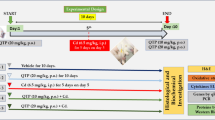
Abstract
Cadmium (Cd) is a toxic environmental contaminant, which bio-accumulate in animals through the food chain. Cerebellum is one of the primary target organs for Cd exposure. In this study, we established a chronic Cd exposure model; 60 chickens were treated with Cd (0 mg/kg, 35 mg/kg, 70 mg/kg) for 90 days. Clinical manifestations indicated that the chicken was depressed and has unstable gait under Cd exposure. Histopathological results indicated that Cd induced neuronal shrunken and indistinct nucleoli, and the number of Purkinje cells decreased significantly. Cerebellar metal contents were analyzed by ICP-MS. We found that Cd caused Cd and Cu accumulation and decreased the content of Se, Fe, and Zn, suggesting that Cd disturbed metal homeostasis. Besides, Cd treatment group also showed high levels of malondialdehyde (MDA) and hydrogen peroxide (H2O2) content and inhibited selenoprotein transcriptome, suggesting that Cd exposure resulted in oxidative stress. Notably, low-dose Cd exposure activated MTF1 mRNA and protein expression and its target metal-responsive genes, including MT1, MT2, DMT1, ZIP8, ZIP10, TF, and ATP7B which indicate cellular adaptive response against Cd-induced damage. On the other hand, 70 mg/kg Cd downregulated MTF1-mediated metal response, which was involved in Cd-induced cerebellar injury in chicken. In conclusion, our data demonstrated that molecular mechanisms are associated with Cd-induced cerebellar injury due to disturbing MTF1-mediated metal response.
Graphical abstract
This study indicated that the cerebellum is one of the target organs of Cd-induced toxicity. Additionally, Cd exposure induced metal dyshomeostasis in chicken’s cerebellum, whereas this study found that lower level of Cd dose triggered the activation of the cytoprotective mechanism through activating the expression of MTF1 which regulate MT1, MT2, DMT1, ZIP8, ZIP10, TF, and ATP7B expressions in cerebellum. However, MTF1-mediated metal response was inhibited under the exposure of high dose of Cd, which ultimately caused cerebellar injury. The present study provides a new insight that Cd through disturbed MTF1-mediated metal response disrupts metal homeostasis that induced cerebellar injury.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is a widespread hazardous pollutant that has a long biological half-life in humans (20–30 years) (Satarug et al. 2010). Metallurgy and the plastic industry, mining, and battery production released Cd into the environment that polluted agricultural land and food (Anetor 2012). Cd through the food chain accumulates into the kidney, liver, and other organs and resulted in irrevocable injury to the target organs; moreover, Cd exposure also caused certain carcinogenic and increased non-carcinogenic health risk (Wang et al. 2021). China paid high attention to food security issues that caused by Cd (Feng et al. 2020). Notably, Cd has been linked to the development of neurological diseases via triggering oxidative stress, which caused cellular damage and altered gene transcription, which ultimately led to cell apoptosis (Jomova and Valko 2011; Lopez et al. 2003). Moreover, it has been confirmed that Cd can cause nervous system injury and neurological disorders that lead to learning disabilities and cognitive dysfunction (Karri et al. 2016). It is worth noting that selenium (Se) can reduce the accumulation of Cd in the brain of chickens and reduce oxidative stress and histopathological damage (Liu et al. 2014). Previous study has shown that exogenous Se addition inhibits the absorption of Cd (Costa et al. 2020), suggesting Se might reduce the concentration of Cd and thereby reduce the toxicity of Cd. Importantly, selenoprotein was associated with antioxidant roles and promote the growth of nerve cells (Adedara et al. 2020). However, the effect of Cd toxicity on the nervous system has not been comprehensively investigated, especially in cerebellum.
It is worth noting that metal transcription factor 1 (MTF1) is the indicator that can respond higher and lower levels of metal content and protects cells against oxidative and hypoxic stresses (Park and Jeong 2018). MTF1 regulates expression of its target genes in response to heavy metal load, through binding metal response elements (MREs) in the respective enhancer/promoter regions; in addition, it plays a protective role in the oxidative stress response (Wimmer et al. 2005). Former researchers have proposed that low affinity interactions between zinc and specific zinc fingers in MTF-1 reversibly regulate its binding to the metal response elements in the mouse metallothionein-I promoter. Besides, MTF1 can activate metallothionein expression in response to the heavy metals Cd (Smirnova et al. 2000). Previous study indicated that the sturdy induction of metallothionein-1 and 2 (MT1 and MT2) genes by Zn and Cd requires the specific transcription factor MTF1 (Wimmer et al. 2005), and MTs bind and sequester metals that are involved in zinc, ferrum, and copper homeostasis and alleviating oxidative stress and adjusting metal homeostasis (Chen et al. 2020; Forcella et al. 2020).
As stated above, although some signaling pathways have been involved in Cd-induced neurotoxicity, however, the role of MTF1-mediated metal response in the underlying mechanism of Cd-induced cerebellar injury is still unclear. The present study explored the novel mechanism of Cd-induced cerebellar injury in chicken via adjusting MTF1-mediated metal response.
