Abstract
Purpose
Obtaining an objective, reproducible, and accurate assessment of volume status is one of the more difficult tasks in the perioperative arena. Since its advent in 2020, the Venous Excess Ultrasound (VExUS) score has gained popularity in the minimally invasive assessment of venous congestion. The VExUS exam has been well described as an additional series of images (hepatic vein, portal vein, and intrarenal vein) obtained with a phased-array probe during a transthoracic echocardiogram. Nevertheless, there are no descriptions of comprehensive VExUS exams performed using transesophageal echocardiography (TEE)—a modality that is routinely employed in patients undergoing cardiac surgery.
Clinical features
We describe techniques to acquire and interpret a comprehensive TEE-supported VexUS exam, which may be used to optimize the perioperative care of cardiac surgical patients.
Conclusion
Given the risks of fluid overload in critically ill cardiac surgery patients, TEE-supported VExUS examination may be a way to reduce morbidity in this population.
Résumé
Objectif
L’obtention d’une évaluation objective, reproductible et précise du statut volémique est l’une des tâches les plus difficiles dans l'arène périopératoire. Depuis son introduction en 2020, le score VExUS (pour Venous Excess Ultrasound, soit échographie de l’excès veineux) a gagné en popularité dans l’évaluation minimalement invasive de la congestion veineuse. L’examen échographique VExUS a été bien décrit en tant que série supplémentaire d’images (veine hépatique, veine porte et veine intrarénale) obtenues à l’aide d’une sonde type « phased-array » lors d’un échocardiogramme transthoracique. Néanmoins, il n’existe aucune description d’examens VExUS complets réalisés à l’aide d’une sonde d’ETO (échocardiographie transœsophagienne), une modalité couramment utilisée chez les patient·es bénéficiant d’une chirurgie cardiaque.
Caractéristiques cliniques
Nous décrivons des techniques permettant d’acquérir et d’interpréter un examen VexUS complet par ETO, qui peut être utilisé pour optimiser les soins périopératoires de la patientèle en chirurgie cardiaque.
Conclusion
Compte tenu des risques de surcharge hydrique chez la patientèle gravement malade en chirurgie cardiaque, l’examen VExUS basé sur l’ETO peut être un moyen de réduire la morbidité dans cette population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Description of technique
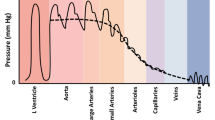
The Venous Excess Ultrasound (VExUS) exam was originally described in 20201 as a minimally invasive assessment of venous congestion using an additional series of images (hepatic vein, portal vein, and intrarenal vein) obtained with a phased-array probe during transthoracic echocardiography.2 Because fluid overload has been associated with suboptimal outcomes in cardiac surgical patients3 and the vast majority of cardiac surgical patients receive a comprehensive perioperative transesophageal echocardiogram (TEE), we aimed to describe the techniques and interpretation of a TEE-supported VExUS exam. A simplified VExUS algorithm is provided in Fig. 1.
Patient consent was obtained for both the acquisition and publication of images. Three patients with differing levels of venous congestion were included in this case series. All images were obtained during comprehensive transesophageal echocardiography (TEE) exams performed as part of cardiothoracic surgical cases. Of note, the views were obtained with standard TEE windows (distal esophageal and transgastric) and only slight additional probe manipulations, so minimal additional risk was incurred. In patients with contraindications to or difficulty with transgastric probe advancement, a TEE-guided VExUS exam should not be performed.
View 1: Inferior vena cava long- and short-axis
We first obtained this view to determine whether further VExUS imaging was warranted, as an inferior vena cava (IVC) diameter < 2 cm is thought to represent the absence of venous congestion (VExUS grade 0). Of note, positive pressure ventilation generally increases IVC diameter,4 so TEE-guided IVC measurements performed during the VExUS exam must be interpreted with caution. From the midesophageal window, the probe was mechanically rotated clockwise to focus on the right atrium and then advanced until the inferior cavoatrial junction, IVC, and confluence of the hepatic veins were visualized. Alternatively, the IVC can be visualized from the transgastric window by flexing the probe and rotating clockwise until the inferior cavoatrial junction is seen. We prefer to use multiplanar imaging to obtain orthogonal views of the intrahepatic cava approximately 2 cm from the confluence of the hepatic veins as previously described,1 and use M-mode to estimate IVC diameter. If multiplanar imaging is unavailable, advancing the omniplane to 50–80° typically displays the IVC in long-axis. In the attached images (Figs 2 and 3), the IVC measures well over 2 cm, indicating a need for further imaging of the hepatic, portal, and/or renal veins. In contrast, an IVC diameter < 2 cm would represent a VExUS score of 0, and the exam could be stopped in that case.
