Abstract
Purpose
We sought to evaluate the synergistic risk of postoperative thrombosis in patients with a history of COVID-19 who undergo major surgery. Major surgery and SARS-CoV-2 infection are independently associated with an increased risk of thrombosis, but the magnitude of additional risk beyond surgery conferred by a COVID-19 history on the development of perioperative thrombotic events has not been clearly elucidated in the literature.
Methods
We conducted a retrospective cohort study among commercially insured adults in the USA from March 2020 to June 2021 using the Optum Labs Data Warehouse (OLDW), a longitudinal, real-world data asset containing deidentified administrative claims and electronic health records. We compared patients with prior COVID-19 who underwent surgery with control individuals who underwent surgery without a COVID-19 history and with control individuals who did not undergo surgery with and without a COVID-19 history. We assessed the interaction of surgery and previous COVID-19 on perioperative thrombotic events (venous thromboembolism and major adverse cardiovascular events) within 90 days using multivariable logistic regression and interaction analysis.
Results
Two million and two-hundred thousand eligible patients were identified from the OLDW. Patients in the surgical cohorts were older and more medically complex than nonsurgical population controls. After adjusting for confounders, only surgical exposure—not COVID-19 history—remained associated with perioperative thrombotic events (adjusted odds ratio [aOR], 4.07; 95% confidence interval [CI], 3.81 to 4.36). The multiplicative interaction term (aOR, 1.25; 95% CI, 0.96 to 1.61) and the synergy index (0.76; 95% CI, 0.56 to 1.04) suggest minimal effect modification of prior COVID-19 on surgery with regards to overall thrombotic risk.
Conclusions
We found no evidence of synergistic thrombotic risk from previous COVID-19 in patients who underwent selected major surgery relative to the baseline thrombotic risk from surgery alone.
Résumé
Objectif
Nous avons cherché à évaluer le risque synergique de thrombose postopératoire chez les patient·es ayant des antécédents de COVID-19 qui bénéficient d’une intervention chirurgicale majeure. La chirurgie majeure et l’infection par le SRAS-CoV-2 sont indépendamment associées à un risque accru de thrombose, mais l’ampleur du risque supplémentaire d’apparition de complications thrombotiques périopératoires, au-delà de la chirurgie et conféré par des antécédents de COVID-19, n’a pas été clairement élucidée dans la littérature.
Méthode
Nous avons mené une étude de cohorte rétrospective auprès d’adultes assuré·es commercialement aux États-Unis de mars 2020 à juin 2021 à l’aide de la base de données Optum Labs Data Warehouse (OLDW), un actif de données longitudinales du monde réel contenant des requêtes administratives anonymisées et des dossiers de santé électroniques. Nous avons comparé les patient·es ayant déjà souffert de COVID-19 et ayant bénéficié d’une intervention chirurgicale avec des personnes témoins ayant bénéficié d’une intervention chirurgicale sans antécédents de COVID-19 et avec des personnes témoins n’ayant pas bénéficié de chirurgie, avec et sans antécédents de COVID-19. Nous avons évalué l’interaction de la chirurgie et des antécédents de COVID-19 avec les complications thrombotiques périopératoires (thromboembolie veineuse et événements cardiovasculaires indésirables majeurs) dans les 90 jours à l’aide d’une régression logistique multivariée et d’une analyse des interactions.
Résultats
Deux millions deux cent mille personnes admissibles ont été identifiées à partir du registre OLDW. Les patient·es des cohortes chirurgicales étaient plus âgé·es et présentaient une plus grande complexité médicale que les personnes témoins de la population non chirurgicale. Après ajustement pour tenir compte des facteurs de confusion, seule l’exposition chirurgicale – et non les antécédents de COVID-19 – est restée associée aux complications thrombotiques périopératoires (rapport de cotes ajusté [RCa], 4,07; intervalle de confiance [IC] à 95 %, 3,81 à 4,36). Le terme d’interaction multiplicative (RCa, 1,25; IC 95 %, 0,96 à 1,61) et l’indice de synergie (0,76; IC 95 %, 0,56 à 1,04) suggèrent une modification minimale de l’effet d’un diagnostic antérieur de COVID-19 sur la chirurgie en matière de risque thrombotique global.
