Abstract
To make the interpretation of the complex xCsBr.(100 − x)(CaO:SiO2) glasses easier to study, the structure of glasses in a binary composition CaO–SiO2 is being initially investigated. The changes in the crystallization behavior of glasses and the local environment surrounding silicon atoms could be easily followed using X-ray diffraction (XRD) and nuclear magnetic resonance (NMR) spectroscopy. The XRD pattern of the binary 50CaO–50SiO2 glass showed that its structure was amorphous. But when (CaO:SiO2) is replaced with CsBr, some sharp diffraction lines appear in system of the ternary xCsBr.(100 − x)(CaO:SiO2) glass composition. A Polycrystalline Cs2Ca(SiO3)2 structure is the primary phase in CsBr rich glasses. The results based on transmission electron microscopy (TEM-EDP) and X-ray diffraction pattern (XRD) are in excellent agreement, indicating that crystalline-clustered species develop in glasses enriched with CsBr. Both the NMR and FTIR spectra are clearly defined, and they contain different features that distinguish between different silicate structural subunits. One and two bridging oxygen atoms (BO) can be found in the main SiO4 structural units. Such units become less shielded due to increasing of nonbridging oxygen atoms (NBO) in the silicate network by increasing CsBr at the expense of both SiO2 and CaO. There is a good correlation between the data obtained from FTIR and NMR spectroscopy. Both techniques could differentiate between BO and NBO involved in the silicate structural units.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The effect of thermal heat treatments (THT) can result in the formation of the crystalline Ca2SiO4 and CaSiO3 polymorphs in the amorphous silicate glasses containing calcium oxide [1, 2]. The cement chemistry community is quite interested in producing materials under the application of a thermal heat treatment process [2,3,4]. Inorganic stabilizers such as metal halides, in addition to the (THT) methods, have a direct impact on the stability and crystallinity of the prepared materials. The well-formed structural units that make up the host glass network can be controlled through addition of specific concentrations from halides in the host glass matrix. Additionally, the stability and crystallization of a glass network are affected by the indirect manner connected to the ion exchange process. For instance, when the metal halide salts and a metal oxide are introduced to the primary glass formers, such as P2O5, TeO2, SiO2, B2O3, etc., an exchange reaction between the two types in the primary glass forming network may occur. The ion exchange interaction (IXI) between the halogen atom from the metal halide and the oxygen atom from the modifier oxide changes the physical properties and structure of the produced glass samples [4].
An inversion structure with crystalline phases can be created as a result of IXI process [2,3,4]. In this situation, X-ray diffraction analyses showed that the base glass that is free from halogens, is entirely amorphous [5]. The amorphous glass network becomes polycrystalline with the addition of metal halides. For instance, more open spaces might be formed when CdO is increased at the expense of Na2O and P2O5 [5] using invert phosphate glasses. This may contribute to structural weakness, which is manifested in an increase in glass-free volume (Vf). The concentration of (NBO) should probably grow with increasing CdO at the expense of a concentration of similar magnitude from P2O5 and Na2O, according to increasing the latter in the glass network. The formation of the cadmium invert phosphate glasses results in the remaining of the amorphous structure even at high ion exchange rates.
The main aim of this study is obtaining new cesium invert crystalline silicate glasses, as a first trial, through ion exchange process between oxygen atoms from CaO and bromine ions from CsBr. In this case, opened spaces in the host glass matrix might be increased, to play the main role of increasing both the number and mobility of charge carriers [4, 6]. Increasing the latter recommend the studied material to be used as row material shared in the manufacturing of solid state batteries.
2 Experimental details
Glass samples in the system xCsBr.(100 − x)(CaO:SiO2) were prepared using analytical grade materials of purity around 99.9% of SiO2, CaCO3, and CsBr from Aldrich company. Glasses were formed and obtained using the molar formula xCsBr.(100 − x) (CaO:SiO2), where x = 0, 5, 15, 30, and 40 mol%, Table 1. The melting process was carried out in crucibles made of silica at melting temperature between 1250 and 1560°C in an electric furnace. To ensure homogeneity, the melts were shacked multiple times. In an electric furnace, the resulting glasses were annealed at 400 °C for 4 h to reduce the internal stresses and strains formed in the bulk of as prepared glasses.
To measure X-ray diffraction, a Shimadzu X-ray diffractometric is employed (the apparatus type Dx-30, Metallurgy institute, El Tebbin, Cairo, Egypt). By comparing the maximum peak and intensity values to patterns in the international powder diffraction file (PDF) database of the joint committee for powder diffraction standards (JCPDS), the material type can be identified.