View 2: Hepatic vein imaging
Imagers may need to slightly advance the probe to image the hepatic veins, taking care to ensure favourable alignment of the pulsed wave sample volume at least 1–2 cm within the hepatic vein. If difficulty is encountered with orientation, colour Doppler with a reduced scale (< 30 cm·sec–1) can be used to help identify the hepatic veins. If the distal-esophageal window is used to visualize the hepatic veins, the IVC is seen in the near field and hepatic venous flow is towards the probe during systole in a normal exam, thus appearing red for most of the cardiac cycle. Alternatively, the transgastric window can be used to visualize the hepatic veins. In this case, the hepatic veins may be seen in the near field with the IVC in the far field relative to the TEE probe, similar to a typical subcostal IVC view used in transthoracic echocardiography. Using this transgastric view, hepatic venous flow will be away from the probe (blue on colour Doppler) during systole in noncongested patients. The advantage of the transgastric approach is that the portal vein or its branches may be seen in cross section in the near field and the probe omniplane angle can be adjusted between 20–60° to display these structures in long-axis. With slight probe manipulation (mechanical rotation and/or depth modulation), both hepatic and portal venous structures may be visualized with this approach, yielding a view like the transthoracic VExUS subcostal or right midaxillary transhepatic view.
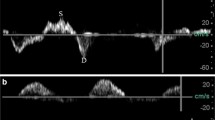
A pulsed wave Doppler gate placed in the hepatic vein in a patient with no congestion will show systolic predominance (Fig. 4), whereas patients with mild congestion may show diastolic predominance with mild systolic blunting (Fig. 5) and patients with more severe congestion may show complete reversal of systolic vs diastolic flow velocities (Fig. 6). As previously noted,5 hepatic venous waveforms will be reversed compared with those acquired with transthoracic imaging protocols and may be altered after cardiopulmonary bypass.6 Additionally, nonsinus rhythms and severe tricuspid regurgitation may alter hepatic venous waveforms and increase difficulty of VExUS interpretation.
View 3: Portal venous imaging
After visualizing hepatic venous flow, the probe may be advanced slightly to visualize the portal vein. Occasionally, slight mechanical rotation of the probe may be necessary. Our team finds colour flow Doppler helpful in localizing the portal vein and in discriminating between hepatic and portal venous flow. In the attached video (Electronic Supplementary Material [ESM] eVideo 1), the TEE probe is withdrawn slightly from the deep transgastric position (where the portal vein can be clearly seen) to visualize the confluence of the hepatic veins. Imagers may also use the increased echodensity of portal veins (also seen in ESM eVideo 1) compared with hepatic veins to differentiate between the two structures. Finally, application of pulsed wave Doppler will reveal characteristic low-velocity portal venous flow. Reduction of the wall filter and/or use of tissue Doppler imaging may aid in detecting portal venous flow. Doppler waveforms may show low-velocity flow with minimal pulsatility (Fig. 7) in noncongested patients or marked portal venous pulsatility in patients with significant venous congestion (Fig. 8). Portal venous flow by Doppler ultrasound will generally be found below the baseline with TEE. As previously noted, increased pulsatility in the portal venous circulation is representative of systemic venous congestion and/or right ventricular dysfunction7 and portends a higher risk of perioperative complications after cardiac surgery.8 In patients whose portal vein cannot be imaged, a splenic venous Doppler signal has previously been detected with TEE and may similarly reflect splanchnic drainage congestion.9
View 4: Renal venous imaging
Visualization of the kidney using TEE can prove difficult in some patients but is generally possible. From the prior position, the TEE probe should be mechanically rotated approximately 180° until the descending aorta is visualized. Thereafter, the probe is advanced and often mechanically rotated counterclockwise until the left kidney is visualized as previously described.10 Alternatively, the kidney may be visualized in longitudinal cross-section by maintaining an omniplane of 90° and mechanically rotating the TEE probe counter-clockwise from the descending aortic short axis view. The TEE probe is then advanced until the kidney is seen. This approach is a surrogate for the transthoracic VExUS renal vessel view obtained from posterior axillary line.
