Abstract
Enhancing the characteristics of amorphous semiconductor thin films is imperative for a multitude of applications. The present study examines the impact of Cd nanolayers deposited on a thin film of amorphous Si. The X-ray test showed that the thin silicon film had less of an amorphous structure after a nanolayer of cadmium was added. There has also been the appearance of new phases, and as the thickness of the Cd nanolayer increases, so does the intensity of these phases. Using a field emission scanning electron microscope, it was seen that nanoparticles were developed and subsequently transformed into clusters as the thickness of the Cd nanolayer grew. The absorbance was maximized at 750 nm and near-infrared region after depositing Cd nanolayers, while transmittance was reduced, especially at 100 nm thicknesses. The energy gap was reduced, with a decrease from 5.1 to 1.8 electron volts (eV). However, an increase in the band tails was also noted, rising from 0.7 to 4.9 eV. An increase in the values of the refractive index (n) and extinction coefficient (k) was observed following the deposition of Cd nanolayers of different thicknesses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The deposition of thin metallic coatings onto dielectric substrates has been the subject of a great deal of research in recent years since the interface between the two plays a crucial role in many processes, including microelectronic bias, solar cells, and many others. The variations in coating thickness were the focus of several of these experiments, while others looked more closely at the uniformity and adherence of the metallic compounds to the substrate [1]. For fundamental scientific research and usage in preparation for new solid-state electrical equipment, the production and search for substances with nanofilms and nanoparticles on the surface are appealing [2]. When metal is grown on top of semiconductors, the resulting film thickness is on the nanometer scale [3]. Some interesting things about this film are that it has a large magneto-optical Kerr response, charge transport anisotropy, and less scattering losses because the metal layer's surface is smooth, has largely controllable effective optic parameters, and has better resistance to wear and tear than metal films. This film also has benefits like low scattering losses [4,5,6,7,8,9].
In addition, the growth of metal–semiconductor hybrid structures has introduced novel prospects within the fields of optoelectronics and photonics. The unique properties of these materials work together to create the way for the creation of novel devices with enhanced performance. As an example, the development of plasmonic devices is a good one. These devices use the surface plasmons made by the metal layer to make the interactions between light and matter in the semiconductor layer stronger. These devices have demonstrated significant promise in various applications, including sensing, energy conversion, and information processing. Moreover, the ability to modify the metal layer's thickness and composition within these structures provides the potential for specific device development and enhancement. Because of this, metal–semiconductor hybrid structures look like a very promising way for technology to move forward [10,11,12,13,14]. In 2014, Stefaniuk et al. discovered that thin layers of Ag on Ge condensation coatings have about twice the resistance of the original structures that were deposited on Ti or Ni sublayers [15]. Based on recent research findings, it has been observed that there is a tendency for Ge atoms to accumulate close to the surface of the silver layer, coinciding with the increase in ohmic losses.
This unique idea represents the process of deposition of the Si thin film by thermal vacuum evaporation [16,17,18]. Also, the production of metal nanostructures with unique metamaterial properties. The metamaterial properties of metal nanostructures, such as a high refractive index and enhanced light–matter interactions, enable the development of novel devices with unique functionalities, such as absorbers, superlenses, and cloaking devices.
To attain this objective, Si thin coatings were deposited on glass substrates via thermal evaporation. The Cd nanolayers were then deposited on the Si thin films using the same technique, with varying thicknesses between 25 and 100 nm. Controlling the thickness of the Cd layer by varying the deposition duration via thermal evaporation is crucial for understanding the alteration in properties. Various techniques, including X-ray diffraction (XRD), scanning electron microscopy (SEM), and UV–Vis spectroscopy, were used to analyze the composition and optical properties of thin films. The results demonstrated that the deposition of Cd nanolayers significantly impacted the thin film. In this study, a novel Cd element was used to improve the characteristics of semiconductor material. The researchers found that the addition of Cd significantly enhanced the morphology and optical properties of the semiconductor material. This breakthrough has potential applications in various fields, such as electronics, solar cells, and optoelectronics.
