Abstract
Purpose
There is evidence that cholinergic imbalance secondary to neuroinflammation plays a role in the pathophysiology of sepsis-associated encephalopathy (SAE). Blood acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) activities have been proposed as surrogate parameters for the cholinergic function of the central nervous system. Viral sepsis is associated with systemic inflammation and BChE has been reported to be of prognostic value in a small cohort of COVID-19 patients. Nevertheless, the prognostic value of AChE in patients with viral sepsis remains unclear.
Methods
We investigated the role of AChE and BChE activities as prognostic biomarkers of SAE and mortality in patients with viral vs nonviral sepsis enrolled in two prospective cohort studies. We quantified the AChE and BChE activities in whole blood of patients at two time points in the acute phase of viral sepsis (N = 108) and compared them with the activities in patients with nonviral sepsis (N = 117) and healthy volunteers (N = 81). Patients were observed until discharge from the intensive care unit (ICU).
Results
Three days after sepsis onset, the median [interquartile range] levels of AChE and BChE were reduced in both patients with viral sepsis (AChE, 5,105 [4,010–6,250] U·L−1; BChE, 1,943 [1,393–2,468] U·L−1) and nonviral sepsis (AChE, 4,424 [3,630–5,055] U·L−1; BChE, 1,095 [834–1,526] U·L−1) compared with healthy volunteers (AChE, 6,693 [5,401–8,020] U·L−1; BChE, 2,645 [2,198–3,478] U·L−1). Patients with viral sepsis with SAE during their ICU stay had lower AChE activity three days after sepsis onset than patients without SAE (4,249 [3,798–5,351] U·L−1 vs 5,544 [4,124–6,461] U·L−1). Butyrylcholinesterase activity seven days after sepsis onset was lower in patients with viral sepsis who died in the ICU than in surviving patients (1,427 [865–2,181] U·L−1 vs 2,122 [1,571–2,787] U·L−1).
Conclusion
Cholinesterase activities may be relevant prognostic markers for the occurrence of SAE and mortality in the ICU in patients with viral sepsis.
Study registration
This study constitutes an analysis of data from the ongoing studies ICROS (NCT03620409, first submitted 15 May 2018) and ICROVID (DRKS00024162, first submitted 9 February 2021).
Résumé
Objectif
Certaines données probantes soutiennent que le déséquilibre cholinergique secondaire à la neuroinflammation joue un rôle dans la physiopathologie de l’encéphalopathie associée au sepsis (EAS). Les activités de l’acétylcholinestérase (AChE) et de la butyrylcholinestérase (BChE) sanguines ont été proposées comme paramètres de substitution de la fonction cholinergique du système nerveux central. Le sepsis viral est associé à une inflammation systémique et il a été rapporté que la BChE possédait une valeur pronostique dans une petite cohorte atteinte de COVID-19. Néanmoins, la valeur pronostique de l’AChE chez les patient·es atteint·es de sepsis viral reste incertaine.
Méthode
Nous avons étudié le rôle des activités de l’AChE et de la BChE en tant que biomarqueurs pronostiques de l’EAS et de la mortalité chez les patient·es atteint·es de sepsis viral vs non viral recruté·es dans deux études de cohorte prospectives. Nous avons quantifié les activités de l’AChE et de la BChE dans le sang total de patient·es à deux moments de la phase aiguë du sepsis viral (N = 108) et les avons comparées aux activités chez les patient·es atteint·es de sepsis non viral (N = 117) et chez des volontaires sain·es (N = 81). Les patient·es ont été observé·es jusqu’à leur sortie de l’unité de soins intensifs (USI).
Résultats
Trois jours après l’apparition du sepsis, les taux médians [écart interquartile] d’AChE et BChE étaient réduits tant chez la patientèle atteinte de sepsis viral (AChE, 5105 [4010–6250] U·L−1; BChE, 1943 [1393–2468] U·L−1) et de sepsis non viral (AChE, 4424 [3630–5055] U·L−1; BChE, 1095 [834–1526] U·L−1) par rapport aux volontaires sain·es (AChE, 6693 [5401–8020] U·L−1; BChE, 2645 [2198–3478] U·L−1). Les patient·es atteint·es de sepsis viral avec EAS pendant leur séjour aux soins intensifs avaient une activité AChE plus faible trois jours après l’apparition du sepsis que les personnes sans EAS (4249 [3798–5351] U·L−1 vs 5544 [4124–6461] U·L−1). L’activité de la butyrylcholinestérase sept jours après l’apparition du sepsis était plus faible chez les patient·es atteint·es de sepsis viral décédé·es à l’USI que chez les personnes ayant survécu (1427 [865–2181] U·L-1 vs 2122 [1571–2787] U·L-1).
Conclusion
Les activités des cholinestérases pourraient constituer des marqueurs pronostiques pertinents pour la survenue d’EAS et la mortalité en soins intensifs chez la patientèle atteinte de sepsis viral.