Materials and Methods
Study Approval and Reagents
The animal protocol was approved by the Animal Care and Use Committee of Northeast Agricultural University. All procedures were performed in accordance with the ethical standards of the institution. In addition, the study was in accordance with Good Laboratory Practice (Trial) published by the China Food and Drug Administration (CFDA). Furthermore, CdCl2 was provided by Tianjin Zhi-yuan Chemical Reagent Company, China; the assay kits of hydrogen peroxide (H2O2), catalase (CAT), glutathione peroxidase (GSH-PX), total superoxide dismutase (T-SOD), malondialdehyde (MDA), and total antioxidant capacity (T-AOC) contents were supplied by Nanjing Jian-cheng Bioengineering Institute, P.R. China.
Animals and Treatment
Chicks were obtained from Xian Feng Chick Farm (Harbin, P.R. China). The chicks were equally divided into 3 groups (20 chickens/group) including the control group (basic diet), low dose group (35 mg/kg CdCl2), and high dose group (70 mg/kg CdCl2). Furthermore, chicks were caged separately. All treatments were given access to water and standard chow and given by oral gavage for 3 months. Cerebellum from each chicken was cautiously divided and stored at −80 °C for subsequence experiments.
Assessments of Oxidative Stress–Related Markers
This study detected the MDA, H2O2, T-AOC, T-SOD, CAT, and GSH-PX according to the manufacture’s instruction; the optimal operating conditions were described by Zhao and Talukder et al. (Talukder et al. 2021; Zhao et al. 2018).
Histopathological Analysis
Cerebellums were fixed in 10% neutral buffered formalin solution and then processed for paraffin. Five-µm-thick sections were prepared for staining with hematoxylin and eosin (H&E) for microscopic observation. All slides of the kidneys were examined under an optical microscope at 200 × and 400 × magnifications.
Detection of Trace Elements
The contents of 5 metals, including Cd, Fe, Cu, Se, and Zn in the cerebellum, were detected using inductively coupled plasma mass spectrometry (ICP-MS). The optimal operating conditions were described by Jin et al. (2018). Procedures are described in Supplementary 1.1.
Western Blot Analysis
Protein samples were extracted from the cerebellum tissues and quantified using commercially available kits (Beyotime institute of biotechnology, P.R. China). Detailed treatment methods were performed as previously described (Zhang et al. 2019). MTF1 (1:700) antibody was purchased from Nan-Jing AnYan Biotechnology Co., Ltd; SepSecS (1:500) antibody was synthesized from Jin-Long Li Lab; GPX4 (1:800) antibody was purchased from Abclonal, USA; β-actin (1:1000, Beijing Biosynthesis Biotechnology Co., Ltd); and secondary antibody against rabbit IgG was purchased from Santa Cruz, CA.
RNA Extraction and Quantitative Real-time PCR Analysis
An animal RNAout commercial kit (3070, Tiandz, Inc, China) was used to extract total mRNA from cerebellum tissues (50 mg). The specific oligonucleotide primers sequence was designed by Oligo 7.0 and listed in Table S1. qRT-PCR program was performed by LineGene 9600 (Bioer Technology Co. Ltd). The value indicated relative mRNA expression level compared to Con by the 2−△△CT method normalized to β-actin (1 and 2).
Statistical Analysis
Statistical analysis of all data was performed using GraphPad Prism 5.1 (GraphPad Software, Inc., USA). Results were analyzed using one-way ANOVA and Tukey’s post hoc pairwise comparison. Asterisks (*) indicates statistical significance compared to the control group, *P < 0.05, **P < 0.01, and ***P < 0.001. Each experiment was performed in triplicate and data are means ± standard deviation (SD). PCoA analysis was using CANOCO 5.0 software. Correlation analysis was accomplished using R Programming Language version 4.0.2. Heatmaps were plotted using R Programming Language version 3.4.1.
Results
Effect of Cd on the Histopathological Changes in the Cerebellar Tissues
The histopathological observation of the cerebellum is shown in Fig. 1A. Cerebellar sections stained with H&E exhibited normal histological structure in the Purkinje cell layer and regular morphology in control group. Some neurons were slightly shrunken; the number of Purkinje cell decreased significantly in Cd 35 mg/kg group (P < 0.05) (Fig. 1B). On the other hand, Cd induced neuronal shrunken and indistinct nucleoli. Besides, the number of Purkinje cell decreased significantly in 70 mg/kg group (Fig. 1B) (P < 0.001). These results suggested that Cd induced cerebellar injury. As shown in Fig. 1C, dose-dependent cerebellar injury was observed as determined by through PCoA analysis.
Effect of Cd on the cerebellum histopathological changes. A The histopathological morphology in control, 35 mg/kg, and 70 mg/kg groups (H&E, 200 × , 400 ×). B The number of Purkinje cells in control, 35 mg/kg, and 70 mg/kg groups. C The PCoA analysis in control, 35 mg/kg, and 70 mg/kg groups. Notes: yellow triangle: Purkinje cells. Values are expressed as the mean ± SD. Significant differences between Cd-treated groups and control groups are denoted as follows: *P < 0.05, **P < 0.01, ***P < 0.001
Effects of Cd on Homeostasis of Trace Elements
There are 5 trace elements in chicken’s cerebellum by ICP-MS. As shown in Fig. 2A, B, the content of Cd and Cu was increased significantly, especially in 70 mg/kg group (Fig. 2A, B) (P < 0.05, P < 0.01, P < 0.001). As shown in Fig. 2C−E, the content of Se, Fe, and Zn was decreased (Fig. 2C−E) (P < 0.05, P < 0.01, P < 0.001). As shown in Fig. 2F, heatmap showed the content of 5 trace elements in cerebellum. As shown in Fig. 2G, the correlation analysis showed that Cd positively correlated with Cu. In contrast, Cd had a negative correlation with Se, Zn, and Fe. Among these elements, Cd had a negative correlation with Se (Fig. 2G) (P < 0.05).