2 Experimental details
First, a thin film layer was made on a quartz substrate using an EDWARDS E306A thermal vacuum evaporator and a diffusion pump at a high vacuum of 10–5 mbar. This process resulted in the deposition of multilayers of varying thicknesses. The very first layer is a film made entirely of silicon that has a thickness of 150 nm. The second layer is comprised of Cd and has three distinct thicknesses: 25 nm, 50 nm, and 100 nm. Evaporation was accomplished with the use of a tungsten spiral to maintain a high current that gives enough power to separate semiconducting components. This was done even though the melting temperature of silicon is rather high.
X-ray diffraction data were taken with a Panalytical X'Pert PROMRD diffractometer that had a 2theta range from 10° to 80° and a Cu-Ka that was set at 1.54056 A wavelength. This was done so that different thin film properties could be studied.
For this investigation of the microstructure of the thin coatings, a field emission scanning electron microscope (FESEM) model Quanta FEG 250 was used. The optical investigation of the thin films was carried out with the assistance of a JASCO 570-designed UV–Vis spectrophotometer.
3 Result and discussion
3.1 Structural properties
3.1.1 X-ray
Figure 1 shows how the XRD patterns changed when a Cd growth layer of different thicknesses hit the Si thin film. The Si hump display around (2Ө = 220) indicates a completely amorphous structure of the thin film after depositing a layer of Cd with a thickness of 25 nm. The new diffraction peak close to 31.79o is assigned to the plane (002). The peak's intensity increased with an increased thickness of the Cd layer, and the new phase appeared close to (2Ө = 600) in the corresponding plane (112), with a hexagonal structure. The intensities of the phases increase with increasing Cd nanolayer thickness, and the new phases appear at different (2Ө) [19]. The lattice parameters of the hexagonal crystalline structure of the cadmium nanolayer determined from the XRD graph were a = 3.286 Å and c = 5.613 Å [20]. The above behavior resulted from a rise in nucleation sites, which converted to agglomerate with the increased cadmium thickness layer, then increased crystalline phases in samples.
The X-ray spectra were used to figure out the crystallite size (D) of the nanoparticles in the structural thin films by using the Scherrer equation [21]:
where λ is the wavelength of the applied X-ray, Ө is the Bragg angle of the used X-ray peak in degrees, β is the full width at half maximum (FWHM) of the given diffraction peak, and k is the dimensionless shape factor with a value close to unity.
The values of crystalline size, lattice strain, and dislocation density are tabulated in Table 1. It is noted that the crystalline phases were influenced by the thickness ratio between the metal and semiconductor layers. This proves that the Cd metal provides phases of crystallization on the amorphous surface Si layer.
The behavior of dislocation density and lattice strain has opposite effects on crystallite sizes. The broadening of peaks increased due to multiple lattice distortions and refinement, likely explaining this increase. The following relation for the hexagonal crystal structure was used to calculate lattice constants 'a' and 'c' from X-ray investigation [22]:
where h, k, and l represent the Miller indices. Using the following formula, dislocation density (δ), which can be described as the number of dislocation lines per unit mass, was calculated [23]:
Employing the following equation, the cell volume of the created films was calculated:
The different structural parameters for Cd nanolayers deposited on Si thin films were determined and methodically depicted in Table 1.
