Abstract
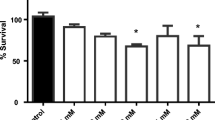
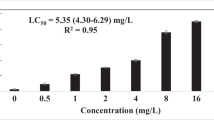
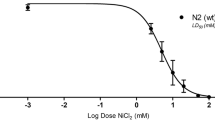
The toxicity of diuron herbicide and its metabolites has been extensively investigated; however, their precise toxic mechanisms have yet to be fully appreciated. In this context, we evaluated the toxic mechanism of diuron, 3,4-dichloroaniline (DCA) and 3-(3,4-dichlorophenyl)-1-methylurea (DCPMU), using Caenorhabditis elegans (C. elegans) in the L1 larval stage. For this purpose, worms were acutely exposed to the test chemicals with a preliminary concentration range of 0.5 to 500 μM and first analyzed for lethality (%). Next, the highest concentration (500 μM) was considered for survival (%), reactive oxygen and nitrogen species (RONS), glutathione (GSH) and ATP levels, autophagy index, behavior, and dopaminergic neurodegeneration parameters. Interestingly, increased lethality (%) was found for all chemicals at the higher concentrations tested (100 and 500 μM), with significant differences at 500 μM DCA (p < 0.05). A decrease in the median survival was observed mainly for DCA. Although no changes were observed in RONS production, GSH levels were significantly increased upon diuron and DCA treatment, likely reflecting an attempt to restore the redox status. Moreover, diuron and its metabolites impaired ATP levels, suggesting an alteration in mitochondrial function. The latter may trigger autophagy as an adaptive survival mechanism, but this was not observed in C. elegans. Dopaminergic neurotoxicity was observed upon treatment with all the tested chemicals, but only diuron induced alterations in the worms’ locomotor behavior. Combined, these results indicate that exposure to high concentrations of diuron and its metabolites elicit distinct adverse outcomes in C. elegans, and DCA in particular, plays an important role in the overall toxicity observed in this experimental model.









Similar content being viewed by others
Data Availability
Please contact the authors for data requests.
References
Alister CA, Kogan M (2010) Rainfall effect on dissipation and movement of diuron and simazine in a vineyard soil. Planta Daninha 28:1059–1071
APVMA (2011) Review of the mammalian toxicology and metabolism/toxicokinetics of diuron. Australian Pesticides and Veterinary Medicines Authority
Aspernig H, Heimbucher T, Qi W et al (2019) Mitochondrial perturbations couple mTORC2 to autophagy in C. elegans. Cell Rep 29:1399–1409
Brenner S (1974) Genetics of Caenorhabditis-elegans. Genetics 77:71–94
Cardoso APF, Catalano SMI, Sanches M et al (2013) Dose–response of diuron [3-(3,4-dichlorophenyl)-1,1-dimethylurea] in the urothelial mucosa of Wistar rats. Toxicology 312:1–5
da Rocha MS, Arnold LL, Luiza M et al (2014) Diuron-induced rat urinary bladder carcinogenesis: mode of action and human relevance evaluations using the International Programme on Chemical Safety framework. Crit Rev Toxicol 1–14
da Rocha MSD, Arnold LL, Dodmane PR et al (2013) Diuron metabolites and urothelial cytotoxicity: in vivo, in vitro and molecular approaches. Toxicology 314:238–246
da Rocha MSD, Arnold LL, Pennington KL et al (2012) Diuron-induced rat bladder epithelial cytotoxicity. Toxicol Sci 130:281–288
da Rocha MSd, Nascimento MG, Cardoso APF et al (2010) Cytotoxicity and regenerative proliferation as the mode of action for diuron-induced urothelial carcinogenesis in the rat. Toxicol Sci 113:37–44
Danish Q (SAR) Database (2022) Diuron (CAS no. 330–54–1). Available in: https://qsardb.food.dtu.dk/db/index.html
Demine S, Renard P, Arnould T (2019) Mitochondrial uncoupling: a key controller of biological processes in physiology and diseases. Cells 8:795
