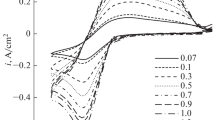
Abstract
The work is aimed at studying the kinetics of silicon electrodeposition from different chloride melts in order to select both optimal melt compositions and their electrolysis parameters. The study was carried out in KCl, KCl-CsCl and LiCl–KCl-CsCl melts with the addition of K2SiF6 at different temperatures depending on the melting point of each system. The optimal deposition potentials and current densities for electrolysis were determined by voltammetry. The LiCl–KCl-CsCl based melts are characterized by the highest silicon electrodeposition rates. Moreover, lowering lithium chloride content increase electrodeposition rates at the same other conditions. In LiCl–KCl-CsCl melt containing lithium chloride, the electrodeposition rate is highest in an electrolyte with a reduced LiCl content due to the lowest decomposition rate of the additive. Galvanostatic and potentiostatic electrolysis was carried out in the melts with different composition that have the highest rate of electrodeposition. It was found that continuous deposits occur in the LiCl–KCl-CsCl melt, while in other melts silicon is deposited in the form of fibers and dendrites. Fibers with a diameter of up to 0.7 μm were obtained in LiCl-free melts, and films consisting mainly of spherical grains with a diameter of up to 1 μm were obtained in a melt with lithium chloride.
Similar content being viewed by others
Data Availability
The authors declare that the data supporting the findings of this study are available within the article and its supplementary information are available by request.
References
Cohen U (1977) Some prospective applications of silicon electrodeposition from molten fluorides to solar cell fabrication. J Electronic Mat 6:607–643. https://doi.org/10.1007/BF02660341
Korenko M, Vasková Z, Priščák J, Šimko F, Ambrová M, Shi Zh (2015) Density, viscosity and electrical conductivity of the molten cryolite electrolytes (Na3AlF6–SiO2) for solar grade silicon (Si–SoG) electrowinning. SILICON 7:261–267. https://doi.org/10.1007/s12633-014-9214-2
Juzeliunas E, Fray DJ (2020) Silicon electrochemistry in molten salts. Chem Reviews 120:1690. https://doi.org/10.1021/acs.chemrev.9b00428
Rachedi N, Hadjersi T, Moulai F, Dokhane N (2022) Diamond-like carbon - coated silicon nanowires as a supercapacitor electrode in an aqueous LiClO4 electrolyte. SILICON 14:2533–2543. https://doi.org/10.1007/s12633-021-01046-y
Yasuda K, Maeda K, Hagiwara R, Homma T, Nohira T (2017) Silicon electrodeposition in a water-soluble KF–KCl molten salt: Utilization of SiCl4 as Si source. J Electrochem Soc 164:D67–D71. https://doi.org/10.1149/2.0641702JES
Zhuk SI, Isaev VA, Grishenkova OV, Isakov AV, Apisarov AP, Zaikov YuP (2017) Silicon electrodeposition from chloride-fluoride melts containing K2SiF6 and SiO2. J Serb Chem Soc 82(1):51–62. https://doi.org/10.2298/JSC160712109Z
Zhuk SI, Isakov AV, Apisarov AP, Grishenkova OV, Isaev VA, Vovkotrub EG, Zaikov YuP (2017) Electrodeposition of continuous silicon coatings from the KF-KCl-K2SiF6 melts. J Electrochem Soc 164(8):H5135–H5138. https://doi.org/10.1149/2.0171708jes
Yang X, Ji L, Zou X, Lim T, Zhao J, Yu ET, Bard AJ (2017) Toward cost-effective manufacturing of silicon solar cells: Electrodeposition of high-quality Si films in a CaCl2-based molten salt. Angew Chemie Int Ed 129:15274–15278. https://doi.org/10.1002/anie.201707635
Cho SK, Lim T (2021) Catalyst-mediated doping in electrochemical growth of solar silicon. Electrochim Acta 367:137472. https://doi.org/10.1016/j.electacta.2020.137472
Yu Zh, Wang N, Fang Sh, Qi X, Gao Zh, Yang J, Lu Sh (2020) Pilot-plant production of high-performance silicon nanowires by molten salt electrolysis of silica. Ind Eng Chem Res 59:1–8. https://doi.org/10.1021/acs.iecr.9b04430
Xu L, Tian C, Bao C, Liu T, Xia H (2023) Potential of silicon and carbon nanocages (C38, F-C38, Cl-C38, Si38, F-Si38, Cl-Si38) as anode materials in Li-ion battery and Mg-ion battery. Silicon (in press). https://doi.org/10.1007/s12633-023-02580-7
Rani S, Dhaka R, Shukla AK (2023) Next-generation cost- effective photovoltaic cell with tailored structural and optical properties of silicon nanowires with silver nanoparticle deposition. Silicon (in press). https://doi.org/10.1007/s12633-023-02601-5