The analysis of 5 trace elements in chicken’s cerebellum. A The content of Cadmium in chicken’s cerebellum. B The content of copper in chicken’s cerebellum. C The content of Selenium in chicken’s cerebellum. D The content of Iron in chicken’s cerebellum. E The content of Zinc in chicken’s cerebellum. F The content of Fe, Zn, Cu, Se, and Cd in chicken’s cerebellum. G Correlation analysis between Cd and Fe, Zn, Cu, and Se. From − 1 (red) to + 1 (blue) relative to correlation analysis for 5 trace elements in chicken’s cerebellum with red indicating negative correlation, blue indicating positive correlation, and white indicating no correlation. Values are expressed as the mean ± SD. Significant differences between Cd-treated groups and control groups are denoted as follows: *P < 0.05, **P < 0.01, ***P < 0.001
Effect of Cd on Oxidative Stress in Chicken’s Cerebellum
The markers of oxidative stress results showed that the oxidation products H2O2 and MDA were increased significantly, especially in 70 mg/kg group (Fig. 3A, B) (P < 0.05, P < 0.01, and P < 0.001). On the other hand, T-SOD, GSH-PX, CAT, and T-AOC level were decreased (Fig. 3C−F) (P < 0.01 and P < 0.001). Above all, these results suggested that Cd caused oxidative stress in cerebellum.
Effect of Cd on oxidative stress indices. A H2O2 content. B MDA content. C T-SOD activity. D GSH-PX activity. E CAT activity. F T-AOC level. Values are expressed as the mean ± SD. Significant differences between Cd-treated groups and control groups are denoted as follows: *P < 0.05, **P < 0.01, ***P < 0.001
Effect of Cd on Selenoprotein Transcriptome
The expressions of selenoprotein biosynthesis–related factors and selenoprotein transcriptome were tested by qRT-PCR and western blot. The results showed that the expression of selenoprotein biosynthesis–related factors and selenoprotein transcriptome decreased significantly (Fig. 4B−G, L, M) (P < 0.05, P < 0.01 and P < 0.001) along with the content of Se decreased. In addition, we demonstrated that at the protein level, the protein expression of SepSecS and Gpx4 was consistent with mRNA expression (Fig. 4I, J) (P < 0.01 and P < 0.001). Furthermore, heatmap showed that the mRNA expression of selenoprotein biosynthesis–related factors and selenoprotein transcriptome under Cd exposure in Fig. 4H, K. Above all, these results suggested that Cd inhibited selenoprotein transcriptome in the cerebellum.
Effect of Cd-induced selenoprotein biosynthetic related factors and selenoprotein expression. A The content of selenium in the cerebellum. B Relative mRNA expression of SARS. C Relative mRNA expression of PSTK. D Relative mRNA expression of SepSecS. E Relative mRNA expression of SecP43-1. F Relative mRNA expression of SPS. G Relative mRNA expression of SBP2. H The heatmap of 6 selenoprotein biosynthetic related factors. I The relative protein expression of SepSecS. J The relative protein expression of Gpx4. L, M The 24 selenoproteins mRNA expression level. K The heatmap of 24 selenoproteins mRNA expression. From − 1 (red) to + 1 (green) relative to values for chicken’s cerebellum in the Con group. The color scale represented the relative mRNA levels, with green indicating up-regulated genes, red indicating down-regulated genes, and black indicating unchanged genes. Values are expressed as the mean ± SD. Significant differences between Cd-treated groups and control groups are denoted as follows: *P < 0.05, **P < 0.01, ***P < 0.001
Effect of Cd on the MTF1-Mediated Metal Response
The result of MTF1-mediated metal response–related genes shows that Cd-induced MTF1, MT1, MT2, and DMT1 increased significantly in low-dose (35 mg/kg) Cd group, whereas high-dose Cd group was considerably decreased when compared with the control group (Fig. 5B, D−F) (P < 0.05, P < 0.01 and P < 0.001).
Effect of Cd on MTF1-mediated metal response. A The content of Cd in the cerebellum. B Relative mRNA expression of MTF1. C Relative Protein expression of MTF1. D Relative mRNA expression of MT1. E Relative mRNA expression of MT2. F Relative mRNA expression of DMT1. Values are expressed as the mean ± SD. Significant differences between Cd-treated groups and control groups are denoted as follows: *P < 0.05, **P < 0.01, ***P < 0.001
It is worth noting that the tendency expression of MTF1 protein is consistent with gene expression. Concretely, in the low-dose Cd group, it is increased significantly, whereas high Cd group was considerably decreased when compared with the control group (Fig. 5C) (P < 0.01).
