Abstract
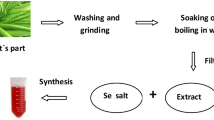
In this study, the authors aimed to expand a sustainable and gainful methodology for the purpose of synthesizing silver nanoparticles (AgNPs) using polyphenol bioactive compounds extracted from Eichhornia crassipes. The eco-friendliness of this method was also emphasized. The ethanol leaf extract with silver nitrate solution heated and filtered then the AgNPs produced were extensively the samples were thoroughly nanoparticles charaterized using a range of analytical techniques, including UV (nanoparticles wavelength measured), FTIR (functional groups), SEM (morphology, size, range, etc.,), TEM (morphological size of nanoparticles), XRD (crystaline sturcture), EDX (elemental composition), and AFM (irregular shape). In results the characterization process revealed the presence of secondary metabolites, total phenol content (TPC) observed that the highest value of 212.47 ± 7.07 (GAE). Additionally, through GC–MS analysis 12 major bioactive compounds were identified; In UV-Visible spectrum analysized its confirmed that size of nanoparticles in 448 nm absorption of the surface Plasmon resonance band by observed. The dimension of AgNPs was analyzed through SEM was found 11 nm. TEM and SAED analyses revealed the high crystallinity of AgNPs with homogeneous polycrystalline components and lattice spacing at 0.295. XRD patterns displayed four Bragg reflections at 38.45 (111), 46.35 (200), 64.75 (220), and 78.05 (301). Energy synthesis in EDX was observed in 8.79 nm. The AFM analysis the irregular shapes noticed. The synthesized AgNPs tested antimicrobial properties against various strains of microorganisms, including Staphylococcus aureus and Fusarium graminium. The synthesized AgNPs demonstrated potent antimicrobial activity, as evidenced by the highest zones of inhibition observed against Staphylococcus aureus (16.8 ± 0.03 mm) bacteria and Fusarium graminium (12.01 ± 0.01 mm) fungi at a concentration of 80 µg/mL. Our findings concluded that polyphenolic compounds are mostily in high amount reported in fresh fruits and vegetables so for that its having biological properties, same polyphenolic compounds we identified from leaf ethanolic extract of E. crassipes.
Similar content being viewed by others
Data Availability
No data was used for the research described in the article.
References
Kumari K, Bauddh K (2023) Chapter 18 - Phytoremediation of inorganic contaminants from the aquatic ecosystem using Eichhornia crassipes. In: Shukla SK, Kumar S, Madhav S, Mishra PK (eds) Metals in Water. Elsevier, pp 353–368. https://doi.org/10.1016/B978-0-323-95919-3.00001-X
Nguyen DTC, Van Tran T, Nguyen TTT, Nguyen DH, Alhassan M, Lee T (2022) New frontiers of invasive plants for biosynthesis of nanoparticles towards biomedical applications: A review. Science of The Total Environment:159278
Soto KM, López-Romero JM, Mendoza S, Peza-Ledesma C, Rivera-Muñoz EM, Velazquez-Castillo RR, Pineda-Piñón J, Méndez-Lozano N, Manzano-Ramírez A (2023) Rapid and facile synthesis of gold nanoparticles with two Mexican medicinal plants and a comparison with traditional chemical synthesis. Mater Chem Phys 295:127109. https://doi.org/10.1016/j.matchemphys.2022.127109
Murphy JW, Guentzel JL (2023) Total mercury and methylmercury concentrations in water hyacinth (Eichhornia crassipes) from a South Carolina coastal plain river. Aquatic Botany 184:103597. https://doi.org/10.1016/j.aquabot.2022.103597