Enregistrement de l’étude
Cette étude constitue une analyse des données des études en cours ICROS (NCT03620409, première soumission le 15 mai 2018) et ICROVID (DRKS00024162, première soumission le 9 février 2021).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
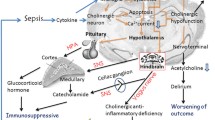
Sepsis-associated encephalopathy (SAE) is a frequent organ dysfunction in sepsis, associated with poor outcome.1 Various descriptions of SAE exist, but all are commonly defined as a reversible global impairment of consciousness in patients with sepsis excluding other causes.2 Neuroinflammation secondary to systemic inflammation is associated with an imbalance of the neurotransmitter acetylcholine (ACh) in the central nervous system (CNS).3,4,5 This cholinergic imbalance has been proposed as a possible cause of SAE.4
In the peripheral nervous system, ACh modulates the host immune response via the cholinergic anti-inflammatory pathway.6 In homeostasis, inflammation sensed by the vagus nerve leads to the secretion of ACh via vagal nerve endings. Acetylcholine binds to nicotinic, α-bungarotoxin-sensitive receptors on macrophages and lymphocytes, inhibiting the synthesis of proinflammatory cytokines and counterbalancing the sensed inflammation.6,7,8,9,10 A functional cholinergic anti-inflammatory pathway has been reported to be protective in experimental sepsis.11
Cholinergic activity in the CNS can be evaluated by measuring the activities of the ACh-degrading enzymes acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) in whole blood12,13 as their activities in blood correlate with those in the CNS.14,15,16 Acetylcholinesterase is present in the nervous system and in the cytosol of erythrocytes.17 Butyrylcholinesterase is produced in the liver and present in the plasma.18
The general objective of this study was to evaluate the prognostic value of AChE and BChE activities in patients with viral sepsis. Because of its lower prevalence compared with bacterial sepsis, viral sepsis has received less scientific attention as a separate sepsis entity despite calls for the adoption of a personalized approach to sepsis therapy.19 The COVID-19 pandemic, during which a persistent subset of patients required intensive care,20 made the need for a better understanding of viral sepsis evident. Influenza is another common cause of viral sepsis. Severe COVID-19 and influenza are characterized by many common immunological abnormalities, including systemic hyperinflammation referred to as cytokine storm.21
Hitherto, cholinesterase activities have rarely been assessed in viral sepsis. A prospective cohort study of 54 patients with severe COVID-19 found reduced levels of BChE activity compared with a historical healthy control group of 40 volunteers.22 Acetylcholinesterase activity has not been assessed in viral sepsis. Because viral sepsis is associated with systemic inflammation, we hypothesized a cholinergic imbalance in patients with viral sepsis—as seen in reduced cholinesterase activities—and a prognostic relevance thereof. The specific aims of this study were to evaluate 1) the effects of viral sepsis vs nonviral sepsis on AChE and BChE activities, 2) the prognostic values of AChE and BChE activities for the occurrence of SAE, and 3) the prognostic values of AChE and BChE activities for intensive care unit (ICU) mortality. To address these aims, we conducted an interim analysis of two ongoing prospective sepsis cohort studies including > 300 critically ill and healthy volunteers.23,24 We quantified AChE and BChE activities in patients at two time points in the acute phase of viral sepsis compared with nonviral sepsis and healthy volunteers.
Methods
Study design
This study constitutes a subanalysis of the ongoing prospective studies Identification of cardiovascular and molecular prognostic factors for the medium-term and long-term outcomes of sepsis (ICROS, NCT03620409)23 and Identification of cardiovascular and molecular prognostic factors for the morbidity and mortality in COVID-19-sepsis (ICROVID, DRKS00024162),24 in accordance with the Declaration of Helsinki. The Ethics Committee of the Friedrich Schiller University Jena (Jena, Germany) approved both studies (ICROS: 5276-09/17, 2017-10-10 and ICROVID: 2020-2052-BO, 2021-01-21). This subanalysis of cholinesterase activities was specified a priori in the protocols of both studies.23,24 Patients were enrolled from May 2018 to November 2022 in the ICU of the Department of Anesthesiology and Intensive Care Medicine, Jena University Hospital and met the Sepsis-3 criteria.25 Patients were allocated to an analytical cohort depending on clinical, microbiological, and virological findings. COVID-19 and influenza were diagnosed by positive polymerase chain reaction. Written informed consent was obtained by medical doctors of the study team not involved in the patient care from either the patient or the patient's legal proxy if the patient was incapacitated. Demographic and clinical data were collected from the digital charts and files at sepsis onset (T0), 3 ± 1 days (T1), and 7 ± 1 days (T2) after sepsis onset and during the entire ICU stay. Blood samples were collected at T1 and T2. Healthy volunteers were recruited in ICROS from May 2018 to June 2022. They were not formally matched with patients with nonviral sepsis but a target age range and gender proportion similar to that of patients with nonviral sepsis were taken into consideration during recruitment.