Effect of Cd on Zn, Fe, and Cu Transporters
The result showed that zinc exporters including ZNT3, ZNT5, and ZNT10 mRNA expression were decreased significantly, especially in 70 mg/kg group (Fig. 6A−C) (P < 0.01 and P < 0.001), while zinc importers including ZIP8 and ZIP10 expressions were significantly increased in 35 mg/kg group when compared with the control group (Fig. 6D, E) (P < 0.001). However, the expression of the zinc importers was decreased significantly in the 70 mg/kg group when compared with the control group (Fig. 6D, E) (P < 0.05). As for Fe and Cu transporters, the result showed that Cd exposure significantly increased the level of iron importer TF and copper exporter ATP7B expression in the 35 mg/kg group when compared with the control group (Fig. 6F, H) (P < 0.001), while their expressions were significantly decreased in 70 mg/kg group (Fig. 6F, H) (P < 0.05, P < 0.001).
Effect of Cd on Zn, Fe and Cu related transporters. A Relative mRNA expression of ZNT3. B Relative mRNA expression of ZNT5. C Relative mRNA expression of ZNT10. D Relative mRNA expression of ZIP8. E Relative mRNA expression of ZIP10. F Relative mRNA expression of TF. G Relative mRNA expression of FPN1. H Relative mRNA expression of ATP7B. I Relative mRNA expression of CTR1. J Correlation analysis of MTF1-mediated metal response. From − 1 (red) to + 1 (blue) relative to correlation analysis of MTF1-mediated metal response–related genes in chicken’s cerebellum with red indicating negative correlation, blue indicating positive correlation, and white indicating no correlation. Values are expressed as the mean ± SD. Significant differences between Cd-treated groups and control groups are denoted as follows: *P < 0.05, **P < 0.01, ***P < 0.001
However, the expression of iron exporter FPN1 and copper importer CTR1 was considerably decreased, especially in 70 mg/kg group (Fig. 6G, I) (P < 0.01 and P < 0.001). Furthermore, the result of correlation analysis between MTF1 and Zn-, Fe-, and Cu-related transporters showed that MTF1 had a positive correlation with Zn-, Fe-, and Cu-related transporters. Among them, MTF1 had a significant positive correlation with MT1 (Fig. 6J) (P < 0.001), ZIP8, and ATP7B (Fig. 6J) (P < 0.05).
Discussion
Cd is an environmental contaminant, which threatens the health of human beings and wild animals (Berglund and Nyholm 2011; Zhu et al. 2018). It can pass the blood–brain barrier (BBB) and enter the brain, causing neurotoxic effects (Mendez-Armenta et al. 2001). Besides, a recent study has shown that Cd exposure can cause neuronal death (Wei et al. 2015). Notably, Cd-induced neurotoxicity includes cell death, receptor dysfunction, and behavioral changes (Wang et al. 2014). Former researches have proposed that Cd exposure decreased cerebellar cell viability and caused degeneration in cortical neurons in the rat brain (Varmazyari et al. 2020). Thus, these studies suggested that Cd through destructing the normal histological structure induced neuro-organ injury. The result of this study found that Cd induced neuronal shrunken, darkly stained pyknotic neurons; the number of Purkinje cell decreased, and dendrites of neurons were observed, which indicate that Cd caused injury in cerebellar neurons.
Cerebellum is one of the major nervous organs that maintain coordination in animals. Notably, previous study indicated that higher Cd accumulation was found in cerebellum when compared with cerebellum in peacock blennies (Naija et al. 2017). Furthermore, it has been reported that with the dose of Cd increased, the content of Cd and Pb was also increased, while the contents of Se, Cr, and Fe were decreased in chicken’s pectorals (Qu et al. 2020). Cd not only can interfere the capability of cells to manage transition metals and disturb the homeostatic (Ammendola et al. 2014), but also disrupt the homeostasis of trace elements that resulted in oxidative damage in chicken’s kidney (Ge et al. 2019). It is worth noting that Cd exposure significantly increased the content of Cd in the chicken’s brain (Zhang et al. 2016). Consistent with previous study, in this study, we had detected 5 elements by ICP-MS in chicken’s cerebellum; the results found that the content of Cd and Cu was enhanced in cerebellum, while Cd treatment significantly reduced the contents of Se, Zn, and Fe in the 35mg/kg group; this situation gets more serious in the 70mg/kg group. These findings suggested that Cd disordered metal homeostasis in chicken cerebellum.
Se is a wholesome trace element that plays a critical role in biological antioxidative metabolism process (Xiong et al. 2020). Besides, Se is an integral part of some enzymes including glutathione peroxidases, deiodinases, and thioredoxin reductases (Zoidis et al. 2018). It could prevent oxidative injury of toxins (Zhang et al. 2020a). Se not only has positive effects of animal, such as resistance to oxidative damage and reduction of chronic inflammatory (Dehkordi et al. 2017), but also have a strong function that against Cd triggered oxidative stress. Notably, previous study also documented that Cd exposure depleted the content of Se in chicken’s kidney (Ge et al. 2019). Consistent with previous research, this study found that chronic Cd treatment obviously decreased Se content and inhibited selenoprotein transcriptome and increased H2O2 and MDA contents, while antioxidative stress indexes decreased in chicken’s cerebellum. These results suggest that Cd caused oxidative stress in chicken’s cerebellum.