Das RK, Pachapur VL, Lonappan L, Naghdi M, Pulicharla R, Maiti S, Cledon M, Dalila LMA, Sarma SJ, Brar SK (2017) Biological synthesis of metallic nanoparticles: plants, animals and microbial aspects. Nanotechnol Environ Eng 2(1):18. https://doi.org/10.1007/s41204-017-0029-4
Usui H, Shimizu Y, Sasaki T, Koshizaki N (2005) Photoluminescence of ZnO nanoparticles prepared by laser ablation in different surfactant solutions. J Phys Chem B 109(1):120–124. https://doi.org/10.1021/jp046747j
Yan J, Abdelgawad AM, El-Naggar ME, Rojas OJ (2016) Antibacterial activity of silver nanoparticles synthesized In-situ by solution spraying onto cellulose. Carbohyd Polym 147:500–508. https://doi.org/10.1016/j.carbpol.2016.03.029
Fozia F, Ahmad N, Buoharee ZA, Ahmad I, Aslam M, Wahab A, Ullah R, Ahmad S, Alotaibi A, Tariq A (2022) Characterization and Evaluation of Antimicrobial Potential of Trigonella incise (Linn) Mediated Biosynthesized Silver Nanoparticles. Molecules 27(14):4618
Rezazadeh NH, Buazar F, Matroodi S (2020) Synergistic effects of combinatorial chitosan and polyphenol biomolecules on enhanced antibacterial activity of biofunctionalized silver nanoparticles. Sci Rep 10(1):1–13
Galdopórpora JM, Ibar A, Tuttolomondo MV, Desimone MF (2021) Dual-effect core–shell polyphenol coated silver nanoparticles for tissue engineering. Nano-Struct Nano-Objects 26:100716. https://doi.org/10.1016/j.nanoso.2021.100716
Pandian A, Semwal D, Semwal R, Malaiyandi M, Sivaraj C, Vijayakumar S (2017) Total Phenolic Content, Volatile Constituents and Antioxidative Effect of Coriandrum sativum, Murraya koenigii and Mentha arvensis. Nat Prod J 7:65–74. https://doi.org/10.2174/2210315506666161121104251
Khane Y, Benouis K, Albukhaty S, Sulaiman GM, Abomughaid MM, Al Ali A, Aouf D, Fenniche F, Khane S, Chaibi W (2022) Green synthesis of silver nanoparticles using aqueous Citrus limon zest extract: Characterization and evaluation of their antioxidant and antimicrobial properties. Nanomaterials 12(12):2013
Naseem K, Zia Ur Rehman M, Ahmad A, Dubal D, AlGarni TS (2020) Plant extract induced biogenic preparation of silver nanoparticles and their potential as catalyst for degradation of toxic dyes. Coatings 10(12):1235
Asgary V, Shoari A, Baghbani-Arani F, Shandiz SAS, Khosravy MS, Janani A, Bigdeli R, Bashar R, Cohan RA (2016) Green synthesis and evaluation of silver nanoparticles as adjuvant in rabies veterinary vaccine. Int J Nanomed 11:3597
Shah M, Nawaz S, Jan H, Uddin N, Ali A, Anjum S, Giglioli-Guivarc’h N, Hano C, Abbasi BH (2020) Synthesis of bio-mediated silver nanoparticles from Silybum marianum and their biological and clinical activities. Mater Sci Eng C, Mater Biol Appl 112:110889. https://doi.org/10.1016/j.msec.2020.110889
Sumra AA, Zain M, Saleem T, Yasin G, Azhar MF, Zaman QU, Budhram-Mahadeo V, Ali HM (2023) Biogenic Synthesis, Characterization, and In Vitro Biological Evaluation of Silver Nanoparticles Using Cleome brachycarpa. Plants 12(7):1578
Nagarajan S, Arjun P, Raaman N, Shah A, Sobhia ME, Das TM (2012) Stereoselective synthesis of sugar-based β-lactam derivatives: docking studies and its biological evaluation. Tetrahedron 68(14):3037–3045. https://doi.org/10.1016/j.tet.2012.02.017
Periasamy S, Jegadeesan U, Sundaramoorthi K, Rajeswari T, Tokala VNB, Bhattacharya S, Muthusamy S, Sankoh M, Nellore MK (2022) Comparative Analysis of Synthesis and Characterization of Silver Nanoparticles Extracted Using Leaf, Flower, and Bark of Hibiscus rosasinensis and Examine Its Antimicrobicidal Activity. J Nanomater 2022:1–10
