Abstract
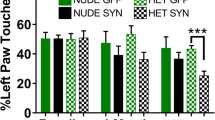
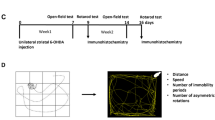
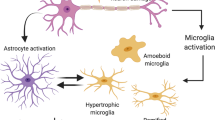
Parkinson’s disease (PD) is characterized by progressive degeneration of dopaminergic neurons accompanied by an inflammatory reaction. The neuron-derived chemokine fractalkine (CX3CL1) is an exclusive ligand for the receptor CX3CR1 expressed on microglia. The CX3CL1/CX3CR1 signaling is important for sustaining microglial activity. Using a recently developed PD model, in which the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) toxin is delivered intranasally, we hypothesized that CX3CR1 could play a role in neurotoxicity and glial activation. For this, we used CX3CR1 knock-in mice and compared results with those obtained using the classical PD models through intraperitonal MPTP or intrastriatal 6-hydroxydopamine (6-OHDA). The striatum from all genotypes (CX3CR1+/+, CX3CR1+/GFP and CX3CR1-deficient mice) showed a significant dopaminergic depletion after intranasal MPTP inoculation. In contrast to that, we could not see differences in the number of dopaminergic neurons in the substantia nigra of CX3CR1-deficient animals. Similarly, after 6-OHDA infusion, the CX3CR1 deletion decreased the amphetamine-induced turning behavior observed in CX3CR1+/GFP mice. After the 6-OHDA inoculation, a minor dopaminergic neuronal loss was observed in the substantia nigra from CX3CR1-deficient mice. Distinctly, a more extensive neuronal cell loss was observed in the substantia nigra after the intraperitoneal MPTP injection in CX3CR1 disrupted animals, corroborating previous results. Intranasal and intraperitoneal MPTP inoculation induced a similar microgliosis in CX3CR1-deficient mice but a dissimilar change in the astrocyte proliferation in the substantia nigra. Nigral astrocyte proliferation was observed only after intraperitoneal MPTP inoculation. In conclusion, intranasal MPTP and 6-OHDA lesion in CX3CR1-deficient mice yield no nigral dopaminergic neuron loss, linked to the absence of astroglial proliferation.






Similar content being viewed by others
References
Aguiar AS Jr, Tristao FS, Amar M, Chevarin C, Lanfumey L, Mongeau R, Corti O, Prediger RD, Raisman-Vozari R (2013) Parkin-knockout mice did not display increased vulnerability to intranasal administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Neurotox Res 24(2):280–287. doi:10.1007/s12640-013-9389-0
Aguiar AS Jr, Tristao FS, Amar M, Chevarin C, Glaser V, de Paula Martins R, Moreira EL, Mongeau R, Lanfumey L, Raisman-Vozari R, Latini A, Prediger RD (2014) Six weeks of voluntary exercise don’t protect C57BL/6 mice against neurotoxicity of MPTP and MPP(+). Neurotox Res 25(2):147–152. doi:10.1007/s12640-013-9412-5
Asensio VC, Campbell IL (1999) Chemokines in the CNS: plurifunctional mediators in diverse states. Trends Neurosci 22(11):504–512
Bannon MJ (2005) The dopamine transporter: role in neurotoxicity and human disease. Toxicol Appl Pharmacol 204(3):355–360
Blandini F, Nappi G, Tassorelli C, Martignoni E (2000) Functional changes of the basal ganglia circuitry in Parkinson’s disease. Prog Neurobiol 62(1):63–88
Blesa J, Phani S, Jackson-Lewis V, Przedborski S (2012) Classic and new animal models of Parkinson’s disease. J Biomed Biotechnol 2012:845618. doi:10.1155/2012/845618
Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM (2006) Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci 9(7):917–924