MTF1 is a transcription factor that not only regulates metal transporters including Zn, Fe, and Cu (Cuillel et al. 2014; Fu et al. 2017b; Nemmiche and Guiraud 2016), but also can induce the expression of MTs. MTs are a family of stress proteins that are involved in the process of detoxification and anti-oxidation (Sheng et al. 2015). Besides, MTs are known to protect cells against oxidative stress, especially providing protection against Cd toxicity (Shen et al. 2019). Furthermore, MTs play an essential role in protection against Cd toxicity in snails (Dvorak et al. 2019). Former researches have proposed that MTs participate in the homeostasis of Zn, Fe, and Cu and thus protecting metal toxicity and oxidative damage (Chen et al. 2011; Menezo et al. 2011). It is worth noting that the expressions of MT and MTF1 proteins were firstly found in the cattle group which had low Cd concentration in cattle kidney and liver tissues (Buranasinsup et al. 2011). Besides, MT1 and MT2 expressions were induced in liver and lung cells even upon low-dose Cd exposure (Shen et al. 2019). It has been reported that MTF1 is a molecular target of Cd-induced cerebral toxicity (Talukder et al. 2021). Thus, MTF1 and MTs are involved in Cd toxicity. In keeping with previous study, this study showed that Cd activated the MTF1 expression along with the increase of MTs in low-dose Cd exposure, whereas high-dose Cd inhibited MTs and MTF1, which suggest that MTF1 can be activated by low-dose Cd, which induced MT expression.
Increasing evidence suggests that changes in the activity of specific Zn transporters might play a role in regulating the general Zn homeostasis (Cai et al. 2018). Notably, zinc transporters are crucial for the MTF1-mediated metal response (Grzywacz et al. 2015; Muraina et al. 2020). The main function of the ZNT family is to promote the transfer of Zn from cytoplasm into the extracellular, as well as the compartmentalization of zinc in various organelles to reduce the content of cytoplasmic zinc for detoxification (Palmiter and Findley 1995). Besides, the function of ZIP family is to promote the transfer of Zn from extracellular into the cytoplasm that maintain the homeostasis of zinc (Troche et al. 2015). It has been reported that Cd accumulation within the cell induced Zn starvation, which may be due to Cd compete with Zn for the metal binding site (Ammendola et al. 2014). Notably, previous study also documented that Cd-induced alterations of Zn homeostasis in rat hippocampal neurons, lead to the hippocampal neurons injury, and Zn supplementation able to partial protection against Cd neurotoxicity (Ben Mimouna et al. 2019). Consistent with previous study, in this study, the result showed that Cd exposure led to reduction in Zn level, and low-dose Cd activated the MTF1 protein expression level along with the increase of MTs and ZIP expression, which suggests that MTF1-mediated metal response was activated by low-dose Cd, which tries to adjust Zn homeostasis.
Fe metabolism disorder plays a key role in neurodegeneration (Bi et al. 2020). Transferrin (TF) and ferroportin 1 (FPN1) are the MTF1-regulated proteins, which are involved in Fe homeostasis (Fu et al. 2017a). Besides, it has been reported that DMT1 was involved in neuronal cell Fe homeostasis (Aral et al. 2020). The proper homeostasis of Fe is managed by Fe importer and exporter, which prevent Fe overload or Fe starvation in cells. FPN1 at the basal membrane exports Fe into the circulation; TF carries Fe to various tissues and cells (Tsuji 2020). Previous study reported that MTF1 and TF have strong positive correlation, suggesting that these genes are involved in transportation of iron in fish liver (Fu et al. 2017b). Cu is essential for the central nervous system (Zhang et al. 2020b). Cu uptake transporter CTR1 and efflux transporters ATP7B were involved in Cu homeostasis. The function of ATP7B is to transport excess Cu in the cytoplasm, and the function of CTR1 is to transport Cu from extracellular transport to the cytoplasm when Cu deficiency in the cytoplasm (Kitada et al. 2008). What is noteworthy is that MTF1 can upregulate ATP7B expression and eliminate Cu through serum ceruloplasmin and bile (Hardyman et al. 2016). However, Cd exposure induced disordered iron homeostasis on HEL cells (Wang et al. 2020) and causes a marked depletion of the content of Fe in rat’s liver and kidney (Whanger 1979). Besides, Cd exposure disturbed Cu and manganese homeostasis, and with the dose of Cd increased, Cu deposition on hepatopancreas was observed (Nica et al. 2019), suggesting that Cd exposure disrupts Cu homeostasis. In line with previous study, this study showed that Cd exposure led to reduction in Fe level and enhanced the content of Cu. In addition, MTF1-mediated metal response was activated by low-dose Cd, which means that MTF1-mediated metal response tries to adjust Fe and Cu homeostasis. However, it was inhibited under the high-dose Cd exposure. Above all, these results suggest that Cd exposure disrupted homeostasis via inhibiting MTF1-mediated metal response to further induce cerebellar injury.