Definition of sepsis-associated encephalopathy
Because of the lack of concurrent diagnostic criteria3,26 and considering definitions in other studies,12,27,28,29 SAE was defined as a positive Confusion Assessment Method for the ICU (CAM-ICU) and/or Glasgow Coma Scale (GCS) < 15 and/or description of altered mental state or delirium in the doctor’s or nurse’s notes, all not attributed to other encephalopathies or medication.29 Patients with other causes of encephalopathy, including structural neurologic disease, mental-state-altering medication, and metabolic disorder were excluded from analysis. Sedation was defined as the use of any dose of a sedative (e.g., midazolam, clonidine, remifentanil, sufentanil, and propofol). If the patient was under moderate or deep sedation (target Richmond Agitation Sedation Scale [RASS] < –1), these sections of the chart were not evaluated for the presence of SAE. Patients with light sedation (target RASS –1 to 0) were considered to have SAE if they showed signs of hypoactive delirium (GCS < 9) and/or hyperactive delirium.
Measurement of cholinesterase activities and laboratory parameters
Point-of-care testing of cholinesterase activity was performed with assay kits and the device LISA-ChE® model 98LCHE003, s SN 22 (both, Dr. F. Köhler Chemie GmbH, Germany) in 10 µl ethylenediaminetetraacetic acid whole blood. The measurement is based on a colorimetric method, a variant of the Ellman method improved by Worek et al.30,31 The concentrations of hemoglobin (Hb), albumin, and cholinesterase (ChE) were measured photometrically and C-reactive protein (CRP), procalcitonin (PCT), ferritin, and interleukin-6 (IL-6) using immunoassays at the Institute for Clinical Chemistry and Laboratory Diagnostic of Jena University Hospital.
Statistical analyses
We report median and interquartile ranges [IQRs] for continuous demographic, clinical, and laboratory data and absolute and relative frequencies for categorical variables. For intergroup comparisons, we applied Mann–Whitney U tests for continuous variables and Chi square tests for categorical variables.
Because of repeated measurements, the associations were assessed using Spearman’s rank correlation coefficients including 95% confidence intervals and P values for each study time point. We used receiver operating characteristic analysis32 to obtain thresholds (i.e., cut-offs) of AChE and BChE levels for predicting ICU mortality. The optimal cut-off was chosen based on Youden’s Index.33
Statistical analysis was performed using Graphpad Prism 9.5.1 (GraphPad Software Inc., San Diego, CA, USA), R 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria), and R-Studio 1.4.1717 (PBC, Boston, MA, USA). Missing data were few, perceived missing at random and handled with pairwise deletion. We applied a significance level of 5% and report two-sided P values.
Results
Demographic and clinical characteristics
We analyzed a total of N = 108 patients with viral sepsis from the studies ICROS (n = 51) and ICROVID (n = 57). Patients with nonviral sepsis (N = 117) and healthy volunteers (N = 81) from ICROS served as controls (Fig. 1). In patients with viral sepsis, SARS-CoV-2 (n = 106) and influenza virus (n = 2) were the cause of sepsis. In patients with nonviral sepsis, microbiological findings showed bacteria (n = 87), fungi (n = 35), and/or parasites (n = 1) as causative agents. When no pathogen was identified (n = 22), clinical and laboratory findings indicated a nonviral origin. Demographic data are summarized in Table 1. Patients with nonviral sepsis were older and had lower weight and body mass index (BMI), and higher sequential organ failure assessment scores at T0 and T1 than viral sepsis patients. In viral sepsis, fewer patients required vasopressors and sedation at T1 but more patients required ventilation at T1 and T2 than patients with nonviral sepsis did (Table 1).
Overview of patient inclusion and analytical cohorts from the recruiting studies ICROS and ICROVID. Created with BioRender.com.
ICROS = Identification of cardiovascular and molecular prognostic factors for the medium-term and long-term outcomes of sepsis; ICROVID = Identification of cardiovascular and molecular prognostic factors for the morbidity and mortality in COVID-19-sepsis
There were no differences between patients with viral sepsis and nonviral sepsis in comparison with healthy volunteers regarding age and sex (Table 1). Patients with viral sepsis and nonviral sepsis had a higher weight and BMI than healthy volunteers.
Acetylcholinesterase and butyrylcholinesterase activities during the acute phase of sepsis
We measured the activities of AChE and BChE in whole blood of patients with sepsis and healthy volunteers and report AChE activity per hemoglobin as well as hemoglobin concentrations (Fig. 2). Patients with viral sepsis and nonviral sepsis showed lower activities in AChE and BChE than healthy volunteers at both study time points (Fig. 2A and 2B). Patients with nonviral sepsis showed lower levels of both enzyme activities than patients with viral sepsis at both time points (Fig. 2A and 2B). Acetylcholinesterase activity per hemoglobin was higher in patients with nonviral sepsis than in healthy volunteers at T1 (Fig. 2C). Hemoglobin was reduced in patients with viral sepsis and nonviral sepsis at both time points compared with healthy volunteers, with lower concentrations in nonviral sepsis compared with viral sepsis (Fig. 2D).