Saif OM, Zekry AH, Abouelatta M, Shaker A (2023) A comprehensive review of tandem solar cells integrated on silicon substrate: III/V vs perovskite. Silicon (in press). https://doi.org/10.1007/s12633-023-02466-8
Gor BM (2023) Hydrogen storage in porous silicon – A review. SILICON 15:4663–4673. https://doi.org/10.1007/s12633-023-02355-0
Yasuda K, Kato T, Norikawa Yu, Nohira T (2021) Silicon electrodeposition in a water-soluble KF-KCl molten salt: Properties of Si films on graphite substrates. J Electrochem Soc 168:112502. https://doi.org/10.1149/1945-7111/ac3272
Zou X, Ji L, Ge J, Sadoway DR, Yu ET, Bard AJ (2019) Electrodeposition of crystalline silicon films from silicon dioxide for low-cost photovoltaic applications. Nat Comm 10:5772. https://doi.org/10.1038/s41467-019-13065-w
Nikolaev A, Mullabaev A, Suzdaltsev A, Zaikov Y (2023) Efficiency of electrochemical methods of purification and control over the oxide concentration in halide melts: PbCl2. Processes 11:636. https://doi.org/10.3390/pr11020636
Gevel T, Zhuk S, Suzdaltsev A, Zaikov Yu (2022) Silicon electrodeposition from the KCl-K2SiF6 melt. Ionics 28:3537–3545. https://doi.org/10.1007/s11581-022-04573-9
Gevel T, Zhuk S, Leonova N, Leonova A, Trofimov A, Suzdaltsev A, Zaikov Yu (2021) Electrochemical synthesis of nano-sized silicon from KCl-K2SiF6 melts for powerful lithium-ion batteries. Applied Science 11(22):10927. https://doi.org/10.3390/app112210927
Pavlenko OB, Ustinova YuA, Zhuk SI, Suzdaltsev AV, Zaikov YuP (2022) Silicon electrodeposition from low-melting LiCl-KCl-CsCl melts. Rus Met (Metally) 2022(8):818–824. https://doi.org/10.1134/S0036029522080109
Ustinova Yu, Pavlenko O, Gevel T, Zhuk S, Suzdaltsev A, Zaikov Yu (2022) Electrodeposition of silicon from the low-melting LiCl-KCl-CsCl-K2SiF6 electrolytes. J Electrochem Soc 169(3):032506. https://doi.org/10.1149/1945-7111/ac5a1c
Pavlenko OB, Suzdaltsev AV, Parasotchenko YA, and Zaikov YuP. (2023) Electrochemical synthesis and characterization of silicon thin films for energy conversion. Silicon (in press). https://doi.org/10.1007/s12633-023-02615-z.
Redkin A, Korzun I, Reznitskikh O, Yaroslavtseva T, Zaikov Y, Kumkov S (2028) J Thermal Analysis and Calorimetry 131:2021. https://doi.org/10.1007/s10973-017-6650-4
Zachara J, Wigniewski W (1995) Electronegativity force of cations and thermal decomposition of complex fluorides I. Thermal decomposition of fluorosilicates. J Thermal Analysis and Calorimetry 44:363–373. https://doi.org/10.1007/BF02636127
Suzdaltsev A (2022) Silicon electrodeposition for microelectronics and distributed energy: A mini-review. Electrochem 3:760–768. https://doi.org/10.3390/electrochem3040050
Chigondo F (2018) From metallurgical-grade to solar-grade silicon: An overview. SILICON 10:789–798. https://doi.org/10.1007/s12633-016-9532-7
Stepanov VP. (2012) The main issues of electrochemistry of molten salts. Ekaterinburg, UB RAS. ISBN 978–5–7691–2304–7 (in Russian).
Acknowledgements
The research was supported by Russian Science Foundation, agreement 23-23-00361, https://rscf.ru/project/23-23-00361.
Funding
None.
Author information
Authors and Affiliations
Contributions
The manuscript was written by the contributions of all authors. All authors approved the final version of the manuscript. Yulia A. Parasotchenko – experimental data, original draft, visualization, translating. Timofey A. Gevel – electrochemical measurements, electrolysis, XRD analysis. Olga B. Pavlenko – electrolysis. Leonid V. Gorshkov – electrolysis, melts preparation, sediments washing. Natalia M. Leonova – SEM analysis. Andrey V. Suzdaltsev – original idea, methodology, supervision, original draft, reviewing. Yury P. Zaikov – scientific supporting.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Ethical Approval
Not Applicable.
Consent for Publications
All the authors of the manuscript mutually agree on submission and publication in the journal.
Consent to Participate
All the authors contributed to the work and revised the manuscript.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Silicon electrodeposition
• Novel low F chloride electrolytes
• Silicon fibers and films.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Parasotchenko, Y.A., Gevel, T.A., Pavlenko, O.B. et al. Choice of the Composition of the Chloride Melts for the Electrochemical Synthesis of Silicon. Silicon 16, 1025–1032 (2024). https://doi.org/10.1007/s12633-023-02744-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12633-023-02744-5