Abstract
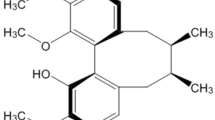
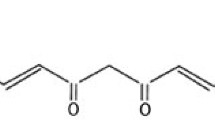
Endoplasmic reticulum stress (ERS) and mitochondrial dysfunction have been suggested to relate with the pathology of Alzheimer’s disease (AD). However, their cross-talk is needed to investigate further. Mitofusin-2 (Mfn2) is a member of mitochondria-associated membrane (MAM), which connects endoplasmic reticulum (ER) and mitochondria. This study investigated the protective effect of curcumin on thapsigargin (TG)-induced ERS and cell apoptosis and the role of Mfn2 on mitochondrial dysfunction. The cell viability of SH-SY5Y cells was decreased and cell damage and apoptosis were increased in a concentration-dependent manner when cells were treated with TG. TG upregulated the protein levels of GRP78, pSer981-PERK, and pSer51-eIF2α. Curcumin attenuated TG-induced damage on cell viability and apoptosis and downregulated the protein levels of GRP78, pSer981-PERK, and pSer51-eIF2α. TG caused the increases in intracellular reactive oxygen species (ROS) and in the protein levels of pSer40-Nrf2 and hemoglobin oxygenase 1 (HO-1). Curcumin decreased the TG-induced intracellular ROS but did not alter the protein levels of pSer40-Nrf2 and HO-1. TG resulted in the upregulation on Mfn2 expression and mitochondrial spare respiratory capacity but the downregulation on mitochondrial basal respiration and ATP production. Curcumin attenuated the TG-induced Mfn2 expression and mitochondrial stress. When Mfn2 was silenced by shRNA interference, curcumin failed to recovery the TG-damaged mitochondrial function. In general, the TG-induced ERS trigged mitochondrial dysfunction and cell apoptosis. Curcumin attenuates TG-induced ERS and the cell damage and apoptosis. Mfn2 is required for curcumin’s protection against the TG-induced damage on mitochondrial functions.






Similar content being viewed by others
References
Akman M, Belisario DC, Salaroglio IC, Kopecka J, Donadelli M, De Smaele E, Riganti C (2021) Hypoxia, endoplasmic reticulum stress and chemoresistance: dangerous liaisons. J Exp Clin Cancer Res 40(1):28
Almanza A, Carlesso A, Chintha C, Creedican S, Doultsinos D, Leuzzi B, Luis A, McCarthy N, Montibeller L, More S, Papaioannou A, Puschel F, Sassano ML, Skoko J, Agostinis P, de Belleroche J, Eriksson LA, Fulda S, Gorman AM, Healy S, Kozlov A, Munoz-Pinedo C, Rehm M, Chevet E, Samali A (2019) Endoplasmic reticulum stress signalling - from basic mechanisms to clinical applications. FEBS J 286(2):241–278
Bobak Y, Kurlishchuk Y, Vynnytska-Myronovska B, Grydzuk O, Shuvayeva G, Redowicz MJ, Kunz-Schughart LA, Stasyk O (2016) Arginine deprivation induces endoplasmic reticulum stress in human solid cancer cells. Int J Biochem Cell Biol 70:29–38
Cao SS, Kaufman RJ (2014) Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid Redox Signal 21(3):396–413
De Brito OM, Scorrano L (2008) Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456(7222):605–610
Devi L, Ohno M (2014a) PERK mediates eIF2 alpha phosphorylation responsible for BACE1 elevation, CREB dysfunction and neurodegeneration in a mouse model of Alzheimer’s disease. Neurobiol Aging 35(10):2272–2281
Devi L, Ohno M (2014b) PERK mediates eIF2alpha phosphorylation responsible for BACE1 elevation, CREB dysfunction and neurodegeneration in a mouse model of Alzheimer’s disease. Neurobiol Aging 35(10):2272–2281
High S, Greenfield JJ, Meacock SL, Oliver JD (1999) Membrane-protein biosynthesis at the endoplasmic reticulum. Biochem Soc Trans 27(6):883–888
Huang HC, Chang P, Lu SY, Zheng BW, Jiang ZF (2015a) Protection of curcumin against amyloid-beta-induced cell damage and death involves the prevention from NMDA receptor-mediated intracellular Ca2+ elevation. J Recept Signal Transduct Res 35(5):450–457