Conclusion
This study revealed that cerebellum is the target organ that Cd accumulated in Cd exposure that inhibited selenoprotein transcriptome, caused oxidative stress, disturbed metals homeostasis, and modulated MTF1 and its target metal-responsive genes, which affected redox homeostasis that was involved in the mechanism of cerebellar injury. In conclusion, Cd through disturbing MTF1-mediated metal response induced cerebellar injury (Fig. 7).
References
Adedara IA, Fabunmi AT, Ayenitaju FC, Atanda OE, Adebowale AA, Ajayi BO, Owoeye O, Rocha JBT, Farombi EO (2020) Neuroprotective mechanisms of selenium against arsenic-induced behavioral impairments in rats. Neurotoxicology 76:99–110
Ammendola S, Cerasi M, Battistoni A (2014) Deregulation of transition metals homeostasis is a key feature of cadmium toxicity in Salmonella. Biometals 27(4):703–714
Anetor JI (2012) Rising environmental cadmium levels in developing countries: threat to genome stability and health. Niger J Physiol Sci 27(2):103–115
Aral LA, Ergun MA, Engin AB, Borcek AO, Belen HB (2020) Iron homeostasis is altered in response to hypoxia and hypothermic preconditioning in brain glial cells. Turk J Med Sci 50(8):2005–2016
Ben Mimouna S, Le Charpentier T, Lebon S, Van Steenwinckel J, Messaoudi I, Gressens P (2019) Involvement of the synapse-specific zinc transporter ZnT3 in cadmium-induced hippocampal neurotoxicity. J Cell Physiol
Berglund AM, Nyholm NE (2011) Slow improvements of metal exposure, health- and breeding conditions of pied flycatchers (Ficedula hypoleuca) after decreased industrial heavy metal emissions. Sci Total Environ 409(20):4326–4334
Bi M, Du X, Jiao Q, Liu Z, Jiang H (2020) Alpha-synuclein regulates iron homeostasis via preventing parkin-mediated DMT1 ubiquitylation in Parkinson’s disease models. ACS Chem Neurosci 11(11):1682–1691
Buranasinsup S, Jangsangthong A, Toniti W (2011) Prediction of cadmium (Cd) toxicity in cattle. J Med Assoc Thai 94(Suppl 5):S50-55
Cai Y, Kirschke CP, Huang L (2018) SLC30A family expression in the pancreatic islets of humans and mice: cellular localization in the beta-cells. J Mol Histol 49(2):133–145
Chen D, Zhang D, Yu JC, Chan KM (2011) Effects of Cu2O nanoparticle and CuCl2 on zebrafish larvae and a liver cell-line. Aquat Toxicol 105(3–4):344–354
Chen PH, Wu J, Ding CC, Lin CC, Pan S, Bossa N, Xu Y, Yang WH, Mathey-Prevot B, Chi JT (2020) Kinome screen of ferroptosis reveals a novel role of ATM in regulating iron metabolism. Cell Death Differ 27(3):1008–1022
Costa LC, Luz LM, Nascimento VL, Araujo FF, Santos MNS, Franca CFM, Silva TP, Fugate KK, Finger FL (2020) Selenium-ethylene interplay in postharvest life of cut flowers. Front Plant Sci 11:584698
Cuillel M, Chevallet M, Charbonnier P, Fauquant C, Pignot-Paintrand I, Arnaud J, Cassio D, Michaud-Soret I, Mintz E (2014) Interference of CuO nanoparticles with metal homeostasis in hepatocytes under sub-toxic conditions. Nanoscale 6(3):1707–1715
Dehkordi AJ, Mohebbi AN, Aslani MR, Ghoreyshi SM (2017) Evaluation of nanoselenium (Nano-Se) effect on hematological and serum biochemical parameters of rat in experimentally lead poisoning. Hum Exp Toxicol 36(4):421–427
Dvorak M, Schnegg R, Niederwanger M, Pedrini-Martha V, Ladurner P, Lindner H, Kremser L, Lackner R, Dallinger R (2019) Cadmium pathways in snails follow a complementary strategy between metallothionein detoxification and auxiliary inactivation by phytochelatins. Int J Mol Sci 21(1)
Feng L, Yan H, Dai C, Xu W, Gu F, Zhang F, Li T, Xian J, He X, Yu Y, Ma M, Wang F, He Z (2020) The systematic exploration of cadmium-accumulation characteristics of maize kernel in acidic soil with different pollution levels in China. Sci Total Environ 729:138972
Forcella M, Lau P, Oldani M, Melchioretto P, Bogni A, Gribaldo L, Fusi P, Urani C (2020) Neuronal specific and non-specific responses to cadmium possibly involved in neurodegeneration: a toxicogenomics study in a human neuronal cell model. Neurotoxicology 76:162–173
Fu D, Bridle A, Leef M, Gagnon MM, Hassell KL, Nowak BF (2017a) Using a multi-biomarker approach to assess the effects of pollution on sand flathead (Platycephalus bassensis) from Port Phillip Bay, Victoria. Australia Mar Pollut Bull 119(1):211–219
Fu D, Bridle A, Leef M, Norte Dos Santos C, Nowak B (2017b) Hepatic expression of metal-related genes and gill histology in sand flathead (Platycephalus bassensis) from a metal contaminated estuary. Mar Environ Res 131:80–89