Conclusion
Nous n’avons trouvé aucune preuve de risque thrombotique synergique lié à une COVID-19 antérieure chez les patient·es ayant bénéficié d’une intervention chirurgicale par rapport au risque thrombotique de base lié à la chirurgie seule.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In December 2019, a novel coronavirus identified as SARS-CoV-2 precipitated a global pandemic of COVID-19. Patients presenting with COVID-19 are at increased risk of developing arterial and venous thrombosis arising from endothelial dysfunction and hypercoagulability,1,2,3,4 which can culminate in adverse clinical outcomes from pulmonary emboli, critical limb ischemia, stroke, myocardial infarction, and ultimately multiorgan failure.1,5,6,7,8,9,10 Prior studies have suggested that patients who undergo surgery with active or recent COVID-19 infection have a higher risk of developing complications, including postoperative pneumonia, respiratory failure, pulmonary embolism, sepsis, and death compared with patients who undergo surgery without a COVID-19 history.11,12,13,14,15,16,17,18,19 Due to this increased risk of perioperative adverse events reported in patients who underwent surgery with recent COVID-19 infection, the American Society of Anesthesiologists (ASA) and the Anesthesia Patient Safety Foundation (APSF) recommend waiting seven weeks after SARS-CoV-2 infection in unvaccinated patients prior to selected major surgery.20,21,22
Major adverse cardiovascular events (MACE) and venous thromboembolic events (VTE) have long been recognized as postoperative complications associated with major surgery, independent of COVID-19 history.23,24,25 Nevertheless, the additional risk conferred by a history of COVID-19 beyond the risk of surgery alone on the development of perioperative thrombotic events has not been clearly described. Most of the original studies exploring the impact of COVID-19 on surgical outcomes lack contemporary nonsurgical control groups to serve as a reference for the frequency of thrombotic events in a general population of nonsurgical patients both with and without a COVID-19 history.
Therefore, using an administrative claims dataset representing commercially insured adult patients in the USA, we conducted a retrospective observational cohort study to evaluate the risk of thrombotic events occurring in patients with a recent history of COVID-19 undergoing major surgery compared with patients who underwent surgery without a COVID-19 history as well as control individuals who did not undergo surgery with and without a COVID-19 history. We hypothesized that the risk of MACE and VTE after major surgery would be amplified by residual hypercoagulability from SARS-CoV-2 infection, leading to higher rates of arterial and venous thrombosis in surgical patients with prior COVID-19. We assessed the incidence of postoperative arterial and venous thrombotic events, including ischemic stroke (cerebrovascular accident [CVA]) and myocardial infarction (MI)—composited as MACE—as well as deep vein thrombosis (DVT) and pulmonary embolism (PE)—composited as VTE. We then evaluated the additive and multiplicative interactions between surgical and COVID-19 exposures on these outcomes to explore a possible synergistic effect of COVID-19 history on postoperative thrombotic events.
Methods
Study oversight and data source
We studied a cohort of commercially insured enrollees from the Optum Labs Data Warehouse (OLDW), a longitudinal, real-world data asset composed of deidentified claims and clinical information from over 200 million covered patients across the USA.26 The claims dataset includes inpatient and outpatient claims as well as pharmacy claims dating from 1993 to the present. The University of California, San Francisco (San Francisco, CA, USA), and OLDW jointly deemed this study exempt from approval by the Institutional Review Board. This study abides by the Reporting of Studies Conducted Using Observational Routinely-collected Data extension of the Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines (Electronic Supplementary Material [ESM] eTable 1).
Study participants
Our primary study cohort included adult patients aged 18 yr or older who were previously diagnosed with COVID-19 at any time and who subsequently underwent selected major surgery between 1 March 2020 and 1 June 2021. Selected major surgery was defined using similar Current Procedural Terminology (CPT) codes specified in previous related studies18 (ESM eTable 2). COVID-19 diagnosis was defined using codes from the International Classification of Diseases, 10th revision (ICD-10) (ESM eTable 3).
Control groups
We identified three control cohorts: a surgical control composed of adult patients without a prior COVID-19 diagnosis undergoing the same selected major surgical procedures as the study cohort (“surgical controls”) and two nonsurgical controls drawn from a general population of patients with and without a COVID-19 history (“population controls”). The population controls with a COVID-19 history were defined using the same ICD-10 codes as in the study cohort. The population controls without a COVID-19 history included all patients who underwent an annual physical exam during the study period without any documented COVID-19 history, as defined by CPT codes (ESM eTable 3). Patients were excluded from the nonsurgical population cohorts based on the presence of anesthesia CPT codes denoting surgical exposure (ESM eTable 4).
Index date
For the two surgical cohorts, the index date was defined as the date of surgery. For the COVID-19 population control cohorts, the index date was defined as the date of COVID-19 diagnosis plus 57 days (the median time in days between the date of COVID-19 diagnosis and the date of surgery in the primary study cohort). For the population controls without a COVID-19 history, the index date was defined as the earliest date of a documented annual physical exam occurring within the study period.