Carpentier PA, Begolka WS, Olson JK, Elhofy A, Karpus WJ, Miller SD (2005) Differential activation of astrocytes by innate and adaptive immune stimuli. Glia 49(3):360–374. doi:10.1002/glia.20117
Cavalcanti-Kwiatkoski R, Raisman-Vozari R, Ginestet L, Del Bel E (2010) Altered expression of neuronal nitric oxide synthase in weaver mutant mice. Brain Res 1326:40–50
Cicchetti F, Brownell AL, Williams K, Chen YI, Livni E, Isacson O (2002) Neuroinflammation of the nigrostriatal pathway during progressive 6-OHDA dopamine degeneration in rats monitored by immunohistochemistry and PET imaging. Eur J Neurosci 15(6):991–998
Cook DN, Chen SC, Sullivan LM, Manfra DJ, Wiekowski MT, Prosser DM, Vassileva G, Lira SA (2001) Generation and analysis of mice lacking the chemokine fractalkine. Mol Cell Biol 21(9):3159–3165. doi:10.1128/MCB.21.9.3159-3165.2001
Cordova FM, Aguiar AS Jr, Peres TV, Lopes MW, Goncalves FM, Remor AP, Lopes SC, Pilati C, Latini AS, Prediger RD, Erikson KM, Aschner M, Leal RB (2012) In vivo manganese exposure modulates Erk, Akt and Darpp-32 in the striatum of developing rats, and impairs their motor function. PLoS One 7(3):e33057. doi:10.1371/journal.pone.0033057
Corona AW, Huang Y, O’Connor JC, Dantzer R, Kelley KW, Popovich PG, Godbout JP (2010) Fractalkine receptor (CX3CR1) deficiency sensitizes mice to the behavioral changes induced by lipopolysaccharide. J Neuroinflammation 7:93
Costa G, Frau L, Wardas J, Pinna A, Plumitallo A, Morelli M (2013) MPTP-induced dopamine neuron degeneration and glia activation is potentiated in MDMA-pretreated mice. Mov Disord 28(14):1957–1965. doi:10.1002/mds.25646
Cotter R, Williams C, Ryan L, Erichsen D, Lopez A, Peng H, Zheng J (2002) Fractalkine (CX3CL1) and brain inflammation: implications for HIV-1-associated dementia. J Neurovirol 8(6):585–598. doi:10.1080/13550280290100950
Czlonkowska A, Kohutnicka M, Kurkowska-Jastrzebska I, Czlonkowski A (1996) Microglial reaction in MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) induced Parkinson’s disease mice model. Neurodegeneration 5(2):137–143
Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB (2005) ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 8(6):752–758
Dickson DW (2001) Alpha-synuclein and the Lewy body disorders. Curr Opin Neurol 14(4):423–432
Duvoisin RC (1991) Parkinson’s disease, 3rd edn. Raven Press, New York
Elbaz A, Tranchant C (2007) Epidemiologic studies of environmental exposures in Parkinson’s disease. J Neurol Sci 262(1–2):37–44
Fuhrmann M, Bittner T, Jung CK, Burgold S, Page RM, Mitteregger G, Haass C, LaFerla FM, Kretzschmar H, Herms J (2010) Microglial Cx3cr1 knockout prevents neuron loss in a mouse model of Alzheimer’s disease. Nat Neurosci 13(4):411–413
Ghosh A (2010) Brain APCs including microglia are only differential and positional polymorphs. Ann Neurosci. 17(4):191–199. doi:10.5214/ans.0972.7531.1017410
Goedert M (2001) Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci 2(7):492–501. doi:10.1038/35081564
Graff CL, Pollack GM (2005) Nasal drug administration: potential for targeted central nervous system delivery. J Pharm Sci 94(6):1187–1195. doi:10.1002/jps.20318
Gupta M, Gupta BK, Thomas R, Bruemmer V, Sladek JR Jr, Felten DL (1986) Aged mice are more sensitive to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine treatment than young adults. Neurosci Lett 70(3):326–331
