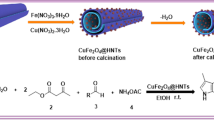
Abstract
Halloysite as an impressive natural eco-friendly nanotube with aluminosilicate structure has been investigated recently due to its unique features such as specific morphology and excellent bio-adaptability. In this research, Fe3O4 nanoparticles have been loaded on the tubular halloysite by co-precipitation method in order to synthesis magnetic halloysite (Hal-Fe3O4). To characterize this recoverable nanocatalyst, applicable analyses such as Fourier-transform infrared (FT-IR) spectroscopy, energy-dispersive X-ray (EDX) analysis, field-emission scanning electron microscopy (FE-SEM) images, X-ray diffraction (XRD) pattern, Thermogravimetric analysis (TGA) and vibrating sample magnetometer (VSM) curves have been carried out. The results confirmed that Fe3O4 nanoparticles with cubic structure, and uniform distribution, were located at halloysite nanotubes (HNTs). This aluminosilicate nanocomposite with high thermal stability, crystalline structure, and stable morphology was evaluated as a heterogeneous catalyst in the symmetrical Hantzsch reaction for the first time. Easy synthesis process, green media, high performance, recoverable catalyst and reusing of the Hal-Fe3O4 as a nanocatalyst for 8 times are the main features of this protocol.
Similar content being viewed by others
References
Maleki A, Hajizadeh Z, Salehi P (2019) Mesoporous halloysite nanotubes modified by CuFe2O4 spinel ferrite nanoparticles and study of its application as a novel and efficient heterogeneous catalyst in the synthesis of pyrazolopyridine derivatives. Sci Rep 9:5552. https://doi.org/10.1038/s41598-019-42126-9
Maleki A, Hajizadeh Z, Firozi-Haji R (2018) Eco-friendly functionalization of magnetic halloysite nanotube with SO3H for synthesis of dihydropyrimidinones. Micropor Mesopor Mater 259:46–53
Maleki A, Hajizadeh Z (2019) Silicon. https://doi.org/10.1007/s12633-019-0069-4
Zhang Y, Tang A, Yang H, Ouyang J (2016) Applications and interfaces of halloysite nanocomposites. Appl Clay Sci 119:8–17
Veerabadran NG, Price RR, Lvov YM (2007) Clay nanotubes for encapsulation and sustained release of drugs. Nano 2:115–120
Xie A, Dai J, Chen X, Zou T, He J, Chang Z, Li C, Yan Y (2016) Hollow imprinted polymer nanorods with a tunable shell using halloysite nanotubes as a sacrificial template for selective recognition and separation of chloramphenicol. RSC Adv 6:51014–51023
Xie Y, Chang PR, Wang S, Yu J, Ma X (2011) Preparation and properties of halloysite nanotubes/plasticized Dioscorea opposita Thunb. starch composites. Carbohydr Polym 83:186–191
Zou M, Du M, Zhang M, Yang T, Zhu H, Wang P, Bao S (2015) Synthesis and deposition of ultrafine noble metallic nanoparticles on amino-functionalized halloysite nanotubes and their catalytic application. Mater Res Bull 61:375–382
Fattahi N, Ramazani A, Kinzhybalo V (2018) Silicon. https://doi.org/10.1007/s12633-017-9757-0
Fattahi N, Ramazani A, Ahankar H, Azimzadeh Asiabi P, Kinzhybalo V (2018) Silicon. https://doi.org/10.1007/s12633-018-9954-5
Maleki A, Rahimi J, Hajizadeh Z, Niksefat M (2019) Synthesis and characterization of an acidic nanostructure based on magnetic polyvinyl alcohol as an efficient heterogeneous nanocatalyst for the synthesis of α-aminonitriles. J Organomet Chem 881:58–65