Inclusion and exclusion criteria
The inclusion and exclusion criteria are fully described in ESM eTable 3. All patients in our study required six months of continuous enrollment before and three months of continuous enrollment after their index date for both insurance and pharmacy claims. Patients were excluded if they had pharmacy claims for anticoagulation or antithrombotic medical therapy within six months to 30 days preceding the index date. New anticoagulant or antithrombotic prescriptions within 30 days of surgery were assumed to be pre-emptive prophylactic measures intended for the postoperative period and were not grounds for exclusion. Patients who experienced a thrombotic event within the six months preceding the index date or whose COVID-19 diagnosis occurred after the surgery date were also excluded. Anticoagulant or antithrombotic medications were defined using the AHFS Pharmacologic-Therapeutic Classification System codes, while historical thrombotic events were defined using ICD-10 codes. Since many patients having selected major surgery take acetylsalicylic acid and/or platelet inhibitor therapy at baseline, patients with these prescriptions were not excluded.
Outcomes
Our primary outcome of interest was the composite endpoint of all VTE (DVT, PE) and MACE (MI, CVA) events. Our secondary outcomes included the incidence of VTE and MACE separately during the same timeframe. We used ICD-10 diagnosis codes to define these events as positive outcomes if they occurred within 90 days following the index date (ESM eTable 5).
Covariates
Demographic covariates included age, sex, race, tobacco use, and geographic region in the USA (West, South, Midwest, or Northeast). Medical covariates included a history of MI, CVA, peripheral vascular disease, and malignancy. All covariates were defined either by direct reporting from the OLDW database or by means of documented ICD-10 codes (ESM eTable 6).
Missing data
The Optum Labs Data Warehouse performs internal data validation and quality checks prior to releasing data to research partners. Any data designated by OLDW as “unknown” (i.e., geographic region, race, etc.) were included in the final analysis and designated accordingly. Due to cell size reporting restrictions in the OLDW Data Use Agreement, whereby authors are instructed not to report counts less than 11, we note that fewer than 11 patients from the nonsurgical SARS-CoV-2-exposed population were removed from the respective control group due to documentation of nonphysiologic ages. The dataset was otherwise essentially complete.
Statistical analysis
Categorical variables were described using counts and percentages, while continuous variables were reported as median and interquartile range [IQR] given nonnormal distributions. We used the Chi square test to compare categorical variables and the nonparametric Kruskal–Wallis test to compare continuous variables across the four cohorts. To assess whether interaction exists between COVID-19 history and surgery on the risk of developing thrombosis postoperatively, we combined our four cohorts into a single cohort for analysis. We tested the multiplicative interaction between surgery and COVID-19 history using multivariable logistic regression with an interaction term and calculated the synergy index to test for additive interaction between COVID-19 history and surgical exposure on our primary outcome after adjusting for the key demographic and medical characteristics described above. Estimation of confidence intervals (CIs) for measures of additive interaction was performed using the delta method described by Hosmer and Lemeshow.27 We conducted all statistical analyses using R version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria) and employed the interactionR package to return estimates for effect modification and interaction analyses as described by Knol and VanderWeele.28,29 These analyses were then repeated for both secondary outcomes.
Results
Patients
The final cohort extraction yielded 2,091 patients with a history of COVID-19 who underwent selected major surgery, 44,095 patients who underwent selected major surgery without a history of COVID-19, 357,545 nonsurgical population controls with a history of COVID-19, and 1,812,704 nonsurgical population controls without a history of COVID-19 (Fig. 1). The patients in the two surgical cohorts were generally older and featured a higher Quan–Charlson comorbidity index, a higher rate of tobacco use, and a more frequent history of MI, CVA, peripheral vascular disease, and malignancy compared with the two nonsurgical population controls. Nonsurgical controls with a history of COVID-19 were more likely to be male than individuals in the other cohorts (Table).
Incidence of thrombotic events
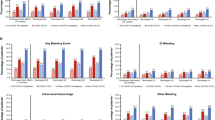
Overall, patients who underwent surgery experienced more thrombotic events than the nonsurgical population controls (Fig. 2). Additionally, patients who underwent surgery with a history of COVID-19 featured a higher incidence of thrombotic events compared with patients who underwent surgery without a history of COVID-19 (5.5% vs 4.5%). Although the overall incidence of thrombosis was lower within the two population controls, patients who did not undergo surgery with a history of COVID-19 similarly had a slightly higher incidence of thrombotic events than patients who did not undergo surgery without a history of COVID-19 (0.4% vs 0.3%). The incidence of MACE was higher than the incidence of VTE across all four cohorts (MACE: 3.1%, 2.7%, 0.3%, and 0.2% vs VTE: 2.8%, 2.1%, 0.1%, and 0.1%, respectively).
