doi:10.1080/15435075.2014.966268
Xu, H., Miao, X., Wu, Q.: High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J. Biotechnol. 126, 499–507 (2006). doi:10.1016/j.jbiotec.2006.05.002
Gao, C., Zhai, Y., Ding, Y., Wu, Q.: Application of sweet sorghum for biodiesel production by heterotrophic microalga Chlorella protothecoides. Appl. Energy. 87, 756–761 (2010). doi:10.1016/j.apenergy.2009.09.006
Wei, A., Zhang, X., Wei, D., Chen, G., Wu, Q., Yang, S.-T.: Effects of cassava starch hydrolysate on cell growth and lipid accumulation of the heterotrophic microalgae Chlorella protothecoides. J. Ind. Microbiol. Biotechnol. 36, 1383–1389 (2009). doi:10.1007/s10295-009-0624-x
Kim, W., Park, J.M., Gim, G.H., Jeong, S.-H., Kang, C.M., Kim, D.-J., Kim, S.W.: Optimization of culture conditions and comparison of biomass productivity of three green algae. Bioprocess Biosyst. Eng. 35, 19–27 (2012). doi:10.1007/s00449-011-0612-1
Kirrolia, A., Bishnoi, N.R., Singh, R.: Response surface methodology as a decision-making tool for optimization of culture conditions of green microalgae Chlorella spp. for biodiesel production. Ann. Microbiol. 64, 1133–1147 (2014). doi:10.1007/s13213-013-0752-4
Cheng, Y., Lu, Y., Gao, C., Wu, Q.: Alga-based biodiesel production and optimization using sugar cane as the feedstock. Energy Fuels. 23, 4166–4173 (2009). doi:10.1021/ef9003818
Onay, M., Sonmez, C., Oktem, H.A., Yucel, A.M.: Thermo-resistant green microalgae for effective biodiesel production: Isolation and characterization of unialgal species from geothermal flora of Central Anatolia. Bioresour. Technol. 169, 62–71 (2014). doi:10.1016/j.biortech.2014.06.078
Gorman, D.S., Levine, R.P.: Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc. Natl. Acad. Sci. USA. 54, 1665–1669 (1965). doi: 10.1073/pnas.54.6.1665
Xiong, W., Li, X., Xiang, J., Wu, Q.: High-density fermentation of microalga Chlorella protothecoides in bioreactor for microbio-diesel production. Appl. Microbiol. Biotechnol. 78, 29–36 (2008). doi:10.1007/s00253-007-1285-1
Abou-shanab, R.A., Raghavulu, S.V., Hassanin, N.M., Kim, S., Kim, Y.J., Oh, S.U., Oh, Y., Jeon, B.: Manipulating nutrient composition of microalgal growth media to improve biomass yield and lipid content of Micractinium pusillum, Afr. J. Biotechnol. 11, 16270–16276 (2012). doi:10.5897/AJB12.2628
Yan, D., Lu, Y., Chen, Y.F., Wu, Q.: Waste molasses alone displaces glucose-based medium for microalgal fermentation towards cost-saving biodiesel production. Bioresour. Technol. 102, 6487–6493 (2011). doi:10.1016/j.biortech.2011.03.036
Miller, G.L.: Use of dinitrosalicyclic acid reagent for determination of reducing sugar. Anal. Chem. 31, 426–428 (1959). doi:10.1021/ac60147a030
Karpagam, R., Raj, K.J., Ashokkumar, B., Varalakshmi, P.: Characterization and fatty acid profiling in two fresh water microalgae for biodiesel production: Lipid enhancement methods and media optimization using response surface methodology. Bioresour. Technol. 188, 177–184 (2015). doi:10.1016/j.biortech.2015.01.053
Uncu, O.N., Cekmecelioglu, D.: Cost-effective approach to ethanol production and optimization by response surface methodology. Waste Manag. 31, 636–643 (2011). doi:10.1016/j.wasman.2010.12.007
Li, Z., Yuan, H., Yang, J., Li, B.: Optimization of the biomass production of oil algae Chlorella minutissima UTEX2341., Bioresour. Technol. 102, 9128–9134 (2011). doi:10.1016/j.biortech.2011.07.004
Gurkok, S., Cekmecelioglu, D., Ogel, Z.B.: Optimization of culture conditions for Aspergillus sojae expressing an Aspergillus fumigatus α-galactosidase. Bioresour. Technol. 102, 4925–4929 (2011). doi:10.1016/j.biortech.2011.01.036
Lakshmikandan, M., Murugesan, A.G.: Enhancement of growth and biohydrogen production potential of Chlorella vulgaris MSU-AGM 14 by utilizing seaweed aqueous extract of Valoniopsis pachynema. Renew. Energy. 96, 390–399 (2016). doi:10.1016/j.renene.2016.04.097
Juneja, A., Ceballos, R., Murthy, G.: Effects of environmental factors and nutrient availability on the biochemical composition of algae for biofuels production: A Review. Energies 6, 4607–4638 (2013). doi:10.3390/en6094607
Kanaga, K., Pandey, A., Kumar, S., Geetanjali: Multi-objective optimization of media nutrients for enhanced production of algae biomass and fatty acid biosynthesis from Chlorella pyrenoidosa NCIM 2738., Bioresour. Technol. 200, 940–950 (2016). doi:10.1016/j.biortech.2015.11.017
Mata, T.M., Martins, A.A., Caetano, N.S.: Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 14, 217–232 (2010). doi:10.1016/j.rser.2009.07.020
Xiufeng, W.Q. L., Han, X., Large-scale biodiesel production from microalga Chlorella protothecoides through heterotrophic cultivation in bioreactors. Biotechnol. Bioeng. (2007). doi:10.1002/bit.
Acknowledgements
We would like to thank to Dr. Melih Onay for his isolation and characterization of microalgal species used in this study. This study was carried out in the following laboratories: Middle East Technical University (METU) Central Laboratory Molecular Biology and Biotechnology R&D Center, METU Biology Department Plant Biotechnology Laboratory and METU Food Engineering Department Bioprocess Laboratory. We would like to thank to TUBITAK Project Number :114Z487 for providing funding to Iskin Kose Engin during this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kose Engin, I., Cekmecelioglu, D., Yücel, A.M. et al. Enhancement of Heterotrophic Biomass Production by Micractinium sp. ME05 . Waste Biomass Valor 9, 811–820 (2018). https://doi.org/10.1007/s12649-017-9846-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-017-9846-8