Fava RM, Cardoso APF, Sanches M et al (2015) Evaluation of early changes induced by diuron in the rat urinary bladder using different processing methods for scanning electron microscopy. Toxicology 333:100–106
Ferrucio B, Franchi CAdS, Boldrin NF et al (2010) Evaluation of diuron (3-[3,4-dichlorophenyl]-1, 1-dimethyl urea ) in a two-stage mouse skin carcinogenesis assay. Toxicol Pathol 38:756–764
Field JA, Reed RL, Sawyer TE et al (2003) Diuron occurrence and distribution in soil and surface and ground water associated with grass seed production. J Environ Qual 32:171–179
Gatidou G, Thomaidis NS (2007) Evaluation of single and joint toxic effects of two antifouling biocides, their main metabolites and copper using phytoplankton bioassays. Aquat Toxicol 85:184–191
Giacomazzi S, Cochet N (2004) Environmental impact of diuron transformation: a review. Chemosphere 56:1021–1032
Gjorgjieva J, Biron D, Haspel G (2014) Neurobiology of Caenorhabditis elegans locomotion: where do we stand? BioSci Adv 1–11
He B, Wang X, Yang C et al (2020) The regulation of autophagy in the pesticide-induced toxicity: Angel or demon? Chemosphere 242:125138
Hodge HC, Downs WL, Panner BS, Smith DW, Maynard EA (1967) Oral toxicity and metabolism of diuron (N-(3,4-dichlorophenyl)-N’, N’-dimethylurea) in rats and dogs. Food Cosmet Toxicol 5:513–531
IBAMA (2020) Relatórios de comercialização de agrotóxicos. Available in: http://ibama.gov.br/agrotoxicos/relatorios-de-comercializacao-de-agrotoxicos
Ihlaseh-Catalano SM, Bailey KA, Hester SD et al (2011) Transcriptional profile of diuron-induced toxicity on the urinary bladder of male Wistar rats to inform mode of action. Toxicol Sci 122:330–338
Ihlaseh-Catalano SM, Bailey KA, Paula A et al (2014) Dose and temporal effects on gene expression profiles of urothelial cells from rats exposed to diuron. Toxicology 325:21–30
Ijomone OM, Miah MR, Akingbade GT et al (2020) Nickel-induced developmental neurotoxicity in C. elegans includes cholinergic, dopaminergic and GABAergic degeneration, altered behaviour, and increased SKN-1 activity. Neurotox Res 37:1018–1028
Klionsky DJ, Abdel-Aziz AK, Abdelfatah S et al (2021) Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition). Autophagy 17:1–382
Krum BN Jr, Martins AC, Queirós L et al (2021) Haloperidol interactions with the dop-3 receptor in Caenorhabditis elegans. Mol Neurobiol 58:304–316
Lima TRR,Lima EdO, Delafiori J et al (2022) Molecular Signatures Associated with Diuron Exposure on Rat Urothelial Mitochondria Toxicology Mechanisms and Methods 32(8):628–635
Meléndez A, Tallóczy Z, Seaman M et al (2003) Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science 301:1387–1391
Meyer D, Williams PL (2014) Toxicity testing of neurotoxic pesticides in Caenorhabditis elegans. J Toxicol Environ Health B Crit Rev 17:284–306
Meyer JN, Leung MCK, Rooney JP et al (2013) Mitochondria as a target of environmental toxicants. Toxicol Sci 134:1–17
Mohammed AM, Huovinen M, Vähäkangas KH (2020) Toxicity of diuron metabolites in human cells. Environ Toxicol Pharmacol 78
Mugova F, Read DS, Riding MJ, Martin FL, Tyne W, Svendsen C, Spurgeon D (2018) Phenotypic responses in Caenorhabditis elegans following chronic low-level exposures to inorganic and organic compounds. Environ Toxicol Chem 37:920–930
Nascimento MG, de Oliveira MLCS, Lima AS et al (2006) Effects of diuron [3-(3,4-dichlorophenyl)-1,1-dimethylurea] on the urinary bladder of male Wistar rats. Toxicology 224:66–73
Neury‐Ormanni J, Doose C, Majdi N, Vedrenne J, Morin S, Höss S, Traunspurger W (2019) Tolerance of free‐living nematode species to imidacloprid and diuron. Invert Biol 138
Orrenius S, Kaminskyy VO, Zhivotovsky B (2013) Autophagy in toxicology: cause or consequence? Annu Rev Pharmacol Toxicol 53:275–297
Osman HC, Sedore CA, Jackson EG et al (2021) Caenorhabditis intervention testing program: the herbicide diuron does not robustly extend lifespan in nematodes. Micro Publication Biology