Comparison of patients with viral sepsis, patients with nonviral sepsis at T1 (3 ± 1 days after the onset of sepsis) and T2 (7 ± 1 days after the onset of sepsis), and of healthy volunteers. (A) AChE activity, (B) BChE activity, (C) AChE activity per hemoglobin, and (D) hemoglobin. Data are displayed as scatter dot plots with median and interquartile range.
*P < 0.05 (Mann–Whitney U test)
AChE = acetylcholinesterase; BChE = butyrylcholinesterase
Acetylcholinesterase and butyrylcholinesterase activities in patients with versus without sepsis-associated encephalopathy
Twenty-four patients with viral sepsis (22%) and 70 patients with nonviral sepsis (60%) developed SAE during their stay in the ICU; there was no significant difference in the ICU length of stay (Table 1). We compared the activity levels of AChE and BChE at T1 and T2 in patients with and without SAE (Fig. 3). Patients with viral sepsis who developed SAE had decreased AChE activity at T1 (Fig. 3A). Patients with nonviral sepsis who developed SAE in the ICU had lower BChE activity at T1 (Fig. 3B).
Comparison of patients with viral sepsis and nonviral sepsis with versus without sepsis-associated encephalopathy during their stay in the intensive care unit at T1 (3 ± 1 days after the onset of sepsis) and T2 (7 ± 1 days after the onset of sepsis). (A) AChE activity, (B) BChE activity. Data are displayed as scatter dot plots with median and interquartile range.
*P < 0.05 (Mann–Whitney U test)
AChE = acetylcholinesterase; BChE = butyrylcholinesterase; ICU = intensive care unit; SAE = sepsis-associated encephalopathy
Acetylcholinesterase and butyrylcholinesterase activities in intensive care unit survivors versus nonsurvivors
Twenty-two patients with viral sepsis (20%) and 18 patients with nonviral sepsis (15%) died in the ICU (Table 1). We compared the activity levels of AChE and BChE at T1 and T2 in patients who survived the ICU and those who did not (Fig. 4). Acetylcholinesterase showed no differences (Fig. 4A). In both viral sepsis and nonviral sepsis, nonsurvivors had decreased activity of BChE at T2 compared with survivors (Fig. 4B). In nonviral sepsis, reduced activity of BChE was also observed at T1. Nonetheless, the diagnostic accuracy of the obtained cut-offs was in the moderate range (Electronic Supplementary Material [ESM] eTables 1 and 2).
Comparison of ICU survivors and nonsurvivors of patients with viral sepsis and nonviral sepsis at T1 (3 ± 1 days after the onset of sepsis) and T2 (7 ± 1 days after the onset of sepsis). (A) AChE activity, (B) BChE activity. Data are displayed as scatter dot plots with median and interquartile range.
*P < 0.05 (Mann–Whitney U test)
AChE = acetylcholinesterase; BChE = butyrylcholinesterase; ICU = intensive care unit
Laboratory parameters and their association with cholinesterase activities
We measured the concentrations of parameters previously reported to affect cholinesterase activity (Table 2). These parameters include Hb because of the presence of AChE on erythrocytes,31 the concentration of plasma cholinesterase (ChE, common designation of BChE), and albumin as a surrogate parameter of synthetic liver function.34 Patients with nonviral sepsis showed lower levels of Hb, ChE, and albumin than patients with viral sepsis (Table 2) at both time points. We assessed the association of these parameters with the cholinesterase activities in viral (Table 3) and nonviral (ESM eTable 3) sepsis. In patients with viral sepsis, there was a weak positive association between AChE activity and Hb, between AChE activity and albumin, and between AChE activity and ChE, while BChE activity was positively associated with albumin and with ChE (Table 3).
We further measured the concentration of inflammatory markers—CRP, PCT, ferritin, and IL-6—in patients with viral sepsis, and compared the concentrations of CRP and PCT with those in patients with nonviral sepsis. We also performed association analyses with cholinesterase activities (Table 2 and Table 3). Patients with viral sepsis showed lower levels of PCT and CRP than patients with nonviral sepsis. In viral sepsis, BChE activity showed more negative associations with inflammatory markers than AChE activity did (Table 3).
Discussion
Cholinergic imbalance in viral and nonviral sepsis
A modulation of the cholinergic anti-inflammatory pathway has been proposed as a mechanism for developing organ dysfunction in severe COVID-19.35 Nevertheless, studies in patients with viral sepsis22 are scarce. In patients with viral and nonviral sepsis, we found a reduction in both AChE and BChE activity compared with healthy volunteers. These reductions were more pronounced in patients with nonviral sepsis. To our knowledge, this is the first description of a reduction in AChE activity in viral sepsis and the first direct comparison with nonviral sepsis, providing new evidence for a possible cholinergic imbalance independent of causative pathogens. Our results are in line those of a study finding reduced levels of BChE activity in 54 patients with severe COVID-19 compared with 40 historical healthy controls.22 Furthermore, our results are consistent with those of other studies in critically ill patients36 and patients with sepsis of any origin37 that found a reduction of BChE activity within the first six37 or 2836 days after the onset of severe inflammation and sepsis.