Ge J, Zhang C, Sun YC, Zhang Q, Lv MW, Guo K, Li JL (2019) Cadmium exposure triggers mitochondrial dysfunction and oxidative stress in chicken (Gallus gallus) kidney via mitochondrial UPR inhibition and Nrf2-mediated antioxidant defense activation. Sci Total Environ 689:1160–1171
Grzywacz A, Gdula-Argasinska J, Muszynska B, Tyszka-Czochara M, Librowski T, Opoka W (2015) Metal responsive transcription factor 1 (MTF-1) regulates zinc dependent cellular processes at the molecular level. Acta Biochim Pol 62(3):491–498
Hardyman JE, Tyson J, Jackson KA, Aldridge C, Cockell SJ, Wakeling LA, Valentine RA, Ford D (2016) Zinc sensing by metal-responsive transcription factor 1 (MTF1) controls metallothionein and ZnT1 expression to buffer the sensitivity of the transcriptome response to zinc. Metallomics 8(3):337–343
Jin X, Jia T, Liu R, Xu S (2018) The antagonistic effect of selenium on cadmium-induced apoptosis via PPAR-gamma/PI3K/Akt pathway in chicken pancreas. J Hazard Mater 357:355–362
Jomova K, Valko M (2011) Advances in metal-induced oxidative stress and human disease. Toxicology 283(2–3):65–87
Karri V, Schuhmacher M, Kumar V (2016) Heavy metals (Pb, Cd, As and MeHg) as risk factors for cognitive dysfunction: A general review of metal mixture mechanism in brain. Environ Toxicol Pharmacol 48:203–213
Kitada N, Takara K, Minegaki T, Itoh C, Tsujimoto M, Sakaeda T, Yokoyama T (2008) Factors affecting sensitivity to antitumor platinum derivatives of human colorectal tumor cell lines. Cancer Chemother Pharmacol 62(4):577–584
Liu LL, Li CM, Zhang ZW, Zhang JL, Yao HD, Xu SW (2014) Protective effects of selenium on cadmium-induced brain damage in chickens. Biol Trace Elem Res 158(2):176–185
Lopez E, Figueroa S, Oset-Gasque MJ, Gonzalez MP (2003) Apoptosis and necrosis: two distinct events induced by cadmium in cortical neurons in culture. Br J Pharmacol 138(5):901–911
Mendez-Armenta M, Barroso-Moguel R, Villeda-Hernandez J, Nava-Ruiz C, Rios C (2001) Histopathological alterations in the brain regions of rats after perinatal combined treatment with cadmium and dexamethasone. Toxicology 161(3):189–199
Menezo Y, Pluntz L, Chouteau J, Gurgan T, Demirol A, Dalleac A, Benkhalifa M (2011) Zinc concentrations in serum and follicular fluid during ovarian stimulation and expression of Zn2+ transporters in human oocytes and cumulus cells. Reprod Biomed Online 22(6):647–652
Muraina IA, Maret W, Bury NR, Hogstrand C (2020) Hatching gland development and hatching in zebrafish embryos: a role for zinc and its transporters Zip10 and Znt1a. Biochem Biophys Res Commun 528(4):698–705
Naija A, Kestemont P, Chenais B, Haouas Z, Blust R, Helal AN, Marchand J (2017) Cadmium exposure exerts neurotoxic effects in peacock blennies Salaria pavo. Ecotoxicol Environ Saf 143:217–227
Nemmiche S, Guiraud P (2016) Cadmium-induced oxidative damages in the human BJAB cells correlate with changes in intracellular trace elements levels and zinc transporters expression. Toxicol in Vitro 37:169–177
Nica DV, Draghici GA, Andrica FM, Popescu S, Coricovac DE, Dehelean CA, Gergen II, Kovatsi L, Coleman MD, Tsatsakis A (2019) Short-term effects of very low dose cadmium feeding on copper, manganese and iron homeostasis: a gastropod perspective. Environ Toxicol Pharmacol 65:9–13
Palmiter RD, Findley SD (1995) Cloning and functional characterization of a mammalian zinc transporter that confers resistance to zinc. EMBO J 14(4):639–649
Park C, Jeong J (2018) Synergistic cellular responses to heavy metal exposure: a minireview. Biochim Biophys Acta Gen Subj 1862(7):1584–1591
Qu KC, Li HQ, Tang KK, Wang ZY, Fan RF (2020) Selenium mitigates cadmium-induced adverse effects on trace elements and amino acids profiles in chicken pectoral muscles. Biol Trace Elem Res 193(1):234–240
Satarug S, Garrett SH, Sens MA, Sens DA (2010) Cadmium, environmental exposure, and health outcomes. Environ Health Perspect 118(2):182–190
Shen X, Liu W, Chen Y, Guo Y, Gao M, Chen W, Liu Y, Liu S (2019) Diagnostic significance of metallothionein members in recognizing cadmium exposure in various organs under low-dose exposure. Chemosphere 229:32–40
Sheng Z, Yang WX, Zhu JQ (2015) Metallothionein from Pseudosciaena crocea: expression and response to cadmium-induced injury in the testes. Ecotoxicology 24(4):779–794
Smirnova IV, Bittel DC, Ravindra R, Jiang H, Andrews GK (2000) Zinc and cadmium can promote rapid nuclear translocation of metal response element-binding transcription factor-1. J Biol Chem 275(13):9377–9384
