Abstract
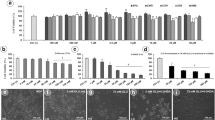
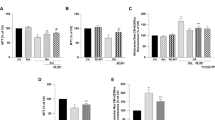
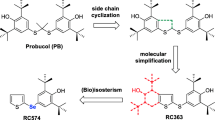
Aldose reductase (AR) catalyzes the conversion of glucose to sorbitol in a NADPH-dependent reaction, thereby increasing the production of reactive oxygen species (ROS). Since AR activation is linked to redox dysregulation and cell damage in neurodegenerative diseases, AR inhibitors (ARIs) constitute promising therapeutic tools for the treatment of these disorders. Among these compounds, the novel substituted triazinoindole derivatives cemtirestat (CMTI) and COTI, as well as the clinically employed epalrestat (EPA) and the pyridoindole-antioxidant stobadine (STB), were tested in both PC12 cells and BV2 microglia exposed to four different neurotoxic models. These include (1) oxidative stress with hydrogen peroxide (H2O2), (2) mitochondrial complex IV inhibition with NaN3, (3) endoplasmic reticulum-stress and lipotoxicity induced by palmitic acid/bovine serum albumin (PAM/BSA), and (4) advanced carbonyl compound lipotoxicity by 4-hydroxynonenal (4-HNE). All toxic compounds decreased cell viability and increased ROS formation in both PC12 and BV2 cells in a concentration-dependent manner (1–1000 μM; NaN3 < H2O2≈PAM/BSA < 4-HNE). In PC12 cells, EPA increased cell viability in all toxic models only at 1 μM, whereas CMTI restored baseline viability in all toxic models. COTI afforded protection against lipotoxicity, while STB only prevented H2O2-induced toxicity. Except for the 4-HNE model, EPA prevented ROS generation in all other toxic models, whereas CMTI, COTI, and STB prevented ROS production in all toxic models. In BV2 cells, EPA and CMTI restored baseline cell viability in all toxic models tested, while COTI and STB did not prevent the loss of viability in the NaN3 model. All ARIs and STB efficiently prevented ROS formation in all toxic models in a concentration-independent manner. The differential protective effects evoked by the novel ARIs and STB on the toxic models tested herein provide novel and relevant comparative evidence for the design of specific therapeutic strategies against neurodegenerative events associated with neurological disorders.






Similar content being viewed by others
References
Baba M, Kimura K, Suda T, Yagihashi S (2006) Aomori Diabetic Study (ADNS) Group. Three-year inhibition of aldose reductase on development of symptomatic neuropathy in diabetic patients. J Peripher Nerv Syst 11:176–178
Balcerczyk A, Bartosz G, Drzewinska J, Piotrowski Ł, Pulaski Ł, Stefek M (2014) Antioxidant action of SMe1EC2, the low-basicity derivative of the pyridoindole stobadine, in cell free chemical models and at cellular level. Interdiscip Toxicol 7:27–32
Chung SS, Ho EC, Lam KS, Chung SK (2003) Contribution of polyol pathway to diabetes-induced oxidative stress. J Am Soc Nephrol 14:S233–S236
Cumaoglu A, Cevik C, Rackova L, Ari N, Karasu C (2007) Effects of antioxidant stobadine on protein carbonylation, advanced oxidation protein products and reductive capacity of liver in streptozotocin-diabetic rats: role of oxidative/nitrosative stress. BioFactors 30:171–178
Elmazoglu Z, Prnova MS, Santamaria A, Stefek M, Karasu C (2020) Combatting nitrosative stress and inflammation with novel substituted triazinoindole inhibitors of aldose reductase in PC12 cells exposed to 6-hydroxydopamine plus high glucose. Neurotox Res. 39:210-226. https://doi.org/10.1007/s12640-020-00305-x
Guz G, Demirogullari B, Ulusu NN, Dogu C, Demirtola A, Kavutcu M, Omeroglu S, Stefek M, Karasu C (2007) Stobadine protects rat kidney against ischaemia/reperfusion injury. Clin Exp Pharmacol Physiol 34:210–216