Acetylcholinesterase and butyrylcholinesterase activities in sepsis-associated encephalopathy
We found that patients with viral sepsis who developed SAE in the ICU had lower AChE activity on day 3 after the onset of sepsis compared with patients without SAE. This finding was not observed in patients with nonviral sepsis who developed SAE in the ICU. These results are in line with those of a previous study,12 showing a significant decrease of AChE activity after three days in patients with sepsis of any origin and suspected SAE. Nevertheless, the authors did not compare AChE activities between patients with and without SAE. Our findings indicate that the significance of AChE activity as a prognostic marker during acute sepsis may be more relevant to viral sepsis than to nonviral sepsis.
In patients with nonviral sepsis with SAE we observed decreased BChE activity on day 3 of sepsis compared with patients without SAE—consistent with other reports of lower levels of BChE associated with postoperative delirium in patients undergoing hip replacement38 and with critical illness.39 Our results indicate that AChE activity in the early phase of sepsis may be of prognostic value for the development of SAE in viral sepsis and BChE activity on day 3 in nonviral sepsis. These findings should be explored further and validated in future studies.
Acetylcholinesterase and butyrylcholinesterase activities and intensive care unit mortality
Acetylcholinesterase activities showed no differences between survivors and nonsurvivors in viral sepsis. Hence, AChE appears unsuitable for predicting mortality, consistent with our results in nonviral sepsis and the literature.40 Butyrylcholinesterase activity was lower seven days after the onset of sepsis in ICU nonsurvivors in both viral sepsis and nonviral sepsis. The plasma concentration41 and the activity22 of BChE have been shown to correlate with disease severity and mortality in severe COVID-19, which we have reproduced in a larger cohort. This supports BChE as a prognostic marker in patients with sepsis. Zivkovic et al. found values of < 1,661 U·L−1 measured at sepsis onset using a similar device to predict mortality (sensitivity 94%; specificity 48%) in patients with sepsis of any origin.37 We found cut-off values with lesser accuracy (ESM eTables 1 and 2) potentially explained by methodological differences in the device used and the timing of measurements. This illustrates both the need for specific cut-off values depending on the diagnostic device and the time dependency of measurements.
The association of butyrylcholinesterase activity and systemic inflammation
A hypothesized pathophysiologic link between increased mortality, organ dysfunction, and reduced BChE activity is inflammation.18,36,42 This link may also hold true for patients with viral sepsis, as BChE activity was negatively correlated with PCT, CRP, and IL-6 in our exploratory study. Our results are consistent with findings in critically ill patients36 showing a negative association with reduced activity of BChE and inflammatory markers. As the association between BChE activity in blood and systemic inflammation does not prove inflammation and cholinergic imbalance in the brain, further possibly preclinical studies are needed to investigate this pathophysiologic link.
Further associations with acetylcholinesterase and butyrylcholinesterase activities
In our study, Hb was reduced in all patients with sepsis. Patients with nonviral sepsis had lower Hb than patients with viral sepsis. We found a weak association between AChE activity and Hb, potentially explained by the presence of AChE in erythrocytes,31 implying Hb as a confounding factor. The employed measurement method reduced the influence of Hb on obtained AChE activities by adapting the measurement wavelength.31 Erythrocytic AChE only contributes to part of the total AChE activity in blood (up to 44%); the other part is derived from nonerythrocytic sources.14 Therefore, the influence of Hb on AChE activity may be limited. Previous studies have assessed the influence of Hb on delirium in critical illness. Anemia presented a risk factor for increased 28-day mortality but was not associated with neurologic dysfunction in a large multicentre cohort study.43,44 We showed positive associations between BChE activity, its concentration in plasma (ChE), and albumin. Butyrylcholinesterase is produced by the liver,45 so its activity is decreased in liver disease, trauma, and sepsis.18,34,37,46 Liver disease has been identified as a risk factor for delirium independent of hepatic encephalopathy47 and a higher prevalence of liver failure has been shown in patients developing SAE.28 Our results indicate a potential link between liver failure and SAE through cholinergic imbalance, which should be examined further in future studies.