The result in Table 1 shows the influence of the Cd nanolayer thickness on Si thin films on structural properties such as crystallite size (D), micro-strain (ε), and dislocation density (δ) for a prominent (002) peak, as shown in Fig. 1. This indicates that the thickness of the Cd nanolayer was increasing; the crystallite size was gradually increasing from 23.92 to 36.6 nm. This is due to the Cd nanolayer acting as a diffusion on the rough surface of the Si thin film, leading to a more compact crystal structure with larger crystallite sizes. In addition, the micro-strain was found to decrease from 0.00562 to 0.00362 as the Cd nanolayer thickness increased, suggesting a reduction in lattice distortions. The dislocation density also showed a decreasing trend, indicating that the Cd nanolayer helped suppress the formation of dislocations within the Si thin films. This means that the Cd nanolayer made the defects on the Si layer less noticeable. As the thickness of the Cd layer rose, these defects started to go away because the Cd nanolayer changed the surface roughness of the Si film, making the surface smoother. Overall, these results show how important the thickness of the Cd nanolayer is in determining the structural properties of Si thin films. The lattice parameters and unit cell volume of the Si thin films were also influenced by the Cd nanolayer thickness. With increasing Cd nanolayer thickness, the lattice parameters increased slightly, indicating a slight expansion of the crystal structure. Similarly, the unit cell volume increased, suggesting an increase in the overall size of the crystal. These results show that the Cd nanolayer not only lessens lattice distortions and dislocation formation, but also changes the Si thin films' overall structural properties. To get the desired structural properties in Si thin films, it is necessary to carefully control and improve the thickness of the Cd nanolayer [23].
3.1.2 Scanning electron microscope (SEM)
Figure 2 shows a SEM image of a pure silicon film containing a nanolayer of cadmium on a silicon film on a glass substrate. The thicknesses of the two films are different. Figure 2a shows that a thin film of pure Si exhibits an amorphous structure, which can be attributed to the random arrangement of particles. Figure 2b illustrates the graphical representation of nanoparticles and their formation during the nucleation phase. The presence of a Cd nanolayer on the surface of the film leads to the promotion of crystallization on the surface of the Si thin film layer, thereby reducing the roughness of the amorphous Si layer. This reduction in roughness is attributed to the strong binding between the Cd nanolayer and the Si atoms, which acts as a barrier, preventing the diffusion of impurities into the Si thin film layer and improving its structural integrity.
It was also seen that as the thickness of the Cd got closer to 50 nm, the number of nanoparticles increased, which led to the formation of an atomic cluster. This indicates that it enhanced the crystallization process. Upon increasing the thickness of the Cd layer to 100 nm, the film's surface exhibited the presence of nanoparticles, leading to a smooth surface of the Si layer. Additionally, it was observed that the presence of Cd nanoparticles exhibited an increase in proportion to the thickness of the Cd thin film. Bright patches were observed on the outermost layer of thin films, which were identified as microparticles. The findings were confirmed by the results of the X-ray examination.
3.1.3 EDAX analysis
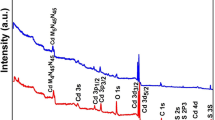
The thin films were quantitatively analyzed using the EDAX method to examine their composition quantitatively. The energy-dispersive X-ray spectroscopy elemental analysis of the Cd/Si thin films with different thicknesses of Cd layer (25 nm, 50 nm, 100 nm) is shown in Fig. 3.
Figure 3 shows an EDAX spectrum, which shows more proof that Cd and Si are in the films made by thermal evaporation deposition. Through EDAx, the relative atomic masses of Si, Cd, and O were found. This backs up what was seen before with X-ray and SEM techniques. These observations indicate that the number of Cd atoms in the films increases as the Cd layer deposition increases. Since the quantity of O decreases with increasing film thickness, the O mass may indicate the formation of CdO.
3.2 Optical properties
The optical properties of pure Si thin films and those with different thicknesses of (Cd) nanolayer deposited by CVD on Si thin films on glass substrates. We did this by measuring the films' ability to transmit light and absorb light in the 200–2500 nm range. The absorption coefficient, optical energy gap for the direct transition, and optical constants, such as extinction coefficient and refractive index, were also calculated.
The absorption, transmission, and reflection spectra of Si before and after adding a Cd layer of different thicknesses in the 200–2500 nm wavelength range are shown in Fig. 4.