Huang HC, Tang D, Lu SY, Jiang ZF (2015b) Endoplasmic reticulum stress as a novel neuronal mediator in Alzheimer’s disease. Neurol Res 37(4):366–374
Huang HC, Tang D, Xu K, Jiang ZF (2014) Curcumin attenuates amyloid-beta-induced tau hyperphosphorylation in human neuroblastoma SH-SY5Y cells involving PTEN/Akt/GSK-3beta signaling pathway. J Recept Signal Transduct Res 34(1):26–37
Huang HC, Xu K, Jiang ZF (2012) Curcumin-mediated neuroprotection against amyloid-beta-induced mitochondrial dysfunction involves the inhibition of GSK-3beta. J Alzheimers Dis 32(4):981–996
Huang HC, Zheng BW, Guo Y, Zhao J, Zhao JY, Ma XW, Jiang ZF (2016) Antioxidative and neuroprotective effects of curcumin in an Alzheimer’s disease rat model co-treated with intracerebroventricular streptozotocin and subcutaneous D-galactose. J Alzheimers Dis 52(3):899–911
Jacquemyn J, Cascalho A, Goodchild RE (2017) The ins and outs of endoplasmic reticulum-controlled lipid biosynthesis. EMBO Rep 18(11):1905–1921
Jaskulska A, Janecka AE, Gach-Janczak K (2020) Thapsigargin-from traditional medicine to anticancer drug. Int J Mol Sci 22(1)
Larrea D, Pera M, Gonnelli A, Quintana-Cabrera R, Akman HO, Guardia-Laguarta C, Velasco KR, Area-Gomez E, Dal Bello F, De Stefani D, Horvath R, Shy ME, Schon EA, Giacomello M (2019) MFN2 mutations in Charcot-Marie-Tooth disease alter mitochondria-associated ER membrane function but do not impair bioenergetics. Hum Mol Genet 28(11):1782–1800
Lee W, Kim DH, Boo JH, Kim YH, Park IS, Mook-Jung I (2005) ER stress-induced caspase-12 activation is inhibited by PKC in neuronal cells. Apoptosis 10(2):407–415
Li JQ, Yu JT, Jiang T, Tan L (2015) Endoplasmic reticulum dysfunction in Alzheimer’s disease. Mol Neurobiol 51(1):383–395
Liang T, Xue F, Hang W, Wen B, Zhang Q, Chen J, Liu X, Chen J (2019) Neuron-specific apolipoprotein E4 (1–272) fragment induces tau hyperphosphorylation and axonopathy via triggering endoplasmic reticulum stress. J Alzheimers Dis 71(2):597–611
Michalak M, Robert Parker JM, Opas M (2002) Ca2+ signaling and calcium binding chaperones of the endoplasmic reticulum. Cell Calcium 32(5–6):269–278
Mueller M, Ahumada-Castro U, Sanhueza M, Gonzalez-Billault C, Court FA, Cardenas C (2018) Mitochondria and calcium regulation as basis of neurodegeneration associated with aging. Front Neurosci 12:470
Naik SR, Thakare VN, Patil SR (2011) Protective effect of curcumin on experimentally induced inflammation, hepatotoxicity and cardiotoxicity in rats: evidence of its antioxidant property. Exp Toxicol Pathol 63(5):419–431
Naon D, Zaninello M, Giacomello M, Varanita T, Grespi F, Lakshminaranayan S, Serafini A, Semenzato M, Herkenne S, Hernandez-Alvarez MI, Zorzano A, De Stefani D, Dorn GW 2nd, Scorrano L (2016) Critical reappraisal confirms that Mitofusin 2 is an endoplasmic reticulum-mitochondria tether. Proc Natl Acad Sci U S A 113(40):11249–11254
Oakes SA, Papa FR (2015) The role of endoplasmic reticulum stress in human pathology. Annu Rev Pathol 10:173–194
Pobre KFR, Poet GJ, Hendershot LM (2019) The endoplasmic reticulum (ER) chaperone BiP is a master regulator of ER functions: getting by with a little help from ERdj friends. J Biol Chem 294(6):2098–2108
Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8(7):519–529
Sagara Y, Wade JB, Inesi G (1992) A conformational mechanism for formation of a dead-end complex by the sarcoplasmic reticulum ATPase with thapsigargin. J Biol Chem 267(2):1286–1292