Ali SG, Ansari MA, Alzohairy MA, Alomary MN, Jalal M, AlYahya S, Asiri SMM, Khan HM (2020) Effect of biosynthesized ZnO nanoparticles on multi-drug resistant Pseudomonas aeruginosa. Antibiotics 9(5):260
Al-Humaid A, Mousa H, El-Mergawi R, Abdel-Salam A (2010) Chemical composition and antioxidant activity of dates and dates-camel-milk mixtures as a protective meal against lipid peroxidation in rats. Am J Food Technol 5(1):22–30
Méndez-Loranca E, Vidal-Ruiz AM, Martínez-González O, Huerta-Aguilar CA, Gutierrez-Uribe JA (2023) Beyond cellulose extraction: Recovery of phytochemicals and contaminants to revalorize agricultural waste. Biores Technol Rep 21:101339. https://doi.org/10.1016/j.biteb.2023.101339
Tunnisa F, Nur Faridah D, Afriyanti A, Rosalina D, Ana Syabana M, Darmawan N, Dewi Yuliana N (2022) Antioxidant and antidiabetic compounds identification in several Indonesian underutilized Zingiberaceae spices using SPME-GC/MS-based volatilomics and in silico methods. Food Chemistry: X 14:100285. https://doi.org/10.1016/j.fochx.2022.100285
Rashed MMA, Mahdi AA, Ghaleb ADS, Zhang FR, YongHua D, Qin W, WanHai Z (2020) Synergistic effects of amorphous OSA-modified starch, unsaturated lipid-carrier, and sonocavitation treatment in fabricating of Lavandula angustifolia essential oil nanoparticles. Int J Biol Macromol 151:702–712. https://doi.org/10.1016/j.ijbiomac.2020.02.224
Dubois LM, Perrault KA, Stefanuto P-H, Koschinski S, Edwards M, McGregor L, Focant J-F (2017) Thermal desorption comprehensive two-dimensional gas chromatography coupled to variable-energy electron ionization time-of-flight mass spectrometry for monitoring subtle changes in volatile organic compound profiles of human blood. J Chromatogr A 1501:117–127. https://doi.org/10.1016/j.chroma.2017.04.026
Delaney P, Sarvothaman VP, Colgan R, Nagarajan S, Deshmukh G, Rooney D, Robertson PKJ, Ranade VV (2022) Removal of single and dual ring thiophene’s from dodecane using cavitation based processes. Ultrasonics Sonochemistry 89:106148. https://doi.org/10.1016/j.ultsonch.2022.106148
Xiao N, Xu H, Jiang X, Sun T, Luo Y, Shi W (2022) Evaluation of aroma characteristics in grass carp mince as affected by different washing processes using an E-nose, HS-SPME-GC-MS, HS-GC-IMS, and sensory analysis. Food Res Int 158:111584. https://doi.org/10.1016/j.foodres.2022.111584
Su H, Kuang X, Ren Y, Luo L (2022) Biostimulants promote biodegradation of n-hexadecane by Raoultella planticola: Generation of lipopeptide biosurfactants. J Environ Chem Eng 10(5):108382. https://doi.org/10.1016/j.jece.2022.108382
Chen X, Cao J, Geng A, Zhang X, Wang H, Chu Q, Yan Z, Zhang Y, Liu H, Zhang J (2023) Integration of GC-MS and LC-MS for metabolite characteristics of thigh meat between fast- and slow-growing broilers at marketable age. Food Chemistry 403:134362. https://doi.org/10.1016/j.foodchem.2022.134362
Xu L, Cheng H, Müller M, Sinclair AJ, Wang W, Guo X, Vetter W, Wang Y (2023) Targeted quantitation of furan fatty acids in edible oils by gas chromatography/triple quadrupole tandem mass spectrometry (GC-TQ/MS). Food Chemistry 404:134521. https://doi.org/10.1016/j.foodchem.2022.134521
Li Y, Cao Z, Yu Z, Zhu Y, Zhao K (2023) Effect of inoculating mixed starter cultures of Lactobacillus and Staphylococcus on bacterial communities and volatile flavor in fermented sausages. Food Sci Human Wellness 12(1):200–211. https://doi.org/10.1016/j.fshw.2022.07.010
Azhagu Madhavan S, Sa V, Ra S, Sb R (2021) Phytochemical Screening and GC–MS Analysis Of Bioactive Compounds Present In Ethanolic Leaf Extract Murraya koenigii. Bull Env Pharmacol Life Sci 10:158–164