Figure 4a shows the optical absorbance of the pure silicon thin film exhibited a significantly low value, reaching a magnitude of 0.1. After depositing a Cd nanolayer of different thicknesses, the observed absorbance went up, especially for a layer that was 100 nm thick. When a nanolayer of Cd was added, there was a noticeable increase in absorbance, especially in the visible spectrum (750 nm) and the near-infrared region. A photobleaching effect starts when the thickness of the Cd nanolayer is increased. This causes these windows to move toward longer wavelengths. The Cd nanolayer reduces the size of the holes, which causes more Cd atoms to gather in the holes. This is what caused the effect that was seen. The augmentation of the concentration of Cd atoms led to the ultimate improvement of the surface of the semiconductor. A photobleaching effect starts when the thickness of the Cd nanolayer is increased. This causes these windows to move toward longer wavelengths. The Cd nanolayer reduces the size of the holes, which causes more Cd atoms to gather in the holes. This is what caused the effect that was seen. The augmentation of the concentration of Cd atoms led to the ultimate improvement of the surface of the semiconductor. Consequently, a reciprocal correlation between the island and the hole can be observed. The previously mentioned aspect depends on the substrate's structure and size. The Cd nanolayer increased absorbance in the visible and IR regions, increasing the efficiency of capturing a broader range of light. Additionally, the Cd layer provides a protective barrier, preventing degradation of the Si thin films and ensuring their longevity in various environmental conditions.
In contrast, the transmittance of the Si film was extremely high but began to decrease after the addition of the Cd nanolayer, reaching a negligible value when the Cd thickness reached 100 nm, as shown in Fig. 4b. This decrease in transmittance results from the addition of Cd, which generates free electrons that absorb some light photons. This suggests that the use of Cd led to the formation of localized states. This optical discovery is consistent with the X-ray findings. The element Cd improved Si's properties, which are beneficial for solar cells and electronic devices.
In addition, the reflectance of the Si thin film increases when a Cd nanolayer is added, as shown in Fig. 4c. The reflectance is highest when the thickness of the Cd nanolayer is 100 nm. This increase in reflectance is because the Cd nanolayer has better optical properties than Si. It has a higher refractive index. As the thickness of the Cd nanolayer increases, it allows for more efficient reflection of light, resulting in the observed increase in reflectance. Also, the Cd nanolayer increases the smoothness of the Si thin film surface, reducing scattering and improving the overall optical quality. This improved smoothness further contributes to the enhanced reflectance of the Si thin film with the addition of the Cd nanolayer.
3.2.1 The absorption coefficient (α)
For the band structure of the current substances, Lambert's formula was used to get the absorption coefficient (α), which was then used to figure out the energy gap values using the equation below [24]:
where R represents optical reflection, T is optical transmission, and d represents the film thickness in nanometers.
The relationship between photon energy (hν) and absorption coefficient α for pure Si film and after adding different Cd thicknesses (25 nm, 50 nm, and 100 nm) is shown in Fig. 5.
When different thicknesses of cadmium nanolayers were added to a pure silicon thin film, the energy of the absorption edge and the values of α went up. Because the films have a 100 nm Cd nanolayer on top of them, the absorption edge has gone red. This is because the nanolayer causes crystalline phases to form close to the valence and conduction bands [25]. The change mentioned earlier led to a change in the electronic structure, which in turn affected the design and optimization of thin film materials for a wide range of uses. The phenomenon of quantum confinement has been observed to result in a reduction in the size of Cd particles and an increase in the energy gap. This characteristic renders the Cd nanolayer a highly promising material for various optoelectronic applications, including solar cells and sensors. When cadmium (Cd) nanolayers are used, the absorption coefficient goes up across a wider range of photon energies. This augmentation facilitates the occurrence of localized phases at the edges of the nanolayer, which has a thickness of 100 nm.
3.2.2 Optical band gap
Both indirect and direct energy gaps involve the transition of electrons across the band gap to the conduction band due to the interaction of the energy of photons with the energy of an electron in the valence band. It can be calculated by employing the following equation [26]:
where A represents a constant, α is the absorption coefficient, hν is the energy of the incident spectrum, Eg represents the material's band gap energy, and n = 1/2 and 2, respectively, for direct and indirect band gaps. The values of indirect and direct band gaps were calculated and recorded in Table 2, which were estimated using the relation between (αhν)2, (αhν)1/2, and photoenergy hν, as shown in Fig. 6.