Hamon M, Fattaccini CM, Adrien J, Gallissot MC, Martin P, Gozlan H (1988) Alterations of central serotonin and dopamine turnover in rats treated with ipsapirone and other 5-hydroxytryptamine1A agonists with potential anxiolytic properties. J Pharmacol Exp Ther 246(2):745–752
Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK, Streit WJ, Salafranca MN, Adhikari S, Thompson DA, Botti P, Bacon KB, Feng L (1998) Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci USA 95(18):10896–10901
Hatori K, Nagai A, Heisel R, Ryu JK, Kim SU (2002) Fractalkine and fractalkine receptors in human neurons and glial cells. J Neurosci Res 69(3):418–426. doi:10.1002/jnr.10304
Heneka MT, Rodriguez JJ, Verkhratsky A (2010) Neuroglia in neurodegeneration. Brain Res Rev 63(1–2):189–211
Hernandez-Romero MC, Delgado-Cortes MJ, Sarmiento M, de Pablos RM, Espinosa-Oliva AM, Arguelles S, Bandez MJ, Villaran RF, Maurino R, Santiago M, Venero JL, Herrera AJ, Cano J, Machado A (2012) Peripheral inflammation increases the deleterious effect of CNS inflammation on the nigrostriatal dopaminergic system. Neurotoxicology 33(3):347–360
Hirsch EC, Hunot S (2009) Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol 8(4):382–397
Hirsch EC, Hunot S, Damier P, Brugg B, Faucheux BA, Michel PP, Ruberg M, Muriel MP, Mouatt-Prigent A, Agid Y (1999) Glial cell participation in the degeneration of dopaminergic neurons in Parkinson’s disease. Adv Neurol 80:9–18
Jackson-Lewis V, Jakowec M, Burke RE, Przedborski S (1995) Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurodegeneration 4(3):257–269
Jellinger KA (1988) Pathology of Parkinson’s syndrome. In: Calne DB (ed) Handbook of experimental pharmacology, vol 88. Springer, New York, pp 47–112
Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR (2000) Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol 20(11):4106–4114
Kadar H, Le Douaron G, Amar M, Ferrie L, Figadere B, Touboul D, Brunelle A, Raisman-Vozari R (2014) MALDI mass spectrometry imaging of 1-methyl-4-phenylpyridinium (MPP +) in mouse brain. Neurotox Res 25(1):135–145. doi:10.1007/s12640-013-9449-5
Kettenmann H, Hanisch UK, Noda M, Verkhratsky A (2011) Physiology of microglia. Physiol Rev 91(2):461–553. doi:10.1152/physrev.00011.2010
Kim YS, Joh TH (2006) Microglia, major player in the brain inflammation: their roles in the pathogenesis of Parkinson’s disease. Exp Mol Med 38(4):333–347
Kinugawa K, Monnet Y, Bechade C, Alvarez-Fischer D, Hirsch EC, Bessis A, Hunot S (2013) DAP12 and CD11b contribute to the microglial-induced death of dopaminergic neurons in vitro but not in vivo in the MPTP mouse model of Parkinson’s disease. J Neuroinflammation 10(1):82
Lazzarini M, Martin S, Mitkovski M, Vozari RR, Stuhmer W, Bel ED (2013) Doxycycline restrains glia and confers neuroprotection in a 6-OHDA Parkinson model. Glia 61(7):1084–1100. doi:10.1002/glia.22496
Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M, McAuliffe WG, Dawson VL, Dawson TM, Przedborski S (1999) Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med 5(12):1403–1409. doi:10.1038/70978
Limatola C, Ransohoff RM (2014) Modulating neurotoxicity through CX3CL1/CX3CR1 signaling. Front Cell Neurosci 8:229. doi:10.3389/fncel.2014.00229
Lundblad M, Usiello A, Carta M, Hakansson K, Fisone G, Cenci MA (2005) Pharmacological validation of a mouse model of l-DOPA-induced dyskinesia. Exp Neurol 194(1):66–75
Mani CS, Bravo FJ, Stanberry LR, Myers MG, Bernstein DI (1996) Effect of age and route of inoculation on outcome of neonatal herpes simplex virus infection in guinea pigs. J Med Virol 48(3):247–252
