Abstract
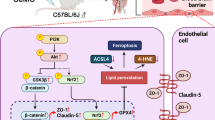
Acetazolamide (AZA) is a carbonic anhydrase inhibitor (CAI) with neuroprotective effects. Hyperhomocysteinemia is associated with blood–brain-barrier (BBB) disruption in brain disorders. A previous study indicated that AZA might have a new role in brain disorders. However, its function in hyperhomocysteinemia-related BBB disruption has not been reported. Here, we aim to clarify the role of AZA in homocysteine (Hcy)-mediated BBB dysfunction using both in vivo and in vitro assays. We found that AZA improved memory and cognitive function, and reduced brain edema in Hcy-stimulated hyperhomocysteinemia model rats. This protective effect of AZA on hyperhomocysteinemia rats was accompanied by improved BBB permeability and increased expression levels of the tight junction proteins, occludin, and claudin-5. The in vitro assay results show that AZA prevented Hcy-induced cell injury and attenuated the increased permeability in Hcy-treated bEnd.3 brain endothelial cells. The Hcy-induced decrease in occludin and claudin-5, and increase in MMP-2 and MMP-9 expression levels were attenuated by AZA in bEnd.3 cells. Moreover, the Hcy-induced downregulation of the Wnt/β-catenin signaling pathway in bEnd.3 cells was abolished by AZA. Inhibition of Wnt/β-catenin by ICG-001 reversed the protective effects of AZA in Hcy-treated bEnd.3 cells. We also prove that this process is mediated by WTAP. These findings suggest that acetazolamide mitigated the Hcy-induced compromised brain vascular endothelial integrity by regulating the activation of the Wnt/β-catenin signaling pathway.








Similar content being viewed by others

Data Availability
Data of this study is available upon reasonable request to the corresponding authors.
References
Abdullahi W, Tripathi D, Ronaldson PT (2018) Blood-brain barrier dysfunction in ischemic stroke: targeting tight junctions and transporters for vascular protection. Am J Physiol Cell Physiol 315(3):C343–C356
Bayat Mokhtari R, Baluch N, Ka Hon Tsui M et al (2017) Acetazolamide potentiates the anti-tumor potential of HDACi, MS-275, in neuroblastoma. BMC Cancer 17(1):156
Cai Z, Hussain MD, Yan LJ (2014) Microglia, neuroinflammation, and beta-amyloid protein in Alzheimer’s disease. Int J Neurosci 124(5):307–321
Cai Z, Qiao PF, Wan CQ, Cai M, Zhou NK, Li Q (2018) Role of blood-brain barrier in Alzheimer’s disease. J Alzheimers Dis 63(4):1223–1234
Cummings JL (2004) Alzheimer’s disease. N Engl J Med 351(1):56–67
Dubchenko E, Ivanov A, Spirina N, Smirnova N, Melnikov M, Boyko A, Gusev E, Kubatiev A (2020) Hyperhomocysteinemia and endothelial dysfunction in multiple sclerosis. Brain Sci 10(9)
Guo F, Hua Y, Wang J, Keep RF, Xi G (2012) Inhibition of carbonic anhydrase reduces brain injury after intracerebral hemorrhage. Transl Stroke Res 3(1):130–137
Kamat PK, Kyles P, Kalani A, Tyagi N (2016) Hydrogen sulfide ameliorates homocysteine-induced Alzheimer’s disease-like pathology, blood-brain barrier disruption, and synaptic disorder. Mol Neurobiol 53(4):2451–2467
Kim J, Kim H, Roh H, Kwon Y (2018) Causes of hyperhomocysteinemia and its pathological significance. Arch Pharm Res 41(4):372–383
Hao JQ, He XY, Yang X, Xiao YC, Duan SQ, Wang H, Bai H, Zhang Y, Shi JY, Zhu XL et al (2022) Acetazolamide alleviate cerebral edema induced by ischemic stroke through inhibiting the expression of AQP4 mRNA. Neurocrit Care 36(1):97–105
Haseloff RF, Dithmer S, Winkler L, Wolburg H, Blasig IE (2015) Transmembrane proteins of the tight junctions at the blood-brain barrier: structural and functional aspects. Semin Cell Dev Biol 38:16–25
Hermann A, Sitdikova G (2021) Homocysteine: biochemistry, molecular biology and role in disease. Biomolecules 11(5)
Hohsfield LA, Humpel C (2010) Homocysteine enhances transmigration of rat monocytes through a brain capillary endothelial cell monolayer via ICAM-1. Curr Neurovasc Res 7(3):192–200
Hooshmand B, Solomon A, Kareholt I, Leiviska J, Rusanen M, Ahtiluoto S, Winblad B, Laatikainen T, Soininen H, Kivipelto M (2010) Homocysteine and holotranscobalamin and the risk of Alzheimer disease: a longitudinal study. Neurology 75(16):1408–1414
Hu S, Wu Y, Zhao B, Hu H, Zhu B, Sun Z, Li P, Du S (2018) Panax notoginseng saponins protect cerebral microvascular endothelial cells against oxygen-glucose deprivation/reperfusion-induced barrier dysfunction via activation of PI3K/Akt/Nrf2 antioxidant signaling pathway. Molecules 23(11)
Laksitorini MD, Yathindranath V, Xiong W, Parkinson FE, Thliveris JA, Miller DW (2021) Impact of Wnt/beta-catenin signaling on ethanol-induced changes in brain endothelial cell permeability. J Neurochem 157(4):1118–1137
Lehmann M, Gottfries CG, Regland B (1999) Identification of cognitive impairment in the elderly: homocysteine is an early marker. Dement Geriatr Cogn Disord 10(1):12–20