Queirós L, Martins AC, Krum BN et al (2021) Assessing the neurotoxicity of the carbamate methomyl in Caenorhabditis elegans with a multi-level approach. Toxicol 451
Refai OD, Blakely R (2019) Blockade and reversal of swimming-induced paralysis in C. elegans by the antipsychotic and D2-type dopamine receptor antagonist azaperone. Neurochem Int 123:59–68
Roubicek DA, de Souza-Pinto NC (2017) Mitochondria and mitochondrial DNA as relevant targets for environmental contaminants. Toxicology 391:100–108
Sawin ER, Ranganathan R, Horvitz HR (2000) C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 26:619–631
Sharma A, Kumar V, Shahzad B et al (2019) Worldwide pesticide usage and its impacts on ecosystem. SN Applied Sciences 1:1446
Simões MdS, Bracht L, Parizotto AV et al (2017) The metabolic effects of diuron in the rat liver. Environ Toxicol Pharmacol 53–61
Smith PK, Krohn RI, Hermanson GT et al (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85
Souza NP, Lima TRR, Pereira LC et al (2020) AOP for urothelial carcinogenesis due to chemical cytotoxicity by mitochondrial impairment. Available in: https://aopwiki.org/aops/335.
Sulston J, Brenner S (1975) Dopaminergic neurons in the nematode Caenorhabditis elegans. Comp Neurol 163:215–226
Sulston J, Hodgkin J (1988) Methods. The Nematode Caenorhabditis elegans. Cold Spring Harbor Monographs
Tejeda-Benitez L, Olivero-Verbel J (2016) Caenorhabditis elegans, a biological model for research in toxicology. In: de Voogt W (ed) Reviews of environmental contamination and toxicology Springer, Cham
U.S. EPA (2003) Registration eligibility decision for diuron. List A. Case 0046. United States Environmental Protection Agency. Washington, DC: Office of prevention, pesticides and toxic substances
Vinod V, Padmakrishnan CJ, Vijayan B et al (2014) How can I halt thee? The puzzles involved in autophagic inhibition. Pharmacol Res 82:1–8
Xing H, Wang Z, Gao X et al (2015) Atrazine and chlorpyrifos exposure induces liver autophagic response in common carp. Ecotoxicol Environ Saf 113:52–58
Yoon DS, Lee M-H, Cha DS (2018) Measurement of intracellular ROS in Caenorhabditis elegans using 2’,7’-dichlorodihydrofluorescein diacetate. Bio-Protoc 8:e2774
Zhou Y, Han X, Bao Y et al (2021) Chronic exposure to environmentally realistic levels of diuron impacts the behaviour of adult marine medaka (Oryzias melastigma). Aquat Toxicol 238:105917
Acknowledgements
The authors are grateful to Breno Pannia Espósito (Institute of Chemistry, University of São Paulo, Brazil) for technical assistance.
Funding
This work was supported by the São Paulo Research Foundation (FAPESP) [Grant No. 2017/25402–5] and the Coordination for the Improvement of Higher Education Personnel (CAPES) [CAPES-PrInt Grant No. 88887.467311/2019–00].
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design, as well as review of the manuscript. Data collection and analysis were performed by Thania Rios Rossi Lima and Airton C. Martins. The first draft of the manuscript was written by Thania Rios Rossi Lima and all authors have approved the final version of the manuscript and assume public responsibility for its content.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Competing Interests
This study was carried out at Albert Einstein College of Medicine, Bronx, NY, USA. After its completion, TRRL assumed job position in a private company. The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lima, T.R.R., Martins, A.C., Pereira, L.C. et al. Toxic Effects Induced by Diuron and Its Metabolites in Caenorhabditis elegans. Neurotox Res 40, 1812–1823 (2022). https://doi.org/10.1007/s12640-022-00596-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-022-00596-2