Madhavan SA, Vinotha P, Uma V (2020) Phytochemical screening and comparative gc–ms analysis of bioactive compounds present in methanolic leaf and latex extract Calotropis gigantea (L). Asian Journal of Advances in Medical Science:1–13
Shanab SM, Shalaby EA, Lightfoot DA, El-Shemy HA (2010) Allelopathic effects of water hyacinth [Eichhornia crassipes]. PLoS ONE 5(10):e13200
De Laet C, Matringe T, Petit E, Grison C (2019) Eichhornia crassipes: a Powerful Bio-indicator for Water Pollution by Emerging Pollutants. Sci Rep 9(1):7326. https://doi.org/10.1038/s41598-019-43769-4
Tian Y, Xu Z, Liu Z, Zhu R, Zhang F, Liu Z, Si X (2022) Botanical discrimination and classification of Mentha plants applying two-chiral column tandem GC-MS analysis of eight menthol enantiomers. Food Research International:112035. https://doi.org/10.1016/j.foodres.2022.112035
Taleghani A (2022) New bioactive compounds characterized by liquid chromatography-mass spectrometry and gas chromatography-mass spectrometry in hydro-methanol and petroleum ether extracts of Prosopis farcta (Banks & Sol) JF Macbr weed. J Mass Spectrometry: JMS 57(9):e4884–e4884
Ganji SM, Singh H, Friedman M (2019) Phenolic content and antioxidant activity of extracts of 12 melon (Cucumis melo) peel powders prepared from commercial melons. J Food Sci 84(7):1943–1948
Abdo MM, Abdel-Hamid MI, El-Sherbiny IM, El-Sherbeny G, Abdel-Aal EI (2023) Green synthesis of AgNPs, alginate microbeads and Chlorella minutissima laden alginate microbeads for tertiary treatment of municipal wastewater. Biores Technol Rep 21:101300. https://doi.org/10.1016/j.biteb.2022.101300
Henglein A (1993) Physicochemical properties of small metal particles in solution:" microelectrode" reactions, chemisorption, composite metal particles, and the atom-to-metal transition. J Phys Chem 97(21):5457–5471
Alzubaidi AK, Al-Kaabi WJ, Ali AA, Albukhaty S, Al-Karagoly H, Sulaiman GM, Asiri M, Khane Y (2023) Green Synthesis and Characterization of Silver Nanoparticles Using Flaxseed Extract and Evaluation of Their Antibacterial and Antioxidant Activities. Appl Sci 13(4):2182
Kumar D, Kumar G, Das R, Agrawal V (2018) Strong larvicidal potential of silver nanoparticles (AgNPs) synthesized using Holarrhena antidysenterica (L.) Wall. bark extract against malarial vector, Anopheles stephensi Liston. Process Safety and Environmental Protection 116. https://doi.org/10.1016/j.psep.2018.02.001
He Y, Wei F, Ma Z, Zhang H, Yang Q, Yao B, Huang Z, Li J, Zeng C, Zhang Q (2017) Green synthesis of silver nanoparticles using seed extract of Alpinia katsumadai, and their antioxidant, cytotoxicity, and antibacterial activities. RSC Adv 7:39842–39851. https://doi.org/10.1039/C7RA05286C
Qais F, Shafiq A, Khan H, Husain F, Khan RA, Alenazi B, Alsalme A, Ahmad I (2019) Antibacterial Effect of Silver Nanoparticles Synthesized Using Murraya koenigii (L.) against Multidrug-Resistant Pathogens. Bioinorg Chem Appl 2019:1–11. https://doi.org/10.1155/2019/4649506
Sabapathi N, Ramalingam S, Aruljothi KN, Lee J, Barathi S (2023) Characterization and Therapeutic Applications of Biosynthesized Silver Nanoparticles Using Cassia auriculate Flower Extract. Plants 12(4):707
Mickymaray S (2019) One-step Synthesis of Silver Nanoparticles Using Saudi Arabian Desert Seasonal Plant Sisymbrium irio and Antibacterial Activity Against Multidrug-Resistant Bacterial Strains. Biomolecules 9:662. https://doi.org/10.3390/biom9110662