Table 3 illustrates a decrease in the energy gap values, specifically from 5.1 to 1.8 eV for the direct energy gap and from 5.55 to 2.95 eV for the indirect energy gap. The effect that was seen can be explained by the fact that when thin Cd films are put down, the Fermi level moves toward the conduction band, which changes the number of carriers. A blueshift in the near-band edge emission in the band gap is often seen. This is because electrons are occupying the conduction band. Table 2 illustrates a decrease in the energy gap values, specifically from 5.1 to 1.8 eV for the direct energy gap and from 5.55 to 2.95 eV for the indirect energy gap. The effect that was seen can be explained by the fact that when thin Cd films are put down, the Fermi level moves toward the conduction band, which changes the number of carriers. A blueshift in the near-band edge emission in the band gap is often seen. This is because electrons are occupying the conduction band. The deposition of the Cd nanolayer increases the smoothness of the surface and allows for enhanced light trapping within the thin film. This leads to the energy gap decreasing and the absorption coefficient increasing. Additionally, the Cd nanolayer also helps to reduce surface recombination, further enhancing the overall performance of the Si thin film.
As a consequence of doping, the Burstein–Moss shift refers to the alteration in the optical band gap of a material, typically a semiconductor [2, 26]. Potential factors contributing to the observed blueshifted phenomenon encompass the possibility of grain size augmentation, thereby widening the band gap.
The absorption coefficient is used to determine the bandgap structure and the specific kind of electronic transitions that contribute to the absorption of photons in the material. From Urbach's formula, it is exponentially reliant on the energy (hν) and Urbach energy EU [27]
Figure 7 shows the relation between ln (α) as a function of photon energy which is the reciprocal of slope equal to Urbach energy and tabulated in Table 2.
According to the data presented in Table 2, there is a noticeable correlation between the thickness of the Cd nanolayer and the magnitude of the Urbach band tail. Specifically, as the thickness of the Cd nanolayer increases, the Urbach band tail values also increase. This relationship is particularly pronounced at higher thicknesses, which can be attributed to the enhanced formation of localized states within the band gap. The EU value serves as an indicator of the level of disorder. The observed decrease in the optical gap can be attributed to the concurrent increase in disorder.
3.2.3 Refractive index and extinction coefficient
Refractive index dispersion plays an important role in optical material research. It is an essential factor in optical communications and the design of spectral dispersion devices. The refractive index of the films was calculated according to the following relationship [28]:
Figure 8 shows the refractive index of layers of silicon (Si) and cadmium (Cd), along with thin films deposited on a glass substrate in a range of thicknesses. It is apparent that the refractive index experienced an increase with higher levels of doping and exhibited nonlinearity as the wavelength was extended. Different layers of the films show big changes in their refractive indices, with the values of n going up as the photon energy goes up, especially at shorter wavelengths. Thin films have a lot of virtual electronic transitions going on, which is a big reason why they changed the way light behaves in a big way. The dispersion in a material arises from its carriers, specifically electrons and holes, as well as phonons. The interaction between phonons and electrons leads to the dispersion of magnetic waves with diverse wavelengths, including those of shorter lengths [29].
Figure 8 shows an increasing trend of the extinction coefficient K of the thin film provided by the equation [20]:
for each film, which is supported by Sellmeier's dispersion relation as Cd content upsurges. As the thickness of the Cd layer went up, the extinction coefficient of the films followed the same pattern as the refractive index. This is in line with what other studies [30, 31] have found. Absorption is the elastic scattering of electromagnetic waves within semiconductors. It includes the Compton scattering, photoelectric, and pair creation effects. This entails augmenting the electronic transitions occurring between the valence and conduction bands.