Incidence of outcomes. This graph compares the incidence of VTE—including PE and DVT—and MACE—including MI and CVA—across “Surgical / COVID-19 History,” “Surgical / No COVID-19 History,” “Non-Surgical / COVID-19 History,” and “Non-Surgical / No COVID-19 History” groups.
CVA = cerebrovascular accident; DVT = deep venous thrombosis; Hx = history; MACE = major adverse cardiovascular events; MI = myocardial infarction; PE = pulmonary embolism; VTE = venous thromboembolism
Association between surgery, previous COVID-19, and outcomes
In univariable analysis, both surgery and COVID-19 history were associated with thrombosis (surgery: odds ratio [OR], 14.25; 95% CI, 13.53 to 15.00; COVID-19 history: OR, 1.19; 95% CI, 1.12 to 1.26), but there did not appear to be any interaction between the two variables (1.04; 95% CI, 0.85 to 1.27). After adjusting for key confounders, only surgery remained associated with the primary outcome (aOR, 4.07; 95% CI, 3.81 to 4.36). The multiplicative interaction term (aOR, 1.25; 95% CI, 0.96 to 1.61) and the synergy index (0.76; 95% CI, 0.56 to 1.04) did not show evidence of interaction between surgery and COVID-19 history on the composite outcome of VTE plus MACE.
When reviewing secondary outcomes, we again found surgery to be much more strongly associated with an increased risk of VTE and MACE than COVID-19 history alone (i.e., in the absence of surgical exposure). Nevertheless, there were important differences when considered alongside our primary analysis, namely that COVID-19 history was associated with greater odds of VTE (aOR, 1.14; 95% CI, 1.04 to 1.26), and yet it appeared slightly protective against MACE (aOR, 0.88; 95% CI, 0.81 to 0.95). Finally, while the interaction terms in the multivariable logistic regression were again not significant for VTE or MACE (aOR, 1.19; 95% CI, 0.89 to 1.57 and 1.17; 95% CI, 0.79 to 1.69, respectively), the synergy index showed an additive interaction between surgical exposure and COVID-19 history for the outcome of VTE only (1.38; 95% CI, 1.03 to 1.85). A forest plot of the primary predictors of interest and interaction terms for all outcomes is presented in Fig. 3. The fully adjusted regression analysis and synergy indices are available in ESM eTable 7.
Multivariable logistic regression outcomes. This forest plot depicts the aORs with 95% CIs from multivariable logistic regression for primary and secondary outcomes. The model adjusts for age, sex, race, tobacco use, and geographic region in the USA (West, South, Midwest, or Northeast), as well as for histories of myocardial infarction, cerebrovascular accident, peripheral vascular disease, and/or malignancy. X axis reports aORs along a logarithmic scale.
aOR = adjusted odds ratio; CI = confidence interval; MACE = major adverse cardiovascular events; VTE = venous thromboembolism
Discussion
In this study, we sought to estimate the association and possible effect modification posed by a history of COVID-19 disease and selected major surgery on postoperative thrombotic events. Specifically, we explored the possible synergistic risk of thrombosis shared between COVID-19 history and routine postoperative recovery, which remains a subject of ongoing debate in the literature. In a cohort of commercially enrolled beneficiaries undergoing selected major surgical procedures, our study did not find evidence of increased risk of composite thrombotic events resulting from the interaction between COVID-19 history and surgery. The risk of thrombotic events in patients who underwent surgery with prior COVID-19 disease was primarily attributable to the patient’s exposure to surgery alone. Together, these findings suggest that the conventional thrombotic risk of selected major surgery may dramatically outweigh any additional risk posed by a history of COVID-19.