Tomaszewska E, Soliwoda K, Kadziola K, Tkacz-Szczesna B, Celichowski G, Cichomski M, Szmaja W, Grobelny J (2013) Detection limits of DLS and UV-Vis spectroscopy in characterization of polydisperse nanoparticles colloids. J Nanomater 2013:60–60
Zhang D, Wang C, Shen L, Shin H-C, Lee KB, Ji B (2018) Comparative analysis of oxidative mechanisms of phloroglucinol and dieckol by electrochemical, spectroscopic, cellular and computational methods. RSC Adv 8(4):1963–1972
Prabu HJ, Johnson I (2015) Plant-mediated biosynthesis and characterization of silver nanoparticles by leaf extracts of Tragia involucrata, Cymbopogon citronella, Solanum verbascifolium and Tylophora ovata. Karbala Int J Modern Sci 1(4):237–246
Chakravarty P, Sarma NS, Sarma H (2010) Removal of lead (II) from aqueous solution using heartwood of Areca catechu powder. Desalination 256(1–3):16–21
Sun Q, Cai X, Li J, Zheng M, Chen Z, Yu C-P (2014) Green synthesis of silver nanoparticles using tea leaf extract and evaluation of their stability and antibacterial activity. Colloids Surf, A 444:226–231
Saligedo TS, Muleta GG, Tsega TW, Tadele KT (2023) Green Synthesis of Copper Oxide Nanoparticles Using Eichhornia Crassipes Leaf Extract, its Antibacterial and Photocatalytic Activities. Curr Nanomater 8(1):58–68
Deeksha B, Sadanand V, Hariram N, Rajulu AV (2021) Preparation and Properties of Cellulose Nanocomposite Fabrics with in situ Generated Silver Nanoparticles by Bioreduction Method. J Biores Bioprod 6(1):75. https://doi.org/10.1016/j.jobab.2021.01.003
Bhutto AA, Kalay Ş, Sherazi STH, Culha M (2018) Quantitative structure–activity relationship between antioxidant capacity of phenolic compounds and the plasmonic properties of silver nanoparticles. Talanta 189:174–181. https://doi.org/10.1016/j.talanta.2018.06.080
Clichici S, David L, Moldovan B, Baldea I, Olteanu D, Filip M, Nagy A, Luca V, Crivii C, Mircea P, Katona G, Filip GA (2020) Hepatoprotective effects of silymarin coated gold nanoparticles in experimental cholestasis. Mater Sci Eng: C 115:111117. https://doi.org/10.1016/j.msec.2020.111117
Chinnathambi A, Alharbi SA, Joshi DVS, Jhanani GK, On-uma R, Jutamas K, Anupong W (2023) Synthesis of AgNPs from leaf extract of Naringi crenulata and evaluation of its antibacterial activity against multidrug resistant bacteria. Environ Res 216:114455. https://doi.org/10.1016/j.envres.2022.114455
Rajkumar G, Sundar R (2022) Biogenic one-step synthesis of silver nanoparticles (AgNPs) using an aqueous extract of Persea americana seed: Characterization, phytochemical screening, antibacterial, antifungal and antioxidant activities. Inorganic Chem Commu 143:109817. https://doi.org/10.1016/j.inoche.2022.109817
Sheny DS, Mathew J, Philip D (2011) Phytosynthesis of Au, Ag and Au–Ag bimetallic nanoparticles using aqueous extract and dried leaf of Anacardium occidentale. Spectrochim Acta Part A Mol Biomol Spectrosc 79(1):254–262. https://doi.org/10.1016/j.saa.2011.02.051
Katta VKM, Dubey RS (2021) Green synthesis of silver nanoparticles using Tagetes erecta plant and investigation of their structural, optical, chemical and morphological properties. Mater Today: Proceed 45:794–798. https://doi.org/10.1016/j.matpr.2020.02.809
Kumar R, Arora N, Gupta M, Katyal P (2022) An experimental approach on characterization techniques of zinc oxide nanoparticles. Mater Today: Proceed 56:2469–2477. https://doi.org/10.1016/j.matpr.2021.08.239
Amutha K, Grace Annapoorani S, Sudhapriya N (2020) Dyeing of textiles with natural dyes extracted from Terminalia arjuna and Thespesia populnea fruits. Industrial Crops Prod 148:112303. https://doi.org/10.1016/j.indcrop.2020.112303