Hajizadeh Z, Maleki A (2018) Poly(ethylene imine)-modified magnetic halloysite nanotubes: A novel, efficient and recyclable catalyst for the synthesis of dihydropyrano[2,3-c]pyrazole derivatives. Mol Catal 460:87–93
Maleki A, Hajizadeh Z, Sharifi V, Emdadi Z (2019) A green, porous and eco-friendly magnetic geopolymer adsorbent for heavy metals removal from aqueous solutions. J Clean Prod 215:1233–1245
Riahi-Madvaar R, Taher MA, Fazelirad H (2017) Synthesis and characterization of magnetic halloysite-iron oxide nanocomposite and its application for naphthol green B removal. Appl Clay Sci 137:101–106
Duan J, Liu R, Chen T, Zhang B, Liu J (2012) Halloysite nanotube-Fe3O4 composite for removal of methyl violet from aqueous solutions. Desalination 293:46–52
Tian X, Wang W, Tian N, Zhou C, Yang C, Komarneni S (2016) Cr(VI) reduction and immobilization by novel carbonaceous modified magnetic Fe 3 O 4 /halloysite nanohybrid. J Hazard Mater 309:151–156
Mu B, Wang W, Zhang J, Wang A (2014) Superparamagnetic sandwich structured silver/halloysite nanotube/Fe3O4nanocomposites for 4-nitrophenol reduction. RSC Adv 4:39439–39445
Hyvönen Z, Hämäläinen V, Ruponen M, Lucas B, Rejman J, Vercauteren D, Demeester J, De Smedt S, Braeckmans K (2012) Elucidating the pre- and post-nuclear intracellular processing of 1,4-dihydropyridine based gene delivery carriers. J Control Release 162:167–175
Rucins M, Kaldre D, Pajuste K, Fernandes MAS, Vicente JAF, Klimaviciusa L, Jaschenko E, Kanepe-Lapsa I, Shestakova I, Plotniece M, Gosteva M, Sobolev A, Jansone B, Muceniece R, Klusa V, Plotniece A (2014) Synthesis and studies of calcium channel blocking and antioxidant activities of novel 4-pyridinium and/or N-propargyl substituted 1,4-dihydropyridine derivatives. C R Chim 17:69–80
Hilgeroth A, Fleischer R, Wiese M, Heinemann FW (1999). J Comput Aided Mol Des 13:233–242
Yiu SH, Knaus EE (1999) Synthesis of valproate, valerate, and 1-methyl-1, 4-dihydropyridyl-3-carbonyloxy ester derivatives of Hantzsch 1,4-dihydropyridines as potential prodrugs and their evaluation as calcium channel antagonist and anticonvulsant agents. Drug Dev Res 48:26–37
Dondoni A, Massi A, Minghini E, Bertolasi V (2004) Multicomponent Hantzsch cyclocondensation as a route to highly functionalized 2- and 4-dihydropyridylalanines, 2- and 4-pyridylalanines, and their N-oxides: preparation via a polymer-assisted solution-phase approach. Tetrahedron 60:2311–2326
Pareek P, Kant R, Shukla S, Ojha K (2010). Open Chem 8:163–173
Maleki A (2018) Green oxidation protocol: Selective conversions of alcohols and alkenes to aldehydes, ketones and epoxides by using a new multiwall carbon nanotube-based hybrid nanocatalyst via ultrasound irradiation. Ultrason Sonochem 40:460–464
Bitaraf M, Amoozadeh A, Otokesh S (2016) A simple and efficient one-pot synthesis of 1,4-dihydropyridines using nano-WO3- supported sulfonic acid as an heterogeneous catalyst under solvent-free conditions. J Chin Chem Soc 63:336–344
Amirheidari B, Seifi M, Abaszadeh M (2016) Evaluation of magnetically recyclable nano-Fe3O4 as a green catalyst for the synthesis of mono- and bis-tetrahydro-4H-chromene and mono and bis 1,4-dihydropyridine derivatives. Res Chem Intermed 42:3413–3423