Mayeux R (2003) Epidemiology of neurodegeneration. Annu Rev Neurosci 26:81–104. doi:10.1146/annurev.neuro.26.043002.094919
McGeer PL, Itagaki S, Akiyama H, McGeer EG (1988) Rate of cell death in parkinsonism indicates active neuropathological process. Ann Neurol 24(4):574–576. doi:10.1002/ana.410240415
Mena MA, Garcia de Yebenes J (2008) Glial cells as players in parkinsonism: the “good,” the “bad,” and the “mysterious” glia. Neuroscientist 14(6):544–560
Meucci O, Fatatis A, Simen AA, Miller RJ (2000) Expression of CX3CR1 chemokine receptors on neurons and their role in neuronal survival. Proc Natl Acad Sci USA 97(14):8075–8080. doi:10.1073/pnas.090017497
Michel PP, Marien M, Ruberg M, Colpaert F, Agid Y (1999) Adenosine prevents the death of mesencephalic dopaminergic neurons by a mechanism that involves astrocytes. J Neurochem 72(5):2074–2082
Miyachi S, Lu X, Imanishi M, Sawada K, Nambu A, Takada M (2006) Somatotopically arranged inputs from putamen and subthalamic nucleus to primary motor cortex. Neurosci Res 56(3):300–308
Mogi M, Harada M, Kondo T, Riederer P, Inagaki H, Minami M, Nagatsu T (1994a) Interleukin-1 beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neurosci Lett 180(2):147–150
Mogi M, Harada M, Riederer P, Narabayashi H, Fujita K, Nagatsu T (1994b) Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci Lett 165(1–2):208–210
Morganti JM, Nash KR, Grimmig BA, Ranjit S, Small B, Bickford PC, Gemma C (2012) The soluble isoform of CX3CL1 is necessary for neuroprotection in a mouse model of Parkinson's disease. J Neurosci 32:14592–14601
Mourlevat S, Troadec JD, Ruberg M, Michel PP (2003) Prevention of dopaminergic neuronal death by cyclic AMP in mixed neuronal/glial mesencephalic cultures requires the repression of presumptive astrocytes. Mol Pharmacol 64(3):578–586
Nagatsu T, Mogi M, Ichinose H, Togari A (2000) Changes in cytokines and neurotrophins in Parkinson’s disease. J Neural Transm Suppl 60:277–290
Nimmerjahn A, Kirchhoff F, Helmchen F (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308(5726):1314–1318
Noelker C, Morel L, Lescot T, Osterloh A, Alvarez-Fischer D, Breloer M, Henze C, Depboylu C, Skrzydelski D, Michel PP, Dodel RC, Lu L, Hirsch EC, Hunot S, Hartmann A (2013) Toll like receptor 4 mediates cell death in a mouse MPTP model of Parkinson disease. Sci Rep 3:1393
O’Hagan DT, Illum L (1990) Absorption of peptides and proteins from the respiratory tract and the potential for development of locally administered vaccine. Crit Rev Ther Drug Carrier Syst 7(1):35–97
Olanow CW, Tatton WG (1999) Etiology and pathogenesis of Parkinson’s disease. Annu Rev Neurosci 22:123–144. doi:10.1146/annurev.neuro.22.1.123
Pabon MM, Bachstetter AD, Hudson CE, Gemma C, Bickford PC (2011) CX3CL1 reduces neurotoxicity and microglial activation in a rat model of Parkinson’s disease. J Neuroinflammation 8:9
Paxinos G, Franklin K (2001) The mouse brain in stereotaxic coordinates, 2nd edn. Academic Press, San Diego
Prediger RD, Batista LC, Medeiros R, Pandolfo P, Florio JC, Takahashi RN (2006) The risk is in the air: intranasal administration of MPTP to rats reproducing clinical features of Parkinson’s disease. Exp Neurol 202(2):391–403