A low colour flow Doppler scale (< 20 cm·sec–1) is often useful to identify renal hilar vasculature (ESM eVideos 2 and 3) before placing a pulsed wave Doppler gate near an interlobar artery to record venous and arterial signals. These signals may be faint but are identified by the mosaic and continuous flow pattern of both the renal artery and renal vein (Figs 9 and 10). If the Doppler filters are not reduced appropriately (Fig. 9), the image quality will be poorer than if the Doppler filters are appropriately reduced (Fig. 10). In the noncongested patient, a monophasic signal with little to no pulsatility (Fig. 9) will be visualized opposite the arterial waveform, but as congestion develops, significant pulsatility will develop in the renal venous waveform (Fig. 10). Depending on orientation, renal venous flow may be visualized above or below the baseline but should be found opposite to renal arterial flow. Observed increases in renal venous pulsatility are likely to represent both an increase in congestion as well as concomitant ventricular dysfunction.11
Discussion
Full TEE-supported VExUS exams are feasible in many patients using echocardiographic windows routinely obtained during a comprehensive perioperative TEE examination. Prior studies have used the VExUS exam to predict acute kidney injury in patients with myocardial infarction12 and cardiorenal syndrome,13 but not in a generalized intensive care unit (ICU) population.14 Given the high incidence of acute kidney injury (AKI) in cardiac surgical patients, VExUS may be used to ascertain volume status and prevent AKI in patients undergoing cardiac surgery,15 and the ability to obtain a full VExUS exam with routine TEE windows has the potential to aid in dissemination of this strategy. Limitations of the TEE-supported VExUS exam include the technical skill required for standardized image acquisition and difficulty validating the exam in the heterogenous patient populations that are seen in cardiac surgery today. Additionally, the VExUS exam, whether performed with transthoracic echocardiography or TEE, suffers from its inability to differentiate the etiology of congestive waveforms as originating from volume overload vs pressure overload states. Future work should focus on comparing the feasibility, quality, and impact of VExUS images obtained by both transesophageal and transthoracic examinations, and ultimately determining whether abnormalities are associated with patient-important outcomes.
References
Beaubien-Souligny W, Rola P, Haycock K, et al. Quantifying systemic congestion with point-of-care ultrasound: development of the venous excess ultrasound grading system. Ultrasound J 2020; 12: 16. https://doi.org/10.1186/s13089-020-00163-w
Stassen J, Falter M, Herbots L, Timmermans P, Dendale P, Verwerft J. Assessment of venous congestion using vascular ultrasound. JACC Cardiovasc Imaging 2023; 16: 426–31. https://doi.org/10.1016/j.jcmg.2022.12.028
Beaubien-Souligny W, Bouchard J, Desjardins G, et al. Extracardiac signs of fluid overload in the critically ill cardiac patient: a focused evaluation using bedside ultrasound. Can J Cardiol 2017; 33: 88–100. https://doi.org/10.1016/j.cjca.2016.08.012
Molokoane-Mokgoro K, Goldstein LN, Wells M. Ultrasound evaluation of the respiratory changes of the inferior vena cava and axillary vein diameter at rest and during positive pressure ventilation in spontaneously breathing healthy volunteers. Emerg Med J 2018; 35: 297–302. https://doi.org/10.1136/emermed-2016-205944
Pinto FJ, Wranne B, St Goar FG, Schnittger I, Popp RL. Hepatic venous flow assessed by transesophageal echocardiography. J Am Coll Cardiol 1991; 17: 1493–8. https://doi.org/10.1016/0735-1097(91)90637-o
Pinto FJ, Wranne B, St Goar FG, et al. Systemic venous flow during cardiac surgery examined by intraoperative transesophageal echocardiography. Am J Cardiol 1992; 69: 387–93. https://doi.org/10.1016/0002-9149(92)90239-u