Limitations and strengths of the study
The obtained results should be evaluated in light of the limitations of this study. Recruitment was from one study centre and the study size was limited. The applicability of results to other regions could be limited. The exclusion criteria of the recruiting studies23,24 yielded a selected cohort of patients, which may limit the generalizability of our results in patients with severe heart disease. The incidence of COVID-19 during recruitment resulted in a high prevalence thereof in the viral sepsis cohort, potentially limiting the applicability of results to other viral infections. Further, Hb and hepatic function may present confounding factors: the prognostic value may be different in patients with anemia and acute or chronic liver disease. The control group of patients with nonviral sepsis showed different patient characteristics; thus, the results should be confirmed in matched cohorts. Different characteristics may result from pathophysiologic differences and predisposing factors in viral and nonviral sepsis. Moreover, the impact of medication such as vasopressors on cholinesterase activity was not assessed. The inability to assess SAE in patients with moderate to deep sedation limits the generalization of findings for sedated patients. Despite its limitations, this study represents the hitherto largest cohort, in which cholinergic imbalance was investigated in viral sepsis. Furthermore, the recruitment of all patients was prospective and the analysis prespecified in published protocols. The analysis not only encompasses comparisons of cohorts but also outcome-relevant subanalyses which may increase the clinical relevance of the results.
Conclusion
The enzymatic activities of AChE and BChE in patients with viral and nonviral sepsis were reduced during the acute phase of sepsis. Patients with viral sepsis who developed SAE in the ICU expressed a reduced activity of AChE. Furthermore, ICU nonsurvivors of both cohorts showed a reduced BChE activity seven days after onset of sepsis, indicating a potential prognostic relevance of BChE.
References
Chen J, Shi X, Diao M, et al. A retrospective study of sepsis-associated encephalopathy: epidemiology, clinical features and adverse outcomes. BMC Emerg Med 2020; 20: 77. https://doi.org/10.1186/s12873-020-00374-3
Eidelman LA, Putterman D, Putterman C, Sprung CL. The spectrum of septic encephalopathy: definitions, etiologies, and mortalities. JAMA 1996; 275: 470–3.
Robba C, Crippa IA, Taccone FS. Septic encephalopathy. Curr Neurol Neurosci Rep 2018; 18: 82. https://doi.org/10.1007/s11910-018-0895-6
Field RH, Gossen A, Cunningham C. Prior pathology in the basal forebrain cholinergic system predisposes to inflammation-induced working memory deficits: reconciling inflammatory and cholinergic hypotheses of delirium. J Neurosci 2012; 32: 6288–94. https://doi.org/10.1523/jneurosci.4673-11.2012
Sonneville R, Verdonk F, Rauturier C, et al. Understanding brain dysfunction in sepsis. Ann Intensive Care 2013; 3: 15. https://doi.org/10.1186/2110-5820-3-15
Tracey KJ. The inflammatory reflex. Nature 2002; 420: 853–9. https://doi.org/10.1038/nature01321
Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000; 405: 458–62. https://doi.org/10.1038/35013070
Bernik TR, Friedman SG, Ochani M, et al. Pharmacological stimulation of the cholinergic antiinflammatory pathway. J Exp Med 2002; 195: 781–8. https://doi.org/10.1084/jem.20011714
Emch GS, Hermann GE, Rogers RC. TNF-alpha activates solitary nucleus neurons responsive to gastric distension. Am J Physiol Gastrointest Liver Physiol 2000; 279: G582–6. https://doi.org/10.1152/ajpgi.2000.279.3.g582
Blalock JE. Shared ligands and receptors as a molecular mechanism for communication between the immune and neuroendocrine systems. Ann N Y Acad Sci 1994; 741: 292–8. https://doi.org/10.1111/j.1749-6632.1994.tb23112.x
Capcha JM, Rodrigues CE, de Souza Moreira R, et al. Wharton's jelly-derived mesenchymal stem cells attenuate sepsis-induced organ injury partially via cholinergic anti-inflammatory pathway activation. Am J Physiol Regul Integr Comp Physiol 2020; 318: R135–47. https://doi.org/10.1152/ajpregu.00098.2018
Zujalovic B, Mayer B, Hafner S, Balling F, Barth E. AChE-activity in critically ill patients with suspected septic encephalopathy: a prospective, single-centre study. BMC Anesthesiol 2020; 20: 287. https://doi.org/10.1186/s12871-020-01204-6
Barth E, Bracht H, Georgieff M, Zujalovic B. AChE and BChE activity as guidance in pharmacological therapy of delirium and cognitive impairment in intensive care patients. Anästh Intensivmed 2019; 60: 233–42.