The powdered samples' NMR spectra were captured using a Joel NMR 500 (11.74T, Mansoura University) spectrometer. To solve the issue of the relaxation time, one plus detection techniques were used. Pulses with a duration of 2–3 s, an angle of around 45°, and substantial delays (25 s) between them were gathered. Up to 1400 scans were accumulated depending on the specific sample. The zirconia rotating sample was capable of rapid rotation at the magic angle at a rate of 7.5 kHz due to two different double-bearing systems.
The FTIR absorbance spectra are measured in the 400–4000 cm−1 region with a spectral resolution of 2 cm−1 using a Mattson 5000 FTIR spectrometer. The resulting spectra were normalized to that of a blank KBr pellet after background and dark currents were removed using a two-point baseline. To minimize the effect of the powder sample’s concentration on the KBr disc, normalization is required. At least three samples of each material were tested. The spectrum of every sample is collected through a collection of 20 scans.
3 Results and discussion
3.1 XRD results
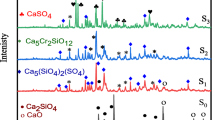
XRD pattern of glass samples of different CsBr concentrations are shown in Fig. 1. The spectra of glasses of 0 and 5 mol% CsBr confirmed the amorphous structure, since there is no any sharp diffraction peaks but just a broad hump lies between 2θ ~ 12°–37° is appeared. On the other hand, the spectra of glasses containing 15, 30 and 40 mol% CsBr (c, d, e) contain some sharp diffraction peaks superimposed on the wide hallo. The intensity of the sharp diffraction lines are gradually increased with increasing CsBr content at expense of both CaO and SiO2 which leads to an increase in the percentage of crystalline species to become maximum at 40 mol% CsBr. Therefore, the samples containing 30 and 40 mol% CsBr are considered as the suitable composition presenting a glass ceramic type, since they contain a specific crystalline Cs2SiO3 and Cs2Ca(SiO3)2 phases [7, 8]. The latter has special interest as a crystalline host for shielding materials against ionized radiations, due to its high chemical stability.
The formation of crystalline structure is considered as a direct result of ion exchange process between Br ions from CsBr and oxygen atoms from CaO. The ion exchange process increases the total modifier contents from Cs2O, CaO and CaBr2 to create more NBO which facilitate the mobility of ions to form a collective crystalline species. This aspect can be clearly clarified from the results based on NMR spectroscopy, which discussed below.
3.2 NMR spectroscopy
29Si NMR and FTIR spectroscopy have been used to investigate the structure of glasses in the system xCsBr (100 − x) (CaO:SiO2) with x = 0,5,15,30 and 40 mol%. Addition of CsBr at expense of both CaO and SiO2 results in the decondensation of the calcium metasilicate chains, which is defined as silicate unit containing two bridging oxygen atoms (BO) named Q2 [9,10,11]. The fraction of Q2 sites of chemical shift lies at about (− 82 ppm), Fig. 2a, b, decreases along with the formation of Q1 and few Q0 structural units of the average chemical shift of value between (−78 and −73 ppm), Fig. 3a, b. It can be detected from Figs. 2 and 3 and Table 2 that there is a clear change in both peak position and intensity of Qn due to change of CsBr from 0 to 40 mol%. The shift of peak to reach − 73 ppm which represents a mixture from Q1 and Q0 sites leads to the formation of orthosilicate groups [12, 13] which are clearly formed upon exchange of both CaO and SiO2 with CsBr. The changes in both spectral feature and chemical shift values of 29Si NMR spectra (Figs. 2 and 3) leads to the conclusion that Cs+ ions are bonded mainly to the less shielded silicate units (Q1 and Q0 sites). Some Q0 [12, 13] species which are present in the glass containing 30 and 40 mol% CsBr are formed by the following disproportionation reaction,
The increase in (Q1 and Q0) at the expense of Q2 leads to conclusion that there is ion exchange between some of oxygen atom from CaO and bromine ion from CsBr which results in formation of Cs2O and CaBr2. This process was also confirmed in PbI2 and Ag2O telluride glasses [14]. The ion exchange process increases the total contents from (CaO and Cs2O) as modifiers which leads to formation of extra nonbridging oxygen atoms (NBO) in the silicate network [12, 13]. Moreover, the bromine ions are inserted intestinally [14] in the silicate network resulting in elongation and weaken Si–O bonds which has a direct effect on NBO formation. In addition, the decreasing SiO2 concentration upon CsBr exchange leads to formation of additional NBO atom which can be considered as agent for enhancing the crystallization process in silicate glasses [13, 14]. Presence of NBO ions and Cs+ with an extremely high concentration leads to formation of electron–hole centers which makes the crystalline glasses useful as a shielding materials against ionized radiation [15].