Denault AY, Azzam MA, Beaubien-Souligny W. Imaging portal venous flow to aid assessment of right ventricular dysfunction. Can J Anesth 2018; 65: 1260–1. https://doi.org/10.1007/s12630-018-1125-z
Denault A, Couture EJ, De Medicis É, et al. Perioperative Doppler ultrasound assessment of portal vein flow pulsatility in high-risk cardiac surgery patients: a multicentre prospective cohort study. Br J Anaesth 2022; 129: 659–69. https://doi.org/10.1016/j.bja.2022.07.053
Silverton NA, Gebhardt BR, Maslow A. The intraoperative assessment of right ventricular function during cardiac surgery. J Cardiothorac Vasc Anesth 2022; 36: 3904–15. https://doi.org/10.1053/j.jvca.2022.05.028
Kararmaz A, Kemal Arslantas M, Cinel I. Renal resistive index measurement by transesophageal echocardiography: comparison with translumbar ultrasonography and relation to acute kidney injury. J Cardiothorac Vasc Anesth 2015; 29: 875–80. https://doi.org/10.1053/j.jvca.2014.11.003
Beaubien-Souligny W, Denault AY. Real-time assessment of renal venous flow by transesophageal echography during cardiac surgery. A A Pract 2019; 12: 30–2. https://doi.org/10.1213/xaa.0000000000000841
Viana-Rojas JA, Argaiz E, Robles-Ledesma M, et al. Venous excess ultrasound score and acute kidney injury in patients with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care 2023; 12: 413–9. https://doi.org/10.1093/ehjacc/zuad048
Bhardwaj V, Vikneswaran G, Rola P, et al. Combination of inferior vena cava diameter, hepatic venous flow, and portal vein pulsatility index: venous excess ultrasound score (VEXUS Score) in predicting acute kidney injury in patients with cardiorenal syndrome: a prospective cohort study. Indian J Crit Care Med 2020; 24: 783–9. https://doi.org/10.5005/jp-journals-10071-23570
Andrei S, Bahr PA, Nguyen M, Bouhemad B, Guinot PG. Prevalence of systemic venous congestion assessed by venous excess ultrasound grading system (VExUS) and association with acute kidney injury in a general ICU cohort: a prospective multicentric study. Crit Care 2023; 27: 224. https://doi.org/10.1186/s13054-023-04524-4
Brown JK, Shaw AD, Mythen MG, et al. Adult cardiac surgery-associated acute kidney injury: joint consensus report. J Cardiothorac Vasc Anesth 2023; 37: 1579–90. https://doi.org/10.1053/j.jvca.2023.05.032
Author contributions
Nathan Waldron, Govind Pandompatam, and Hari Kalagara contributed to all aspects of this manuscript, including study conception and design; acquisition, analysis, and interpretation of data; and drafting the article. Basar Sareyyupoglu contributed to the conception and design of the study.
Acknowledgements
The authors wish to thank Dr. Philip M. Jones for his critical review of this work. This work was supported by the Mayo Clinic in Florida Research Accelerator for Clinicians Engaged in Research (RACER) Program.
Disclosures
All authors have approved the final version of this article.
Funding statement
This work was supported by the Mayo Clinic in Florida Research Accelerator for Clinicians Engaged in Research (RACER) Program.
Editorial responsibility
This submission was handled by Dr. Stephan K. W. Schwarz, Editor-in-Chief, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MPG 620 KB) eVideo 1 Video showing probe manipulation to transition from portal venous to hepatic venous imaging
Supplementary file2 (MPG 1293 KB) eVideo 2 Colour flow Doppler visualization of renal hilar vessels with weak interlobar signals
Supplementary file3 (MPG 1277 KB) eVideo 3 Colour flow Doppler visualization of renal hilar vessels with stronger interlobar signals
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Waldron, N.H., Pandompatam, G., Sareyyupoglu, B. et al. Transesophageal echocardiographic acquisition of the venous excess ultrasound exam—a case series and technical description. Can J Anesth/J Can Anesth 71, 422–430 (2024). https://doi.org/10.1007/s12630-023-02688-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-023-02688-9