Although there was no multiplicative interaction when considering the secondary outcome of VTE, there did appear to be a small additive interaction between COVID-19 history and surgery as originally hypothesized. Nevertheless, while this result is intriguing and offers reasonable biological plausibility, it should be considered exploratory and subject to the important statistical limitations enumerated below. Several putative mechanisms have been described, including dysregulation of the renin-angiotensin-aldosterone system, increased mitogen-activated protein kinase signaling, proinflammatory sequelae of cytokine and complement activation, neutrophil extracellular trapping, and increased oxidative stress, all of which may synergistically predispose to endothelial dysfunction and subsequent hypercoagulability.1,2,3,4 Other recent studies have also highlighted the increased perioperative risk of morbidity and mortality in patients with a history of COVID-19, particularly in the postacute phase 1–6 weeks after SARS-CoV-2 infection.18,19 A Swedish registry revealed history of COVID-19 to be an independent risk factor for venous thrombotic events three to six months after infection in all-comers,8 and studies of nonoperative hospitalized patients revealed substantially increased risk of VTE in the first 30 days after COVID-19 diagnosis compared with uninfected patients, although the incidence of hypercoagulability did not differ dramatically from matched controls with non-SARS-CoV-2 respiratory viral illness.30,31 The occurrence of both venous and arterial thrombotic events in COVID-19 patients was strongly associated with mortality in a recent European network cohort study.16 Given these observational findings as well as compelling international prospective data published by the UK-based COVIDSurg Collaborative, the ASA and APSF currently recommend waiting seven weeks after COVID-19 diagnosis before elective surgery.20,21,22,32 More recent recommendations suggest postponing elective surgery in patients with COVID-19 who are still exhibiting residual pulmonary symptoms of viral infection and recommend accounting for individual risk factors when assessing the optimal timeline for surgery, although individualized thrombotic risk is not specifically emphasized.33
In our study, we found that composite thrombotic events among patients with a history of COVID-19 undergoing major surgery were mostly attributable to the surgical exposure itself, while the effect of a COVID-19 history was not statistically significant. This finding has been replicated across several retrospective single- and multicentre studies in the USA, which failed to show a significant difference in rates of postoperative VTE among elective surgical populations based on COVID-19 history.34,35,36 These observational studies convey some degree of ambiguity, however, as related sequelae are indeed associated with COVID-19 history, including postoperative pneumonia and greater length of hospital stay.34,36 A substudy analysis of an international prospective cohort study found significantly increased rates of postoperative VTE among patients undergoing surgery with active or recent infections (within one to six weeks of diagnosis), contrary to comparable rates of VTE reported observationally among postoperative patients stratified by COVID-19 history as described elsewhere in the literature.19
To date, no major consensus guidelines have delineated the course of persistent hypercoagulability in the COVID-19 recovery period or considered how this may exacerbate the risk of perioperative thromboembolic phenomena.37,38 The Anticoagulation Forum, American College of Chest Physicians, and the Scientific and Standardization Committee of the International Society of Thrombosis and Hemostasis have empirically recommended three-month courses of anticoagulation therapy in the setting of provoked venous thromboembolism from COVID-19, but subsequent recommendations are nonspecific, thereafter relying on standard anticoagulation guidelines and risk stratification.39 The American College of Surgeons has offered guidelines for triaging surgical care in the COVID-19 population, but there is minimal if any dedicated focus on the subacute and long-term risk stratification of thromboembolic events perioperatively. Conventional perioperative risk scoring systems, such as Padua and Caprini systems, fail to account for the major prognostic features of COVID-19.40,41,42 Our study suggests that thrombotic risk alone may not be a compelling rationale for postponing major surgery in this population. Future prospective studies are urgently needed to inform current recommendations and ultimately curate guidelines for elective surgical scheduling in the COVID-19 recovery period.
Previous retrospective studies have reported increased risk of adverse events when selected major surgeries proceed within seven weeks immediately after infection, but they did not distinguish between the risk of COVID-19 exposure and the risk of the surgical procedure itself. While our study is unique in its comparison of COVID-19 surgical cases with both surgical and nonsurgical controls derived from commercially insured patients, it remains limited by the nature of its observational design. Although efforts were made to adjust for important confounders in multivariable regression, residual confounding is likely to persist—especially given the younger age and healthier status of nonsurgical population controls—complicating the interpretation and extrapolation of these findings. By design, high-risk patients were excluded based on previous thrombotic events and pre-existing anticoagulation therapy. Signals with statistical significance should therefore be interpreted cautiously, particularly when the lower CI bound approximates the null value of 1.