Hlavac M, Kovacikova L, Soltesova Prnova M, Šramel P, Addova G, Majekova M, Hanquet G, Bohac A, Stefek M (2020) Development of novel oxotriazinoindole inhibitors of aldose reductase: isosteric sulfur/oxygen replacement in the thioxotriazinoindole cemtirestat markedly improved inhibition selectivity. J Med Chem 63:369–381
Iyer S, Sam FS, DiPrimio N, Preston G, Verheijen J, Murthy K, Parton Z, Tsang H, Lao J, Morava E, Perlstein EO (2019) Repurposing the aldose reductase inhibitor and diabetic neuropathy drug epalrestat for the congenital disorder of glycosylation PMM2-CDG. Dis Model Mech. https://doi.org/10.1242/dmm.040584
Juranek I, Horakova L, Rackova L, Stefek M (2010) Antioxidants in treating pathologies involving oxidative damage: an update on medicinal chemistry and biological activity of stobadine and related pyridoindoles. Curr Med Chem 17:552–570
Karasu C, Cumaoğlu A, Gürpinar AR, Kartal M, Kovacikova L, Milackova I, Stefek M (2012) Aldose reductase inhibitory activity and antioxidant capacity of pomegranate extracts. Interdiscip Toxicol 5:15–20
Konno T, Melo EP, Chambers JE, Avezov E (2021) Intracellular sources of ROS/H(2)O(2) in health and neurodegeneration: spotlight on endoplasmic reticulum. Cells 10:233
Li QR, Wang Z, Zhou W, Fan SR, Ma R, Xue L, Yang L, Li YS, Tan HL, Shao QH, Yang HY (2016) Epalrestat protects against diabetic peripheral neuropathy by alleviating oxidative stress and inhibiting polyol pathway. Neural Regen Res 11:345–351
Mule NK, Singh JN (2018) Diabetes mellitus to neurodegenerative disorders: is oxidative stress fueling the flame? CNS Neurol Disord Drug Targets 17:644–653
Nakayama M, Nakamura J, Hamada Y, Chaya S, Mizubayashi R, Yasuda Y, Kamiya H, Koh N, Hotta N (2001) Aldose reductase inhibition ameliorates pupillary light reflex and F-wave latency in patients with mild diabetic neuropathy. Diabetes Care 24:1093–1098
Pagano G, Polychronis S, Wilson H, Giordano B, Ferrara N, Niccolini F, Politis M (2018) Diabetes mellitus and Parkinson disease. Neurology 90:e1654–e1662
Petrash JM (2004) All in the family: aldose reductase and closely related aldo-keto reductases. Cell Mol Life Sci 61:737–749
Prnova MS, Ballekova J, Majekova M, Stefek M (2015) Antioxidant action of 3-mercapto-5H-1,2,4-triazino[5,6-b]indole-5-acetic acid, an efficient aldose reductase inhibitor, in a 1,1’-diphenyl-2-picrylhydrazyl assay and in the cellular system of isolated erythrocytes exposed to tert-butyl hydroperoxide. Redox Rep 20:282–288
Prnova MS, Kovacikova L, Svik K, Bezek S, Elmazoğlu Z, Karasu C, Stefek M (2020) Triglyceride-lowering effect of the aldose reductase inhibitor cemtirestat-another factor that may contribute to attenuation of symptoms of peripheral neuropathy in STZ-diabetic rats. Naunyn-Schmiedeberg’s Arch Pharmacol 393:651–661
Rackova L, Stefek M, Majekova M (2002) Structural aspects of antioxidant activity of substituted pyridoindoles. Redox Rep 7:207–214
Rackova L, Cumaoglu A, Bagrıacık EU, Stefek M, Maechler P, Karasu C (2011) Novel hexahydropyridoindole derivative as prospective agent against oxidative damage in pancreatic β cells. Med Chem 7:711–717
Schönfeld P, Reiser G (2016) Brain lipotoxicity of phytanic acid and very long-chain fatty acids. Harmful cellular/mitochondrial activities in Refsum disease and X-linked adrenoleukodystrophy. Aging Dis 7:136–149
Shukla K, Pal PB, Sonowal H, Srivastava SK, Ramana KV (2017) Aldose reductase inhibitor protects against hyperglycemic stress by activating Nrf2-dependent antioxidant proteins. J Diabetes Res 2017:6785852