Atack JR, Perry EK, Bonham JR, Perry RH. Molecular forms of acetylcholinesterase and butyrylcholinesterase in human plasma and cerebrospinal fluid. J Neurochem 1987; 48: 1845–50. https://doi.org/10.1111/j.1471-4159.1987.tb05746.x
Ruberg M, Villageois A, Bonnet AM, Pillon B, Rieger F, Agid Y. Acetylcholinesterase and butyrylcholinesterase activity in the cerebrospinal fluid of patients with neurodegenerative diseases involving cholinergic systems. J Neurol Neurosurg Psychiatry 1987; 50: 538–43. https://doi.org/10.1136/jnnp.50.5.538
García-Ayllón M-S, Riba-Llena I, Serra-Basante C, Alom J, Boopathy R, Sáez-Valero J. Altered levels of acetylcholinesterase in alzheimer plasma. PloS One 2010; 5: e8701. https://doi.org/10.1371/journal.pone.0008701
Davis L, Britten JJ, Morgan M. Cholinesterase: its significance in anaesthetic practice. Anaesthesia 1997; 52: 244–60. https://doi.org/10.1111/j.1365-2044.1997.084-az0080.x
Lampón N, Hermida-Cadahia EF, Riveiro A, Tutor JC. Association between butyrylcholinesterase activity and low-grade systemic inflammation. Ann Hepatol 2012; 11: 356–63.
Cohen J, Vincent JL, Adhikari NK, et al. Sepsis: a roadmap for future research. Lancet Infect Dis 2015; 15: 581–614. https://doi.org/10.1016/s1473-3099(15)70112-x
World Health Organization. Severity of disease associated with Omicron variant as compared with Delta variant in hospitalized patients with suspected or confirmed SARS-CoV-2 infection; 2022. Available from URL: https://www.who.int/publications/i/item/9789240051829 (accessed October 2023).
Morris G, Bortolasci CC, Puri BK, et al. The cytokine storms of COVID-19, H1N1 influenza, CRS and MAS compared. Can one sized treatment fit all? Cytokine 2021; 144: 155593. https://doi.org/10.1016/j.cyto.2021.155593
Espeter F, Künne D, Garczarek L, et al. Critically ill COVID-19 patients show reduced point of care-measured butyrylcholinesterase activity—a prospective, monocentric observational study. Diagnostics (Basel) 2022; 12: 2150. https://doi.org/10.3390/diagnostics12092150
Coldewey SM, Neu C, Baumbach P, et al. Identification of cardiovascular and molecular prognostic factors for the medium-term and long-term outcomes of sepsis (ICROS): protocol for a prospective monocentric cohort study. BMJ Open 2020; 10: e036527. https://doi.org/10.1136/bmjopen-2019-036527
Neu C, Baumbach P, Scherag A, Kortgen A, Gotze J, Coldewey SM. Identification of cardiovascular and molecular prognostic factors for the morbidity and mortality in COVID-19-sepsis (ICROVID): protocol for a prospective multi-centre cohort study. PLoS One 2022; 17: e0269247. https://doi.org/10.1371/journal.pone.0269247
Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315: 801–10. https://doi.org/10.1001/jama.2016.0287
Chung HY, Wickel J, Brunkhorst FM, Geis C. Sepsis-associated encephalopathy: from delirium to dementia? J Clin Med 2020; 9: 703. https://doi.org/10.3390/jcm9030703
Eidelman LA, Putterman D, Putterman C, Sprung CL. The spectrum of septic encephalopathy. Definitions, etiologies, and mortalities. JAMA 1996; 275: 470–3.
Sonneville R, de Montmollin E, Poujade J, et al. Potentially modifiable factors contributing to sepsis-associated encephalopathy. Intensive Care Med 2017; 43: 1075–84. https://doi.org/10.1007/s00134-017-4807-z
Yang Y, Liang S, Geng J, et al. Development of a nomogram to predict 30-day mortality of patients with sepsis-associated encephalopathy: a retrospective cohort study. J Intensive Care 2020; 8: 45. https://doi.org/10.1186/s40560-020-00459-y
Ellman GL, Courtney KD, Andres V Jr, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961; 7: 88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Worek F, Mast U, Kiderlen D, Diepold C, Eyer P. Improved determination of acetylcholinesterase activity in human whole blood. Clin Chim Acta 1999; 288: 73–90. https://doi.org/10.1016/s0009-8981(99)00144-8
Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem 1993; 39: 561–77.