From 29Si NMR spectroscopy, both the first and second coordination neighbors of Si nuclei can be determined. Accordingly BO and NBO associated with SiO4 tetrahedral units can be simply followed. From Figs. 2 and 3, it can be documented that the average coordination number of bridged oxygen atoms (BO) around Si atom decreases as CsBr increases which means increasing concentration of (NBO). The samples of 40 mol% CsBr contains the highest number of NBO founds in orthosilicate groups (Q1 + Q0) of chemical shift at about − 73 ppm. Accumulation of NBO around Cs+ cations leads to the formation of cluster species from Cs+ and Si which is called alamosite [16] crystalline phase Cs2SiO3. In such a case the silicate groups should be formed in orthosilicate crystalline type, i.e., highest number of NBO [12, 13, 16,17,18,19,20].
3.3 TEM-EDP investigation
It clear from TEM micrographs, Fig. 4a–c that the most accumulated and precipitated species are appeared in micrograph (c) which represents TEM-EDP of sample containing 40 mol% CsBr. The precipitation of Cs2SiO3 phase increases with increasing CsBr contents. The associated diffraction patterns confirm the enhanced precipitation process with increasing CsBr contents which has been also evidenced from XRD spectra of the same compositions. Generally increasing CsBr content at expense of CaO and SiO2 in silicate glasses will change the feature of the well-formed crystalline units through formation of Cs2SiO3 phase [16, 20]. The nature of glass matrix was highly influenced by the formation of crystalline Cs2SiO3 species, since more ordered network is the main product as was evident from both X-ray and TEM_EDP results. Separated species of larger size are found in glass containing 40 mol% CsBr presented from TEM micrograph, Fig. 4c.
3.4 FTIR analysis
The FTIR spectra of the investigated glasses are displayed in Fig. 5. In the composition of 0 and 5 mol% CsBr, the CaO–SiO2 spectra exhibited a broad absorption band with a peak located at roughly 950 cm−1 which assigned to Si–O vibration in the middle unit, which contains two bridging oxygen atoms, Q2 [16, 17]. The Q1 sites [18, 21, 22], known as chain-end groups, are responsible for the well-defined, high peak (1050 cm−1) in the FTIR spectra of glasses containing 15, 30, and 40% mol% CsBr. It can be assumed that these Q1 sites are connected to mixed Ca2+ and Cs+ ions, since (CaO + Cs)/SiO2 ratio in the glass is extremely high enough to form Q1 species.
The decrease in peak intensity at 940 cm−1 and increasing one at 980 cm−1 confirm that most of cesium and calcium entered the phosphate glass network as a modifier. In some cases Cs2O can be entered with two different coordination's which are tetrahedral CsO4 and octahedral CsO6. The electrostatic field strength of octahedral cesium is smaller than that of tetrahedral one. Then, some of Q units were subjected to more deshielding as a result of the octahedral or modifier cesium oxide. This hypothesis is thought to be the primary cause of the peak shift from 950 to 1050 cm−1. This variation may possibly be a result of the Q1 and Q0 groups' interaction with the octahedral cesium bond. The FTIR spectra of the glass with 30 and 40% CsBr can then be interpreted as showing extraordinarily high concentrations of Q1 and Q0 while glasses with 0 and 5 mol% CsBr had Q2 as a significant species. Other minor peaks may be seen at 1150 and 750 cm−1, respectively, which are attributed to Si–O vibrations in the middle groups of short chains and cycles [18, 21] metasilicate units.
As shown in Fig. 6a–e, line shape simulations or deconvolution techniques can be used to quantitatively calculate the proportion of different Qn species in the silicate network [18,19,20,21,22,23]. The three silicate units Q0, Q1, and Q2 in the 29Si MAS NMR studied spectra, are the average main units. Each Qn fraction can be determined and shown in Fig. 7 after peak deconvolution. The fractions of Q0 and Q1 both increase with increasing CsBr content, the fraction of Q2 units decreases. This behavior leads to the conclusion that the CsBr–CaO exchange characteristic’s tendency to decrease (Q2/Q1 + Q0) ratio which is primarily caused by the local environment's distortion. The formation of additional NBO in conjunction with the addition of CsBr is what ultimately leads to variations in the percentages of Q2 and (Q1 + Q0) with increasing CsBr content. The reversal variations in the peak intensity of the resonance lines of 29Si NMR spectra provide strong support for this result (Fig. 2). Then from the results of NMR and FTIR spectroscopy, it can be confirmed that there are a good agreement between the data obtained from the different techniques. Both confirmed that NBO atom increased upon addition of CsBr at expense of both CaO and SiO2.