Skalska S, Kyselova Z, Gajdosikova A, Karasu C, Stefek M, Stolc S (2008) Protective effect of stobadine on NCV in streptozotocin-diabetic rats: augmentation by vitamin E. Gen Physiol Biophys 27:106–114
Soltesova Prnova M, Medina-Campos ON, Pedraza-Chaverri J, Colín-González AL, Piedra-García F, Rangel-López E, Kovacikova L, Ceylan A, Karasu C, Santamaria A, Stefek M (2020) Antioxidant mechanisms in the neuroprotective action of cemtirestat: studies in chemical models, liposomes and rat brain cortical slices. Neuroscience 443:206–217
Soltesova Prnova M, Svik K, Bezek S, Kovacikova L, Karasu C, Stefek M (2019) 3-Mercapto-5H-1,2,4-triazino[5,6-b]indole-5-acetic acid (cemtirestat) alleviates symptoms of peripheral diabetic neuropathy in Zucker diabetic fatty (ZDF) rats: a role of aldose reductase. Neurochem Res 44:1056–1064
Stefek M, Milackova I, Juskova-Karasova M, Snirc V (2013) Antioxidant action of the hexahydropyridoindole SMe1EC2 in the cellular system of isolated red blood cells in vitro. Redox Rep 18:71–75
Stefek M, Soltesova Prnova M, Ballekova J, Majekova M (2016) Cemtirestat, a novel aldose reductase inhibitor and antioxidant, in multitarget pharmacology of diabetic complications. International Journal of Advances in Science Engineering and Technology IRAJ 4:41–44
Stefek M, Soltesova Prnova M, Majekova M, Rechlin C, Heine A, Klebe G (2015) Identification of novel aldose reductase inhibitors based on carboxymethylated mercaptotriazinoindole scaffold. J Med Chem 58:2649–2657
Ulusu NN, Gök M, Sayin Şakul AA, Ari N, Stefek M, Karasu C (2017) “The ADIC (Antioxidants in Diabetes-Induced Complications) Study Group”. Antioxidant SMe1EC2 modulates pentose phosphate pathway and glutathione-dependent enzyme activities in tissues of aged diabetic rats. Interdiscip Toxicol 10:148–154
Vicente Miranda H, El-Agnaf OM, Outeiro TF (2016) Glycation in Parkinson’s disease and Alzheimer’s disease. Mov Disord 31:782–790
Yeung PKK, Lai AKW, Son HJ, Zhang X, Hwang O, Chung SSM, Chung SK (2017) Aldose reductase deficiency leads to oxidative stress-induced dopaminergic neuronal loss and autophagic abnormality in an animal model of Parkinson’s disease. Neurobiol Aging 50:119–133
Zhang J, Song N, Liu Y, Guo J (2021) Platycodin D inhibits beta-amyloid-induced inflammation and oxidative stress in BV-2 cells via suppressing TLR4/NF-kappaB signaling pathway and activating Nrf2/HO-1 signaling pathway. Neurochem Res. https://doi.org/10.1007/s11064-020-03198-6
Funding
This work was supported by The Scientific and Technological Research Council of Turkey (C.K.; TUBITAK Project No: 215S197; SAS-TUBITAK JRP 2015/7), the Slovak Research and Development Agency (M.S.; Contract No. APVV-15-0455), and the Slovak Grant Agency of the Ministry of Education (M.S.P.; VEGA 2/0005/2018). M.A. was supported by the National Institute of Environmental Health Sciences grants R01ES03771 and R01ES10563.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Research Involving Human and Animal Participants
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Elmazoglu, Z., Prnova, M.S., Stefek, M. et al. Protective Effects of Novel Substituted Triazinoindole Inhibitors of Aldose Reductase and Epalrestat in Neuron-like PC12 Cells and BV2 Rodent Microglial Cells Exposed to Toxic Models of Oxidative Stress: Comparison with the Pyridoindole Antioxidant Stobadine. Neurotox Res 39, 588–597 (2021). https://doi.org/10.1007/s12640-021-00349-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-021-00349-7