Other important limitations include the claims-based nature of the database, the lack of information regarding the severity of symptoms in patients with COVID-19, the heterogeneity of procedures included in our definition of major surgery, and the inability to identify and control for thromboprophylactic measures undertaken in the perioperative period. Furthermore, older adults who are more likely to require major surgery and experience the sequelae of COVID-19 compared with younger patients were underrepresented in our study since commercially insured patients are generally of working age given the employer-based health insurance system in the USA, and this may have resulted in a lower rate of postoperative thrombotic events overall. Therefore, the incidence of thrombotic events after COVID-19 and the combined risk of COVID-19 history and surgery may not be generalizable to older adults. In addition, misclassification bias in the nonsurgical cohorts may be present in our definition of COVID-19 diagnosis given that many patients may have been self-diagnosed using home antigen testing kits without formally presenting to the health care system. This would spuriously elevate thrombotic risk in the population controls presumed to be without COVID-19 and bias the effect size of COVID-19 on our outcomes of interest toward the null. There may also be a degree of detection or measurement bias in that events of interest in nonsurgical population controls may go unrecognized without the clinical surveillance of inpatient hospitalization or postoperative follow-up appointments, and this discrepancy may be further exaggerated in the controls without COVID-19 for whom screening by providers of thrombotic events is likely not as scrupulous. As a corollary, events that are defined by ICD-10 codes are more likely to be detected in hospitalized populations compared with the largely nonhospitalized COVID-19 controls, which may inflate the incidence of outcome detection among hospitalized cohorts, biasing signal away from the null in surgical groups. Furthermore, some studies have shown limited sensitivity in capturing outcomes by ICD-10 codes compared with other nonclaims-based data registries—as low as 20% and 32% sensitivity for VTE and MACE, respectively, compared with the National Surgical Quality Improvement Program, for example43—which may also engender misclassification of the outcome. Finally, there is also a risk of misclassification bias in patients who were found to have MACE postoperatively, since patients with prior MI or CVA were not excluded from our study cohort. This may have contributed to the apparent protective effect of COVID-19 on the risk of developing MACE observed in secondary analysis. These important limitations notwithstanding, the inclusion of both surgical and COVID-19 population controls increases the rigor of our retrospective approach and offers new insights into elective surgical scheduling in the pandemic era. As we did not study long-term thrombotic outcomes, we cannot comment on the impact of continued endothelial dysfunction that has been reported in patients with chronic post–COVID-19 syndromes.44 Future studies should compare thrombotic risk across vaccinated and unvaccinated populations, and prospective or enhanced observational designs may be applied to a narrowed population of interest—perhaps excluding patients with underlying malignancy or limiting surgical exposure to a more focused panel of procedures—to mitigate indication bias.
Conclusion
Our analysis did not show evidence for an increased thrombotic risk from recent COVID-19 exposure in perioperative patients compared with the baseline perioperative risk, which suggests that thrombotic risk alone may not be a compelling rationale for postponing major surgery in this population. Based on this retrospective analysis, thrombotic phenomena following selected major surgery in patients with a history of COVID-19 appear unlikely to directly contribute to increased perioperative morbidity and mortality, and future prospective studies should aim to better understand the drivers of poor outcomes in this population.
References
Ali MA, Spinler SA. COVID-19 and thrombosis: from bench to bedside. Trends Cardiovasc Med 2021; 31: 143–60. https://doi.org/10.1016/j.tcm.2020.12.004
Gómez-Mesa JE, Galindo-Coral S, Montes MC, Muñoz Martin AJ. Thrombosis and coagulopathy in COVID-19. Curr Probl Cardiol 2021; 46: 100742. https://doi.org/10.1016/j.cpcardiol.2020.100742
Leisman DE, Deutschman CS, Legrand M. Facing COVID-19 in the ICU: vascular dysfunction, thrombosis, and dysregulated inflammation. Intensive Care Med 2020; 46: 1105–8. https://doi.org/10.1007/s00134-020-06059-6