Talukder M, Bi SS, Jin HT, Ge J, Zhang C, Lv MW, Li JL (2021) Cadmium induced cerebral toxicity via modulating MTF1-MTs regulatory axis. Environ Pollut 285:117083
Troche C, Eicher SD, Applegate TJ (2015) The influence of dietary zinc source and coccidial vaccine exposure on intracellular zinc homeostasis and immune status in broiler chickens. Br J Nutr 114(2):202–212
Tsuji Y (2020) Transmembrane protein western blotting: impact of sample preparation on detection of SLC11A2 (DMT1) and SLC40A1 (ferroportin). PLoS One 15(7):e0235563
Varmazyari A, Taghizadehghalehjoughi A, Sevim C, Baris O, Eser G, Yildirim S, Hacimuftuoglu A, Buha A, Wallace DR, Tsatsakis A, Aschner M, Mezhuev Y (2020) Cadmium sulfide-induced toxicity in the cortex and cerebellum: in vitro and in vivo studies. Toxicol Rep 7:637–648
Wang B, Xiao JL, Ling YH, Meng XJ, Wu B, Yang XY, Zou F (2014) BNIP3 upregulation by ERK and JNK mediates cadmium-induced necrosis in neuronal cells. Toxicol Sci 140(2):393–402
Wang L, Zheng M, Wang Y, Yuan L, Yu C, Cui J, Zhang S (2020) Activation of integrated stress response and disordered iron homeostasis upon combined exposure to cadmium and PCB77. J Hazard Mater 389:121833
Wang M, Chen Z, Song W, Hong D, Huang L, Li Y (2021) A review on cadmium exposure in the population and intervention strategies against cadmium toxicity. Bull Environ Contam Toxicol 106(1):65–74
Wei X, Qi Y, Zhang X, Gu X, Cai H, Yang J, Zhang Y (2015) ROS act as an upstream signal to mediate cadmium-induced mitophagy in mouse brain. Neurotoxicology 46:19–24
Whanger PD (1979) Cadmium effects in rats on tissue iron, selenium, and blood pressure; blood and hair cadmium in some oregon residents. Environ Health Perspect 28:115–121
Wimmer U, Wang Y, Georgiev O, Schaffner W (2005) Two major branches of anti-cadmium defense in the mouse: MTF-1/metallothioneins and glutathione. Nucleic Acids Res 33(18):5715–5727
Xiong X, Zhang Y, Xing H, Xu S (2020) Ameliorative effect of selenomethionine on cadmium-induced hepatocyte apoptosis via regulating PI3K/AKT pathway in chickens. Biol Trace Elem Res 195(2):559–568
Zhang Q, Zhao Y, Talukder M, Han Y, Zhang C, Li XN, Li JL (2019) Di(2-ethylhexyl) phthalate induced hepatotoxicity in quail (Coturnix japonica) via modulating the mitochondrial unfolded protein response and NRF2 mediated antioxidant defense. Sci Total Environ 651(Pt 1):885–894
Zhang R, Wang L, Zhao J, Wang C, Bao J, Li J (2016) Effects of selenium and cadmium on ion profiles in the brains of chickens. Biol Trace Elem Res 174(1):218–225
Zhang TG, Zhao YL, Li L, Zhou DH (2020a) Antagonistic effects of nano-selenium on broilers hepatic injury induced by Cr(VI) poisoning in AMPK pathway. Environ Sci Pollut Res Int 27(33):41585–41595
Zhang Y, McDermott S, Davis B, Hussey J (2020b) High incidence of brain and other nervous system cancer identified in two mining counties, 2001–2015. Spat Spatiotemporal Epidemiol 32:100320
Zhao Y, Du ZH, Talukder M, Lin J, Li XN, Zhang C, Li JL (2018) Crosstalk between unfolded protein response and Nrf2-mediated antioxidant defense in Di-(2-ethylhexyl) phthalate-induced renal injury in quail (Coturnix japonica). Environ Pollut 242(Pt B):1871–1879
Zhu C, Wen H, Zhang Y, Yin R, Cloquet C (2018) Cd isotope fractionation during sulfide mineral weathering in the Fule Zn-Pb-Cd deposit, Yunnan Province, Southwest China. Sci Total Environ 616–617:64–72
Zoidis E, Seremelis I, Kontopoulos N, Danezis GP (2018) Selenium-dependent antioxidant enzymes: actions and properties of selenoproteins. Antioxidants (Basel) 7(5)
Funding
This study has received assistance from National Natural Science Foundation of China (Nos. 32172932, 32102739), High Level Talent Project of West Anhui University (WGKQ2022031), Key Program of Natural Science Foundation of Heilongjiang Province of China (No. ZD2021C003), China Agriculture Research System of MOF and MARA (No. CARS-35), Distinguished Professor of Longjiang Scholars Support Project (No. T201908), and Heilongjiang Touyan Innovation Team Program and Postdoctoral Research Foundation of China (No. 2021M690925).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bi, SS., Talukder, M., Jin, HT. et al. Cadmium Through Disturbing MTF1-Mediated Metal Response Induced Cerebellar Injury. Neurotox Res 40, 1127–1137 (2022). https://doi.org/10.1007/s12640-022-00474-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-022-00474-x