Srinivasan VV, Pachamuthu MP, Maheswari R (2015) Lewis acidic mesoporous Fe-TUD-1 as catalysts for synthesis of Hantzsch 1,4-dihydropyridine derivatives. J Porous Mater 22:1187–1194
Maleki A, Firozi-Haji R, Hajizadeh Z (2018) Magnetic guanidinylated chitosan nanobiocomposite: A green catalyst for the synthesis of 1,4-dihydropyridines. Int J Biol Macromol 116:320–326
Maleki A, Kamalzare M, Aghaei M (2015) Efficient one-pot four-component synthesis of 1,4-dihydropyridines promoted by magnetite/chitosan as a magnetically recyclable heterogeneous nanocatalyst. J Nanostruct Chem 5:95–105
Luo P, Zhao Y, Zhang B, Liu J, Yang Y, Liu J (2010) Study on the adsorption of Neutral Red from aqueous solution onto halloysite nanotubes. Water Res 44:1489–1497
Hsieh S, Huang B, Hsieh S, Wu C, Wu C, Lin P, Huang Y, Chang C (2010) Green fabrication of agar-conjugated Fe3O4magnetic nanoparticles. Nanotechnology 21:445601–445606
Maleki A, Zand P, Mohseni Z (2016) Fe3O4@PEG-SO3H rod-like morphology along with the spherical nanoparticles: novel green nanocomposite design, preparation, characterization and catalytic application. RSC Adv 6:110928–110934
Dutta AK, Gogoi P, Borah R (2014) Synthesis of dibenzoxanthene and acridine derivatives catalyzed by 1,3-disulfonic acid imidazolium carboxylate ionic liquids. RSC Adv 4:41287–41291
Nasr-Esfahani M, Montazerozohori M, Abdizadeh T (2015) Nanorod vanadatesulfuric acid (VSA NRs)-catalyzed green synthesis of hexahydroacridine-1,8-diones in solvent-free conditions. C R Chim 18:547–553
Heydari A, Khaksar S, Tajbakhsh M, Bijanzadeh HR (2009) One-step, synthesis of Hantzsch esters and polyhydroquinoline derivatives in fluoro alcohols. J Fluor Chem 130:609–614
Khadilkar BM, Gaikar VG, Chitnavis AA (1995) Aqueous hydrotrope solution as a safer medium for microwave enhanced hantzsch dihydropyridine ester synthesis. Tetrahedron Lett 36:8083–8086
Memarian HR, Bagheri M, Döpp D (2004). Monatsh Chem Chem Mon 135:833–838
Eynde JJV, Delfosse F, Mayence A, Van Haverbeke Y (1995) Old reagents, new results: Aromatization of Hantzsch 1,4-dihydropyridines with manganese dioxide and 2,3-dichloro-5,6-dicyano-1,4-benzoquinone. Tetrahedron 51:6511–6516
Wang L, Zhu K-Q, Chen Q, He M-Y (2014). Green Process Synth 3:457–461
Chate AV, Rathod UB, Kshirsagar JS, Gaikwad PA, Mane KD, Mahajan PS, Nikam MD, Gill CH (2016) Ultrasound assisted multicomponent reactions: A green method for the synthesis of N-substituted 1,8-dioxo-decahydroacridines using β-cyclodextrin as a supramolecular reusable catalyst in water. Chin J Catal 37:146–152
Amoozadeh A, Golian S, Rahmani S (2015) TiO2-coated magnetite nanoparticle-supported sulfonic acid as a new, efficient, magnetically separable and reusable heterogeneous solid acid catalyst for multicomponent reactions. RSC Adv 5:45974–45982
Acknowledgements
The authors gratefully acknowledge the partial support from the Research Council of the Iran University of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 1961 kb)
Rights and permissions
About this article
Cite this article
Hajizadeh, Z., Maleki, A., Rahimi, J. et al. Halloysite Nanotubes Modified by Fe3O4 Nanoparticles and Applied as a Natural and Efficient Nanocatalyst for the SymmetricalHantzsch Reaction. Silicon 12, 1247–1256 (2020). https://doi.org/10.1007/s12633-019-00224-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12633-019-00224-3