Prediger RD, Aguiar AS Jr, Rojas-Mayorquin AE, Figueiredo CP, Matheus FC, Ginestet L, Chevarin C, Bel ED, Mongeau R, Hamon M, Lanfumey L, Raisman-Vozari R (2010) Single intranasal administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in C57BL/6 mice models early preclinical phase of Parkinson’s disease. Neurotox Res 17(2):114–129. doi:10.1007/s12640-009-9087-0
Prediger RD, Rojas-Mayorquin AE, Aguiar AS Jr, Chevarin C, Mongeau R, Hamon M, Lanfumey L, Del Bel E, Muramatsu H, Courty J, Raisman-Vozari R (2011) Mice with genetic deletion of the heparin-binding growth factor midkine exhibit early preclinical features of Parkinson’s disease. J Neural Transm 118(8):1215–1225. doi:10.1007/s00702-010-0568-3
Prediger RD, Aguiar AS Jr, Matheus FC, Walz R, Antoury L, Raisman-Vozari R, Doty RL (2012) Intranasal administration of neurotoxicants in animals: support for the olfactory vector hypothesis of Parkinson’s disease. Neurotox Res 21(1):90–116. doi:10.1007/s12640-011-9281-8
Rahman M, Muhammad S, Khan MA, Chen H, Ridder DA, Müller-Fielitz H, Pokorná B, Vollbrandt T, Stölting I, Nadrowitz R, Okun JG, Offermanns S, Schwaninger M (2014) The β-hydroxybutyrate receptor HCA2 activates a neuroprotective subset of macrophages. Nat Commun. 21(5):3944. doi:10.1038/ncomms4944
Rappold PM, Tieu K (2010) Astrocytes and therapeutics for Parkinson’s disease. Neurotherapeutics 7(4):413–423
Rojo AI, Montero C, Salazar M, Close RM, Fernandez-Ruiz J, Sanchez-Gonzalez MA, de Sagarra MR, Jackson-Lewis V, Cavada C, Cuadrado A (2006) Persistent penetration of MPTP through the nasal route induces Parkinson’s disease in mice. Eur J Neurosci 24(7):1874–1884
Sato E, Iikuni N, Yoshio T, Minota S, Kamatani N, Okamoto H (2006) Soluble fractalkine in the cerebrospinal fluid of patients with neuropsychiatric lupus. Ann Rheum Dis 65(9):1257–1259
Savitt JM, Dawson VL, Dawson TM (2006) Diagnosis and treatment of Parkinson disease: molecules to medicine. J Clin Invest 116(7):1744–1754. doi:10.1172/JCI29178
Shan S, Hong-Min T, Yi F, Jun-Peng G, Yue F, Yan-Hong T, Yun-Ke Y, Wen-Wei L, Xiang-Yu W, Jun M, Guo-Hua W, Ya-Ling H, Hua-Wei L, Ding-Fang C (2011) New evidences for fractalkine/CX3CL1 involved in substantia nigral microglial activation and behavioral changes in a rat model of Parkinson’s disease. Neurobiol Aging 32(3):443–458
Shao W, Zhang SZ, Tang M, Zhang XH, Zhou Z, Yin YQ, Zhou QB, Huang YY, Liu YJ, Wawrousek E, Chen T, Li SB, Xu M, Zhou JN, Hu G, Zhou JW (2013) Suppression of neuroinflammation by astrocytic dopamine D2 receptors via alphaB-crystallin. Nature 494(7435):90–94
Song DD, Haber SN (2000) Striatal responses to partial dopaminergic lesion: evidence for compensatory sprouting. J Neurosci 20(13):5102–5114
Sriram K, Matheson JM, Benkovic SA, Miller DB, Luster MI, O’Callaghan JP (2002) Mice deficient in TNF receptors are protected against dopaminergic neurotoxicity: implications for Parkinson’s disease. FASEB J 16(11):1474–1476. doi:10.1096/fj.02-0216fje
Stott SR, Barker RA (2014) Time course of dopamine neuron loss and glial response in the 6-OHDA striatal mouse model of Parkinson’s disease. Eur J Neurosci 39(6):1042–1056. doi:10.1111/ejn.12459
Sugama S, Wirz SA, Barr AM, Conti B, Bartfai T, Shibasaki T (2004) Interleukin-18 null mice show diminished microglial activation and reduced dopaminergic neuron loss following acute 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine treatment. Neuroscience 128(2):451–458. doi:10.1016/j.neuroscience.2004.07.020