Schisterman EF, Perkins NJ, Liu A, Bondell H. Optimal cut-point and its corresponding Youden Index to discriminate individuals using pooled blood samples. Epidemiology 2005; 16: 73–81. https://doi.org/10.1097/01.ede.0000147512.81966.ba
Santarpia L, Grandone I, Contaldo F, Pasanisi F. Butyrylcholinesterase as a prognostic marker: a review of the literature. J Cachexia Sarcopenia Muscle 2013; 4: 31–9. https://doi.org/10.1007/s13539-012-0083-5
Courties A, Boussier J, Hadjadj J, et al. Regulation of the acetylcholine/alpha7nAChR anti-inflammatory pathway in COVID-19 patients. Sci Rep 2021; 11: 11886. https://doi.org/10.1038/s41598-021-91417-7
Zivkovic AR, Schmidt K, Sigl A, Decker SO, Brenner T, Hofer S. Reduced serum butyrylcholinesterase activity indicates severe systemic inflammation in critically ill patients. Mediators Inflamm 2015; 2015: 274607. https://doi.org/10.1155/2015/274607
Zivkovic AR, Decker SO, Zirnstein AC, et al. A sustained reduction in serum cholinesterase enzyme activity predicts patient outcome following sepsis. Mediators Inflamm 2018; 2018: 1942193. https://doi.org/10.1155/2018/1942193
Cerejeira J, Batista P, Nogueira V, Firmino H, Vaz-Serra A, Mukaetova-Ladinska EB. Low preoperative plasma cholinesterase activity as a risk marker of postoperative delirium in elderly patients. Age Ageing 2011; 40: 621–6. https://doi.org/10.1093/ageing/afr053
Hughes CG, Boncyk CS, Fedeles B, et al. Association between cholinesterase activity and critical illness brain dysfunction. Crit Care 2022; 26: 377. https://doi.org/10.1186/s13054-022-04260-1
Tomasi CD, Salluh J, Soares M, et al. Baseline acetylcholinesterase activity and serotonin plasma levels are not associated with delirium in critically ill patients. Rev Bras Ter Intensiva 2015; 27: 170–7. https://doi.org/10.5935/0103-507x.20150029
Nakajima K, Abe T, Saji R, et al. Serum cholinesterase associated with COVID-19 pneumonia severity and mortality. J Infect 2021; 82: 282–327. https://doi.org/10.1016/j.jinf.2020.08.021
Zivkovic AR, Tourelle KM, Brenner T, Weigand MA, Hofer S, Schmidt K. Reduced serum cholinesterase activity indicates splenic modulation of the sterile inflammation. J Surg Res 2017; 220: 275–83. https://doi.org/10.1016/j.jss.2017.07.024
Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med 2013; 369: 1306–16. https://doi.org/10.1056/nejmc1313886
Hemauer SJ, Kingeter AJ, Han X, Shotwell MS, Pandharipande PP, Weavind LM. Daily lowest hemoglobin and risk of organ dysfunctions in critically ill patients. Crit Care Med 2017; 45: e479–84. https://doi.org/10.1097/ccm.0000000000002288
Kaplay SS. Acetylcholinesterase and butyrylcholinesterase of developing human brain. Biol Neonate 1976; 28: 65–73. https://doi.org/10.1159/000240805
Zivkovic AR, Bender J, Brenner T, Hofer S, Schmidt K. Reduced butyrylcholinesterase activity is an early indicator of trauma-induced acute systemic inflammatory response. J Inflamm Res 2016; 9: 221–30. https://doi.org/10.2147/jir.s117590
Denk A, Muller K, Schlosser S, et al. Liver diseases as a novel risk factor for delirium in the ICU-Delirium and hepatic encephalopathy are two distinct entities. PLoS One 2022; 17: e0276914. https://doi.org/10.1371/journal.pone.0276914
Author contributions
Sina M. Coldewey contributed to the conceptualization and design of the study and funding acquisition. Ricardo Esper Treml, Charles Neu, Philipp Baumbach, Markus Engelmann, Claudius Gebhardt, Juliane Götze, and Sina M. Coldewey contributed to study conduction and data acquisition. Philipp Baumbach, Charles Neu, and Ricardo Esper Treml contributed to statistical analysis. Ricardo Esper Treml, Charles Neu, Philipp Baumbach, and Sina M. Coldewey contributed to drafting the manuscript. Sina M. Coldewey, Charles Neu, Philipp Baumbach, Ricardo Esper Treml, Markus Engelmann, Claudius Gebhardt, and Juliane Götze contributed to revising the manuscript prior to submission for important intellectual content. All authors agree to be accountable for all aspects of the work related to the accuracy or integrity of any part of the work.
Acknowledgements
The authors thank the study nurse team of the Department of Anesthesiology and Intensive Care Medicine and the technical assistants at the Institute for Clinical Chemistry and Laboratory Diagnostic of the Jena University Hospital (Jena, Germany) for their support.
Disclosures
The authors declare no competing interests.
Funding statement
The recruiting clinical studies ICROS (Research Group Translational Septomics, Septomics Research Center, grant 03Z22JN12 to SMC) and ICROVID (grant 03COV07 to SMC) were funded by the Federal Ministry of Education and Research (BMBF). Dr. F. Köhler Chemie GmbH provided some of the measurement kits for this study. Both the funding source and manufacturer of the measurement kits were not involved in the study design, the collection, analysis and interpretation of data, or the writing of the report, nor in the decision to submit the article for publication. Ricardo Esper Treml was supported by the clinician-scientist program OrganAge of the Jena University Hospital (grant 413668513) funded by the German Research Foundation (DFG).
Editorial responsibility
This submission was handled by Dr. Patricia S. Fontela, Associate Editor, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Neu, C., Esper Treml, R., Baumbach, P. et al. Cholinesterase activities and sepsis-associated encephalopathy in viral versus nonviral sepsis. Can J Anesth/J Can Anesth 71, 378–389 (2024). https://doi.org/10.1007/s12630-024-02692-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-024-02692-7