Sakai S, Watanabe S, Komine O, Sobue A, Yamanaka K (2021) Novel reporters of mitochondria-associated membranes (MAM), MAMtrackers, demonstrate MAM disruption as a common pathological feature in amyotrophic lateral sclerosis. FASEB J 35(7):e21688
Schwarz DS, Blower MD (2016) The endoplasmic reticulum: structure, function and response to cellular signaling. Cell Mol Life Sci 73(1):79–94
Sehgal P, Szalai P, Olesen C, Praetorius HA, Nissen P, Christensen SB, Engedal N, Moller JV (2017) Inhibition of the sarco/endoplasmic reticulum (ER) Ca(2+)-ATPase by thapsigargin analogs induces cell death via ER Ca(2+) depletion and the unfolded protein response. J Biol Chem 292(48):19656–19673
Senft D, Ronai ZA (2015) UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends Biochem Sci 40(3):141–148
Tadini-Buoninsegni F, Smeazzetto S, Gualdani R, Moncelli MR (2018) Drug interactions with the Ca(2+)-ATPase from sarco(endo)plasmic reticulum (SERCA). Front Mol Biosci 5:36
Walter P, Ron D (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 334(6059):1081–1086
Wang J, Lu L, Chen S, Xie J, Lu S, Zhou Y, Jiang H (2020a) PERK overexpression-mediated Nrf2/HO-1 pathway alleviates hypoxia/reoxygenation-induced injury in neonatal murine cardiomyocytes via improving endoplasmic reticulum stress. Biomed Res Int 2020:6458060
Wang J, Lu L, Chen S, Xie J, Lu S, Zhou Y, Jiang H (2020b) Up-regulation of PERK/Nrf2/HO-1 axis protects myocardial tissues of mice from damage triggered by ischemia-reperfusion through ameliorating endoplasmic reticulum stress. Cardiovasc Diagn Ther 10(3):500–511
Wang K, Zhang W (2021) Mitochondria-associated endoplasmic reticulum membranes: at the crossroad between familiar and sporadic Alzheimer’s disease. Synapse 75(6):e22196
Wang W, Xie Q, Zhou X, Yao J, Zhu X, Huang P, Zhang L, Wei J, Xie H, Zhou L, Zheng S (2015) Mitofusin-2 triggers mitochondria Ca2+ influx from the endoplasmic reticulum to induce apoptosis in hepatocellular carcinoma cells. Cancer Lett 358(1):47–58
Wang Y, Yin H, Wang L, Shuboy A, Lou J, Han B, Zhang X, Li J (2013) Curcumin as a potential treatment for Alzheimer’s disease: a study of the effects of curcumin on hippocampal expression of glial fibrillary acidic protein. Am J Chin Med 41(1):59–70
Wu S, Kaufman RJ (2004) Trans-autophosphorylation by the isolated kinase domain is not sufficient for dimerization or activation of the dsRNA-activated protein kinase PKR. Biochemistry 43(34):11027–11034
Wu Y, Chen Q, Wen B, Wu N, He B, Chen J (2021) Berberine reduces Abeta42 deposition and tau hyperphosphorylation via ameliorating endoplasmic reticulum stress. Front Pharmacol 12:640758
Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen PP, Kayed R, Glabe CG, Frautschy SA, Cole GM (2005) Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem 280(7):5892–5901
Funding
This study was supported by the Academic Research Projects of Beijing Union University (JZ10202001, XP202008, and ZK70202101).
Author information
Authors and Affiliations
Contributions
Han-Chang Huang: leadership responsibility for the research, design on research goals and methodology, and writing original draft; He-Yan Zhou: conducting investigation process and data curation; Yu-Ying Sun: data validation and statistical analysis and revision on draft. Ping Chang: preparation of study materials, reagents, and materials.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, HY., Sun, YY., Chang, P. et al. Curcumin Inhibits Cell Damage and Apoptosis Caused by Thapsigargin-Induced Endoplasmic Reticulum Stress Involving the Recovery of Mitochondrial Function Mediated by Mitofusin-2. Neurotox Res 40, 449–460 (2022). https://doi.org/10.1007/s12640-022-00481-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-022-00481-y