Libby P, Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J 2020; 41: 3038–44. https://doi.org/10.1093/eurheartj/ehaa623
Bikdeli B, Madhavan MV, Gupta A, et al. Pharmacological agents targeting thromboinflammation in COVID-19: review and implications for future research. Thromb Haemost 2020; 120: 1004–24. https://doi.org/10.1055/s-0040-1713152
Iba T, Levy JH, Connors JM, Warkentin TE, Thachil J, Levi M. The unique characteristics of COVID-19 coagulopathy. Crit Care 2020; 24: 360. https://doi.org/10.1186/s13054-020-03077-0
Bunch CM, Moore EE, Moore HB, et al. Immuno-thrombotic complications of COVID-19: implications for timing of surgery and anticoagulation. Front Surg 2022; 9: 889999. https://doi.org/10.3389/fsurg.2022.889999
Katsoularis I, Fonseca-Rodríguez O, Farrington P, et al. Risks of deep vein thrombosis, pulmonary embolism, and bleeding after COVID-19: nationwide self-controlled cases series and matched cohort study. BMJ 2022; 377: e069590. https://doi.org/10.1136/bmj-2021-069590
Malas MB, Naazie IN, Elsayed N, Mathlouthi A, Marmor R, Clary B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: a systematic review and meta-analysis. EClinicalMedicine 2020; 29: 100639. https://doi.org/10.1016/j.eclinm.2020.100639
Katsoularis I, Fonseca-Rodríguez O, Farrington P, Lindmark K, Fors Connolly AM. Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: a self-controlled case series and matched cohort study. Lancet 2021; 398: 599–607. https://doi.org/10.1016/s0140-6736(21)00896-5
Chan NC, Weitz JI. COVID-19 coagulopathy, thrombosis, and bleeding. Blood 2020; 136: 381–3. https://doi.org/10.1182/blood.2020007335
Hanff TC, Mohareb AM, Giri J, Cohen JB, Chirinos JA. Thrombosis in COVID-19. Am J Hematol 2020; 95: 1578–89. https://doi.org/10.1002/ajh.25982
Katneni UK, Alexaki A, Hunt RC, et al. Coagulopathy and thrombosis as a result of severe COVID-19 infection: a microvascular focus. Thromb Haemost 2020; 120: 1668–79. https://doi.org/10.1055/s-0040-1715841
Lax SF, Skok K, Zechner P, et al. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series. Ann Intern Med 2020; 173: 350–61. https://doi.org/10.7326/m20-2566
Kashi M, Jacquin A, Dakhil B, et al. Severe arterial thrombosis associated with COVID-19 infection. Thromb Res 2020; 192: 75–7. https://doi.org/10.1016/j.thromres.2020.05.025
Burn E, Duarte-Salles T, Fernandez-Bertolin S, et al. Venous or arterial thrombosis and deaths among COVID-19 cases: a European network cohort study. Lancet Infect Dis 2022; 22: 1142–52. https://doi.org/10.1016/s1473-3099(22)00223-7
Borrelli MP, Buora A, Scrivere P, Sponza M, Frigatti P. Arterial thrombotic sequalae after COVID-19: mind the gap. Ann Vasc Surg 2021; 75: 128–35. https://doi.org/10.1016/j.avsg.2021.04.009
Deng JZ, Chan JS, Potter AL, et al. The risk of postoperative complications after major elective surgery in active or resolved COVID-19 in the United States. Ann Surg 2022; 275: 242–6. https://doi.org/10.1097/sla.0000000000005308
COVIDSurg Collaborative. Global guidance for surgical care during the COVID-19 pandemic. Br J Surg 2020; 107: 1097–103. https://doi.org/10.1002/bjs.11646
Frangou C. Latest guidance on elective surgery in COVID-19 patients released; 2022. Available from URL: https://www.idse.net/Covid-19/Article/05-22/Latest-Guidance-on-Elective-Surgery-in-COVID-19-Patients-Released/66982?ses=ogst (accessed July 2023).
Anesthesia Patient Safety Foundation. American Society of Anesthesiologists and Anesthesia Patient Safety Foundation joint statement on elective surgery and anesthesia for patients after COVID-19 infection; 2023. Available from URL: https://www.apsf.org/news-updates/asa-and-apsf-joint-statement-on-elective-surgery-and-anesthesia-for-patients-after-covid-19-infection/ (accessed July 2023).
Rohatgi N, Smilowitz NR, Reejhsinghani R. Perioperative cardiovascular considerations prior to elective noncardiac surgery in patients with a history of COVID-19. JAMA Surg 2022; 157: 187–8. https://doi.org/10.1001/jamasurg.2021.6953
Sazgary L, Puelacher C, Buse GL, et al. Incidence of major adverse cardiac events following non-cardiac surgery. Eur Heart J Acute Cardiovasc Care 2020; 10: 550–8. https://doi.org/10.1093/ehjacc/zuaa008
Hawn MT, Graham LA, Richman JS, Itani KMF, Henderson WG, Maddox TM. Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. JAMA 2013; 310: 1462–72. https://doi.org/10.1001/jama.2013.278787
Sweetland S, Green J, Liu B, et al. Duration and magnitude of the postoperative risk of venous thromboembolism in middle aged women: prospective cohort study. BMJ 2009; 339: b4583. https://doi.org/10.1136/bmj.b4583
Optum, Inc. OptumLabs® discovery insights. Available from URL: https://campaign.optum.com/campaign/labs/discovery-insights.html (accessed July 2023).