Figure 7 summarizes the quantitate data obtained from the analysis of FTIR spectra. As can be seen from the figure, the parentage of Q2 decreases and the values of both Q1 and Q0 increase with increasing CsBr content from 0 to 40 mol%. Such changes lead to confirm that less shielded silicate structural units are formed due to increasing of nonbridging oxygen atoms (NBO) in the silicate network by increasing CsBr at the expense of both SiO2 and CaO. This argument was supported from NMR and FTIR techniques that could differentiate between BO and NBO involved in the silicate structural units.
4 Conclusions
The glasses prepared in mol% in the xCsBr.(100 − x)(CaO:SiO2) system have been studied for the first time by several techniques. The XRD pattern of the binary 50CaO–50SiO2 glass showed that its structure was amorphous. Some crystalline phases appear in the ternary xCsBr.(100 − x)(CaO:SiO2) glasses. Data based on (TEM) and X-ray diffraction patterns were in excellent agreement, indicating that the crystalline-clustered species can be developed in glasses enriched with CsBr. Both the NMR and FTIR spectra contain specific different features that can distinguish between different silicate structural subunits. Such units become less shielded and richer with (NBO) in the form of Q0 that are electrically balanced with cesium cations by increasing CsBr at the expense of both SiO2 and CaO. The analysis of FTIR and NMR spectra by peak separating method has a powerful action to distinguish different types of oxygen atoms.
Availability of data and materials
As authors, we are increasingly make our research data available and Data will be made available on request.
References
J Schneider et al Journal of non-crystalline solids 325 164 (2003)
X x Feng and J Chang International Journal of Applied Ceramic Technology 8 547 (2011)
K Garbev, B Gasharova, G Beuchle, S Kreisz and P Stemmermann Journal of the American Ceramic Society 91 263 (2008)
G El-Damrawi Journal of Physics: Condensed Matter 11 6385 (1999)
H Doweidar, G El-Damrawi and E El Agammy Materials Chemistry and Physics 207 259 (2018)
G El Damrawi, A Hassan and A Shahboub Applied Physics A 126 1 (2020)
A Kaur et al Phase Transitions 86 759 (2013)
F Fayon, C Bessada, D Massiot, I Farnan and J Coutures Journal of Non-Crystalline Solids 232 403 (1998)
N M Vedishcheva, B A Shakhmatkin and A C Wright Journal of non-crystalline solids 293 312 (2001)
Y Chen et al Journal of Non-Crystalline Solids 602 122078 (2023)
E A Ibtissam, K Saida and O Hassane Physical Chemistry Research 10 1 (2022)
K V Rao, M Madhu, P Ashok, G A Kumar and R K Guntu Silicon 14 9887 (2022)
W Tan et al Catalysis Today 397 475 (2022)
G S Henderson and J F Stebbins Reviews in Mineralogy and Geochemistry 87 1 (2022)
W Al Mohammedi, G El Damrawi, M Sherbiny and A Mohamed Abdelghany Bulletin of Materials Science 45 90 (2022)
Y Xu, Y Zhang, H Lv, Y Wu and A Zou Journal of Luminescence 252 119316 (2022)
F Detraux, F Finocchi and X Gonze Physical Review B 73 165208 (2006)
C Venkateswaran, H Sreemoolanadhan, B Pant, S Sharma, V Chauhan and R Vaish Journal of Non-Crystalline Solids 550 120289 (2020)
P James, Y Iqbal, U Jais, S Jordery and W Lee Journal of Non-Crystalline Solids 219 17 (1997)
C Venkateswaran, S Sharma, B Pant, V Chauhan and R Vaish Thermochimica Acta 679 178311 (2019)
R F Klein and P A Hays Microgram Journal 13 1 (2016)
G E Damrawi, R M Ramadan and M E Baiomy Silicon 14 4879 (2022)
T S Tavares, J A Torres, M C Silva, F G E Nogueira, A C Da Silva and T C Ramalho Bioprocess and biosystems engineering 41 97 (2018)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical Statement
Authors declare that we have no conflict of interest. We are agreed upon all the Ethical Rules applicable for this journal.
Consent for Publication
Authors agreed to publish this work. This declaration ensures that the Publisher has the Author's permission to publish this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El Damrawi, G., Gharghar, F., Atef, R. et al. Structure role of cesium bromide in calcium silicate glasses and glass ceramics. Indian J Phys 98, 2031–2037 (2024). https://doi.org/10.1007/s12648-023-02984-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12648-023-02984-6