Xue H, Wang X, Xu Q, Dhaouadi F, Sellaoui L, Seliem MK, Lamine AB, Belmabrouk H, Bajahzar A, Bonilla-Petriciolet A (2022) Adsorption of methylene blue from aqueous solution on activated carbons and composite prepared from an agricultural waste biomass: A comparative study by experimental and advanced modeling analysis. Chem Eng J 430:132801
Ovais M, Khalil AT, Raza A, Khan MA, Ahmad I, Islam NU, Saravanan M, Ubaid MF, Ali M, Shinwari ZK (2016) Green synthesis of silver nanoparticles via plant extracts: beginning a new era in cancer theranostics. Nanomedicine 12(23):3157–3177
Raut RW, Mendhulkar VD, Kashid SB (2014) Photosensitized synthesis of silver nanoparticles using Withania somnifera leaf powder and silver nitrate. J Photochem Photobiol, B 132:45–55
Feng QL, Wu J, Chen GQ, Cui F, Kim T, Kim J (2000) A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res 52(4):662–668
Periasamy S, Jegadeesan U, Sundaramoorthi K, Rajeswari T, Tokala VNB, Bhattacharya S, Muthusamy S, Sankoh M, Nellore MK (2022) Comparative Analysis of Synthesis and Characterization of Silver Nanoparticles Extracted Using Leaf, Flower, and Bark of Hibiscus rosasinensis and Examine Its Antimicrobicidal Activity. J Nanomater 2022:8123854. https://doi.org/10.1155/2022/8123854
Nayak D, Ashe S, Rauta PR, Nayak B (2015) Biosynthesis, characterisation and antimicrobial activity of silver nanoparticles using Hibiscus rosa-sinensis petals extracts. IET Nanobiotechnol 9(5):288–293. https://doi.org/10.1049/iet-nbt.2014.0047
Sridhara V, Pratima K, Krishnamurthy G, Sreekanth B (2013) Vegetable assisted synthesis of silver nanoparticles and its antibacterial activity against two human pathogens.
Pallavi S, Rudayni HA, Bepari A, Niazi SK, Nayaka S (2022) Green synthesis of Silver nanoparticles using Streptomyces hirsutus strain SNPGA-8 and their characterization, antimicrobial activity, and anticancer activity against human lung carcinoma cell line A549. Saudi J Biol Sci 29(1):228–238
Singh A, Dar MY, Joshi B, Sharma B, Shrivastava S, Shukla S (2018) Phytofabrication of silver nanoparticles: novel drug to overcome hepatocellular ailments. Toxicol Rep 5:333–342
Acknowledgements
The mentioned statement indicates that the work is supported by the Department of Community Medicine at Saveetha Institute of Medical And Technical Sciences (SIMATS) in Chennai, Tamil Nadu, India.
Funding
The authors express their gratitude to the Department of Biotechnology, New Delhi, India (Grant Number: BT/PR29159/FCB/125/9/2018) for their financial support.
Author information
Authors and Affiliations
Contributions
Azhagu Madhavan Sivalingam:- Work Designed, Writing –Original Draft, Writing-Review & Editing, Grammar Checking, Visualization, Methodology, Formal analysis and Investigation. Arjun Pandian:- Editing, Formal analysis, Methodology, Visualization. Sumathy Rengarajan:- Formal analysis and methodology. Raju Ramasubbu:- Funding sources and formal analysis. All the authors give final approval of the version to be submitted.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sivalingam, A.M., Pandian, A., Rengarajan, S. et al. Polyphenol-compounds From Green Synthesis of Antimicrobial property of Silver Nanoparticles using Eichhornia crassipes: Characterization and Applications. Silicon 15, 7415–7429 (2023). https://doi.org/10.1007/s12633-023-02593-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12633-023-02593-2