Liu L, Wan W, Xia S, Kalionis B, Li Y (2014) Dysfunctional Wnt/beta-catenin signaling contributes to blood-brain barrier breakdown in Alzheimer’s disease. Neurochem Int 75:19–25
Liebner S, Dijkhuizen RM, Reiss Y, Plate KH, Agalliu D, Constantin G (2018) Functional morphology of the blood-brain barrier in health and disease. Acta Neuropathol 135(3):311–336
Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M et al (2008) Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol 183(3):409–417
Lubec B, Labudova O, Hoeger H, Muehl A, Fang-Kircher S, Marx M, Mosgoeller W, Gialamas J (1996) Homocysteine increases cyclin-dependent kinase in aortic rat tissue. Circulation 94(10):2620–2625
Miller JW (1999) Homocysteine and Alzheimer’s disease. Nutr Rev 57(4):126–129
Miller JW (2000) Homocysteine, Alzheimer’s disease, and cognitive function. Nutrition 16(7–8):675–677
Ozcan O, Cosar A (2013) Homocysteine and vitamin B12 levels related to MRI white matter abnormalities in Parkinson’s disease dementia. Neurodegener Dis 12(3):164
Solesio ME, Peixoto PM, Debure L, Madamba SM, de Leon MJ, Wisniewski T, Pavlov EV, Fossati S (2018) Carbonic anhydrase inhibition selectively prevents amyloid beta neurovascular mitochondrial toxicity. Aging Cell 17(4):e12787
Song S, Huang H, Guan X, Fiesler V, Bhuiyan MIH, Liu R, Jalali S, Hasan MN, Tai AK, Chattopadhyay A et al (2021) Activation of endothelial Wnt/beta-catenin signaling by protective astrocytes repairs BBB damage in ischemic stroke. Prog Neurobiol 199:101963
Sweeney MD, Sagare AP, Zlokovic BV (2018) Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol 14(3):133–150
Tawfik A, Samra YA, Elsherbiny NM, Al-Shabrawey M (2020) Implication of hyperhomocysteinemia in blood retinal barrier (BRB) dysfunction. Biomolecules 10(8)
Uldall M, Botfield H, Jansen-Olesen I, Sinclair A, Jensen R (2017) Acetazolamide lowers intracranial pressure and modulates the cerebrospinal fluid secretion pathway in healthy rats. Neurosci Lett 645:33–39
Van Berkel MA, Elefritz JL (2018) Evaluating off-label uses of acetazolamide. Am J Health Syst Pharm 75(8):524–531
Wu X, Zhang L, Miao Y, Yang J, Wang X, Wang CC, Feng J, Wang L (2019) Homocysteine causes vascular endothelial dysfunction by disrupting endoplasmic reticulum redox homeostasis. Redox Biol 20:46–59
Williamson MR, Wilkinson CM, Dietrich K, Colbourne F (2019) Acetazolamide mitigates intracranial pressure spikes without affecting functional outcome after experimental hemorrhagic stroke. Transl Stroke Res 10(4):428–439
Xi G, Keep RF, Hoff JT (1998) Erythrocytes and delayed brain edema formation following intracerebral hemorrhage in rats. J Neurosurg 89(6):991–996
Yang Y, Rosenberg GA (2011) Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke 42(11):3323–3328
Yang Y, Rosenberg GA (2015) Matrix metalloproteinases as therapeutic targets for stroke. Brain Res 1623:30–38
Yen LF, Wei VC, Kuo EY, Lai TW (2013) Distinct patterns of cerebral extravasation by Evans blue and sodium fluorescein in rats. PLoS ONE 8(7):e68595
Zhang S, Leske DA, Lanier WL, Berkowitz BA, Holmes JM (2001) Preretinal neovascularization associated with acetazolamide-induced systemic acidosis in the neonatal rat. Invest Ophthalmol vis Sci 42(5):1066–1071
Zhang J, Tsoi H, Li X, Wang H, Gao J, Wang K, Go MY, Ng SC, Chan FK, Sung JJ, Yu J (2016) Carbonic anhydrase IV inhibits colon cancer development by inhibiting the Wnt signalling pathway through targeting the WTAP-WT1-TBL1 axis. Gut 65:1482–1493
Zhao B, Zhu J, Fei Y, Yin Q, Shen W, Liang B, Zhu X, Li Y (2020) JLX001 attenuates blood-brain barrier dysfunction in MCAO/R rats via activating the Wnt/beta-catenin signaling pathway. Life Sci 260:118221
Acknowledgements
This work was supported by Medical Scientific Research Foundation of Guangdong Province (A2022491) and Climb Plan Foundation of Guangzhou Eighth People’s Hospital, Guangzhou Medical University.
Author information
Authors and Affiliations
Contributions
Chuo Li made substantial contribution to experimental design and data analysis; Chuo Li and Bo Zhang made substantial contribution to investigation and data collection; Chuo Li drafted the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Consent for Publication
All the authors have read and approved the final submission of this study.
Conflict of Interest/Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, C., Zhang, B. The Protective Effects of Acetazolamide Against Homocysteine-Induced Blood–Brain-Barrier Disruption by Regulating the Activation of the Wnt/β-Catenin Signaling Pathway. Neurotox Res 40, 1261–1271 (2022). https://doi.org/10.1007/s12640-022-00551-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-022-00551-1