Assmann S, Hosmer D, Lemeshow S, Mundt K. Confidence intervals for measures of interaction. Epidemiology 1996; 7: 286–90. https://doi.org/10.1097/00001648-199605000-00012
Alli BY. InteractionR: an R package for full reporting of effect modification and interaction. Softw Impacts 2021; 10: 100147. https://doi.org/10.1016/j.simpa.2021.100147
Knol MJ, VanderWeele TJ. Recommendations for presenting analyses of effect modification and interaction. Int J Epidemiol 2012; 41: 514–20. https://doi.org/10.1093/ije/dyr218
Go AS, Reynolds K, Tabada GH, et al. COVID-19 and risk of VTE in ethnically diverse populations. Chest 2021; 160: 1459–70. https://doi.org/10.1016/j.chest.2021.07.025
Cohen K, Ren S, Heath K, et al. Risk of persistent and new clinical sequelae among adults aged 65 years and older during the post-acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ 2022; 376: e068414. https://doi.org/10.1136/bmj-2021-068414
COVIDSurg Collaborative, GlobalSurg Collaborative. Timing of surgery following SARS‐CoV‐2 infection: an international prospective cohort study. Anaesthesia 2021; 76: 748–58. https://doi.org/10.1111/anae.15458
Noll J, Reichert M, Dietrich M, et al. When to operate after SARS-CoV-2 infection? A review on the recent consensus recommendation of the DGC/BDC and the DGAI/BDA. Langenbecks Arch Surg 2022; 407: 1315–32. https://doi.org/10.1007/s00423-022-02495-8
Lung BE, Taka TM, Donnelly M, et al. Prior diagnosis of COVID has no increased complications in total joint arthroplasty. Cureus 2022; 14: e27974. https://doi.org/10.7759/cureus.27974
Vosburg RW, Pratt JS, Kindel T, et al. Bariatric surgery is safe for patients after recovery from COVID-19. Surg Obes Relat Dis 2021; 17: 1884–9. https://doi.org/10.1016/j.soard.2021.07.018
Rosas S, Pollock DC, Roche MW, et al. Patients with previous COVID-19 infection can safely undergo primary total joint arthroplasty. J Arthroplasty 2023: 38: 649–54. https://doi.org/10.1016/j.arth.2022.10.041
Al-Jabir A, Kerwan A, Nicola M, et al. Impact of the coronavirus (COVID-19) pandemic on surgical practice—part 1. Int J Surg 2020; 79: 168–79. https://doi.org/10.1016/j.ijsu.2020.05.022
Soreide K, Hallet J, Matthews JB, et al. Immediate and long-term impact of the COVID-19 pandemic on delivery of surgical services. Br J Surg 2020; 107: 1250–61. https://doi.org/10.1002/bjs.11670
Flaczyk A, Rosovsky RP, Reed CT, Bankhead-Kendall BK, Bittner EA, Chang MG. Comparison of published guidelines for management of coagulopathy and thrombosis in critically ill patients with COVID 19: implications for clinical practice and future investigations. Crit Care 2020; 24: 559. https://doi.org/10.1186/s13054-020-03273-y
Akay T. Perioperative planning in the COVID-19 pandemic: vascular issues. Turk Gogus Kalp Damar Cerrahisi Derg 2019; 28: 244–6. https://doi.org/10.5606/tgkdc.dergisi.2020.09295
Hadid T, Kafri Z, Al-Katib A. Coagulation and anticoagulation in COVID-19. Blood Rev 2021; 47: 100761. https://doi.org/10.1016/j.blre.2020.100761
Cattaneo M, Bertinato EM, Birocchi S, et al. Pulmonary embolism or pulmonary thrombosis in COVID-19? Is the recommendation to use high-dose heparin for thromboprophylaxis justified? Thromb Haemost 2020; 120: 1230–2. https://doi.org/10.1055/s-0040-1712097
McIsaac DI, Hamilton GM, Abdulla K, et al. Validation of new ICD-10-based patient safety indicators for identification of in-hospital complications in surgical patients: a study of diagnostic accuracy. BMJ Qual Saf 2020; 29: 209–216. https://doi.org/10.1136/bmjqs-2018-008852
Andrade BS, Siqueira S, de Assis Soares WR, et al. Long-COVID and post-COVID health complications: an up-to-date review on clinical conditions and their possible molecular mechanisms. Viruses 2021; 13: 700. https://doi.org/10.3390/v13040700
Author contributions
Daniel V. Lazzareschi and Catherine L. Chen contributed to all aspects of this manuscript, including study conception and design; acquisition, analysis, and interpretation of data; and drafting the article. Yanting Luo contributed to the conception and design of the study as well as the acquisition and analysis of data. Nicholas Fong contributed to the analysis of data. Matthieu Legrand and John Boscardin contributed to the conception and design of the study as well as the interpretation of data.
Disclosures
None.
Funding statement
Catherine L. Chen receives research funding from UCSF Anesthesia Research Support, the National Institute of Aging (K23 AG072035, PI: Chen), and the UCSF Pepper Center (P30 AG044281 PI: Covinsky).
Prior conference presentations
Preliminary findings were presented at the American Society of Anesthesiologists 2022 Annual Meeting (21–25 October, New Orleans, LA, USA). Poster: “Postoperative thrombotic events in patients with a history of COVID-19.”
Editorial responsibility
This submission was handled by Dr. Stephan K. W. Schwarz, Editor-in-Chief, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Lazzareschi, D.V., Luo, Y., Fong, N. et al. Postoperative thrombotic events following major surgery in patients with a history of COVID-19: a retrospective cohort analysis of commercially insured beneficiaries in the USA. Can J Anesth/J Can Anesth 71, 55–65 (2024). https://doi.org/10.1007/s12630-023-02639-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-023-02639-4