4 Conclusions
The enhancement of sample properties for diverse applications was achieved through the deposition of a Cd nanolayer with variable thickness onto a Si nanolayer. X-rays showed that adding a nanoscale layer of cadmium increased the crystalline phase and decreased the amorphous layer of silicon. Crystalline phases became more noticeable as the thickness of the Cd nanolayer went up, while the amorphous phases of the Si layer became less noticeable. The X-ray results were supported by the scanning electron microscopy (SEM) images, which showed that there was a link between the thickness of the cadmium layer and the number of nanoparticles. The nanoparticles underwent a conversion process, resulting in the formation of a cluster. The introduction of a nanolayer of Cd with varying thicknesses resulted in an increase in absorbance and a corresponding decrease in transmission. The optical bandgap exhibited a reduction from 5.1 to 1.8 electron volts. When a Cd nanolayer was added, the optical band tails got longer, specifically to 0.76 and 4.95 eV. This made the refractive index and extinction coefficient go up. These changes in the optical properties suggest that the conversion process not only formed a cluster but also induced significant modifications in the electronic structure of the material. The increase in absorbance and decrease in transmission, along with the reduction in optical bandgap, indicate that the Cd nanolayer played a crucial role in altering the light–matter interaction within the cluster.
References
P Prepelita et al. Results Phys. 35 105387 (2022)
Y S Ergashov, D A Tashmukhamedova and E Rabbimov J. Surface Investig. 9 350 (2015)
A Ciesielski et al. Beilstein J. Nanotechnol. 10 238 (2019)
H Neff et al. Thin Solid Films 496 688 (2006)
H Aouani et al. ACS Nano 3 2043 (2009)
V Logeeswaran et al. Nano Lett. 9 178 (2009)
P Melpignano et al. Org. Electron. 11 1111 (2010)
M Mayy et al. J. Appl. Phys. 111 94103 (2012)
R Malureanu and A Lavrinenko Nanotechnol. Rev. 4 259 (2015)
T A Chen et al. ACS Appl. Nano Mater. 4 2211 (2021)
N A A Zainuddin et al. Results Phys. 13 102255 (2019)
P Mosel et al. Optics Express 30 37038 (2022)
H H Chang, P C Jiang and W B Su J. Appl. Phys. 57 08 (2018)
H Yang et al. J. Phys. D: Appl. Phys. 51 085303 (2018)
T Stefaniuk et al. Nanoscale Res Lett 9 1 (2014)
M S AboGhazala et al.ACS Omega 5 27633 (2020)
M A Nawwar et al. Cryst. Growth Des. 23 751 (2022)
M Anawwar et al. RSC Adv. 13 9154 (2023)
Z R Khan, M Zulfequar and M S Khan Mater. Sci. Eng. B 174 145 (2010)
A A I Al-Bassam, A A Al-Juffali and A M Al-Dhafiri J. Cryst. Growth 135 476 (1994)
M D Uplane and S H Pawar Solid State Commun. 46 847 (1983)
A Goswami Thin film fundamentals New age international (1996)
D Sivagamai and B G Priyadarshini Mater. Sci. Energy Technol. 3 709 (2020)
J G Mutitu et al. Optics express 16 15238 (2008)
D G Gromov et al. Appl. Surface Sci. 489 701 (2019)
Z M Gibbs, A LaLonde and G J Snyder New J. Phys. 15 075020 (2013)
S B Aziz et al. Polymers 9 626 (2017)
M Sahana et al. Appl. Phys. Lett. 70 2909 (1997)
P Prepelita, F Garoi and V Craciun Beilstien J. Nanotechnol. 12 354 (2021)
W A S A Neama Procedia-Soc. Behav. Sci. 68 752 (2012)
S H Wemple and M Didomenico Jr J. Appl. Phys. 40 735 (1969)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rabiea, E.A., AboGhazala, M.S. & Zadia, M.M. Structure and optical properties of Si thin film improved by metal Cd nanolayers. Indian J Phys 98, 2923–2932 (2024). https://doi.org/10.1007/s12648-023-03050-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12648-023-03050-x