Sunnemark D, Eltayeb S, Nilsson M, Wallstrom E, Lassmann H, Olsson T, Berg AL, Ericsson-Dahlstrand A (2005) CX3CL1 (fractalkine) and CX3CR1 expression in myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis: kinetics and cellular origin. J Neuroinflammation 2:17
Thomas DM, Francescutti-Verbeem DM, Kuhn DM (2008) Methamphetamine-induced neurotoxicity and microglial activation are not mediated by fractalkine receptor signaling. J Neurochem 106(2):696–705
Tieu K, Ischiropoulos H, Przedborski S (2003) Nitric oxide and reactive oxygen species in Parkinson’s disease. IUBMB Life 55(6):329–335. doi:10.1080/1521654032000114320
Tower DB, Young OM (1973) The activities of butyrylcholinesterase and carbonic anhydrase, the rate of anaerobic glycolysis, and the question of a constant density of glial cells in cerebral cortices of various mammalian species from mouse to whale. J Neurochem 20(2):269–278
Tristao FS, Amar M, Latrous I, Del-Bel EA, Prediger RD, Raisman-Vozari R (2014) Evaluation of nigrostriatal neurodegeneration and neuroinflammation following repeated intranasal 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) administration in mice, an experimental model of Parkinson’s disease. Neurotox Res 25(1):24–32. doi:10.1007/s12640-013-9401-8
Vijitruth R, Liu M, Choi DY, Nguyen XV, Hunter RL, Bing G (2006) Cyclooxygenase-2 mediates microglial activation and secondary dopaminergic cell death in the mouse MPTP model of Parkinson’s disease. J Neuroinflammation 3:6
Wang WF, Wu SL, Liou YM, Wang AL, Pawlak CR, Ho YJ (2009) MPTP lesion causes neuroinflammation and deficits in object recognition in Wistar rats. Behav Neurosci 123(6):1261–1270
Wang AL, Liou YM, Pawlak CR, Ho YJ (2010) Involvement of NMDA receptors in both MPTP-induced neuroinflammation and deficits in episodic-like memory in Wistar rats. Behav Brain Res 208(1):38–46
Weck KE, Kim SS, Virgin HI, Speck SH (1999) B cells regulate murine gammaherpesvirus 68 latency. J Virol 73(6):4651–4661
Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, Vadseth C, Choi DK, Ischiropoulos H, Przedborski S (2002) Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci 22(5):1763–1771
Wynne AM, Henry CJ, Huang Y, Cleland A, Godbout JP (2010) Protracted downregulation of CX3CR1 on microglia of aged mice after lipopolysaccharide challenge. Brain Behav Immun 24(7):1190–1201
Acknowledgments
The authors acknowledge Sabine Stolpe and Tanja Nilsson for their technical support. The authors gratefully thank the financial support and grants provided by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil), CAPES-COFECUB (France/Brazil; 681/2010), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil), Programa Ciência sem Fronteiras (CsF, Brazil), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Brazil), German Academic Exchange Service DAAD-ProBral/CAPES (FK/EADB) and the Max Planck Society (WS). SM was funded through the Cluster of Excellence and DFG Research Center Nanoscale Microscopy and Molecular Physiology of the Brain.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors have no financial or personal conflicts of interest related to this study.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tristão, F.S.M., Lazzarini, M., Martin, S. et al. CX3CR1 Disruption Differentially Influences Dopaminergic Neuron Degeneration in Parkinsonian Mice Depending on the Neurotoxin and Route of Administration. Neurotox Res 29, 364–380 (2016). https://doi.org/10.1007/s12640-015-9557-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-015-9557-5