Abstract
Purpose
Descriptive information on referral patterns and short-term outcomes of patients with respiratory failure declined for extracorporeal membrane oxygenation (ECMO) is lacking.
Methods
We conducted a prospective single-centre observational cohort study of ECMO referrals to Toronto General Hospital (receiving hospital) for severe respiratory failure (COVID-19 and non-COVID-19), between 1 December 2019 and 30 November 2020. Data related to the referral, the referral decision, and reasons for refusal were collected. Reasons for refusal were grouped into three mutually exclusive categories selected a priori: “too sick now,” “too sick before,” and “not sick enough.” In declined referrals, referring physicians were surveyed to collect patient outcome on day 7 after the referral. The primary study endpoints were referral outcome (accepted/declined) and patient outcome (alive/deceased).
Results
A total of 193 referrals were included; 73% were declined for transfer. Referral outcome was influenced by age (odds ratio [OR], 0.97; 95% confidence interval [CI], 0.95 to 0.96; P < 0.01) and involvement of other members of the ECMO team in the discussion (OR, 4.42; 95% CI, 1.28 to 15.2; P < 0.01). Patient outcomes were missing in 46 (24%) referrals (inability to locate the referring physician or the referring physician being unable to recall the outcome). Using available data (95 declined and 52 accepted referrals; n = 147), survival to day 7 was 49% for declined referrals (35% for patients deemed “too sick now,” 53% for “too sick before,” 100% for “not sick enough,” and 50% for reason for refusal not reported) and 98% for transferred patients. Sensitivity analysis setting missing outcomes to directional extreme values retained robustness of survival probabilities.
Conclusion
Nearly half of the patients declined for ECMO consideration were alive on day 7. More information on patient trajectory and long-term outcomes in declined referrals is needed to refine selection criteria.
Résumé
Objectif
On manque d’informations descriptives sur les schémas de références et les devenirs à court terme des patient·es atteint·es d’insuffisance respiratoire n’ayant pas pu recevoir une oxygénation par membrane extracorporelle (ECMO).
Méthode
Nous avons réalisé une étude de cohorte observationnelle prospective monocentrique sur les références vers l’ECMO à l’Hôpital général de Toronto (hôpital d’accueil) pour insuffisance respiratoire grave (COVID-19 et non-COVID-19), entre le 1er décembre 2019 et le 30 novembre 2020. Les données relatives à la référence, à la décision de référence et aux motifs du refus ont été recueillies. Les motifs de refus ont été regroupés en trois catégories mutuellement exclusives sélectionnées a priori : « Trop malade maintenant », « Trop malade avant » et « Pas assez malade ». En ce qui concerne les références refusées, un sondage envoyé aux médecins traitant·es avait pour objectif de recueillir les devenirs des patient·es le jour 7 suivant la référence. Les critères d’évaluation principaux de l’étude étaient le résultat de la référence (accepté/refusé) et le devenir des patient·es (vivant·e/décédé·e).
Résultats
Au total, 193 références ont été incluses; le transfert a été refusé dans 73 % des cas. L’acceptation ou le refus de la référence était influencé par l’âge (rapport de cotes [RC], 0,97; intervalle de confiance [IC] à 95 %, 0,95 à 0,96; P < 0,01) et la participation d’autres membres de l’équipe ECMO à la discussion (RC, 4,42; IC 95 %, 1,28 à 15,2; P < 0,01). Les devenirs des patient·es étaient manquants pour 46 (24 %) des personnes référées (incapacité de localiser les médecins traitant·es ou incapacité des médecins de se souvenir du devenir). À l’aide des données disponibles (95 références refusées et 52 références acceptées; n = 147), la survie jusqu’au jour 7 était de 49 % pour les références refusées (35 % pour la patientèle jugée « trop malade maintenant », 53 % pour celle « trop malade avant », 100 % pour celle « pas assez malade » et 50 % pour les cas où la raison du refus n’était pas déclarée) et 98 % pour les patient·es transféré·es. L’analyse de sensibilité établissant les résultats manquants à des valeurs extrêmes directionnelles a conservé la robustesse des probabilités de survie.
Conclusion
Près de la moitié des patient·es pour lesquel·les un traitement sous ECMO a été refusé étaient en vie au jour 7. Davantage d'informations concernant la trajectoire et les devenirs à long terme des patient·es refusé·es sont nécessaires pour parfaire les critères de sélection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Venovenous extracorporeal membrane oxygenation (ECMO) has been shown to be an effective support strategy for severe acute respiratory failure.1,2 Due to its invasive nature, limited availability, high cost, and resource intensity, ECMO is currently only considered after conventional therapies have failed.3,4 Current evidence-based guidelines consist primarily of expert consensus without formal grading, and patient selection is most often made case-by-case, weighing potential benefits against complications.5,6 For most ECMO centres, the principles guiding the decision-making process include high risk of death despite optimization of conventional treatment, diagnosis of a potentially reversible underlying condition, and the absence of contraindications.6 While the only absolute contraindication is anticipated nonrecovery without an exit strategy, conditions conveying poor prognosis including increased risk for catastrophic bleeding, irreversible neurologic injury, immunosuppression, advanced age, and injurious mechanical ventilation lasting longer than seven days (defined as plateau pressure > 30 cm H2O and fraction of inspired oxygen > 90%) are considered relative contraindications.6 Little is known about the clinical characteristics and outcomes of patients referred but declined for ECMO.7 The decision-making process involved in the selection of patients lacks the information about the patients who are declined for ECMO support. Including such information may help reduce physician-dependent discretionality within ECMO centres, improve selection criteria for ECMO delivery, and verify the efficacy of ECMO in patients with respiratory failure. The objective of this study is to provide information on the referral patterns and outcomes of patients declined for ECMO consideration.
Methods
Institutional research ethics board approval (University Health Network, Toronto, ON, Canada – Research Ethics Board # 18-5424; 13 March 2019) was granted prior to beginning data collection.
We conducted a prospective observational cohort study at Toronto General Hospital (TGH), between 1 December 2019 and 30 November 2020. Extracorporeal membrane oxygenation referrals in the province of Ontario are usually conducted through CritiCall, an organization that facilitates consultation and transfer of patients with critical illnesses requiring expert opinion and therapies not available at the referring centre. The critical care physician on call at TGH would take all ECMO referrals for acute respiratory failure (COVID-19 and non-COVID-19). They would then either decide to accept or decline the referral based on case-by-case assessment (strongly influenced by comorbidities, functional status, duration of injurious mechanical ventilation, advanced age [> 70 yr before the COVID-19 pandemic, > 60 yr during the pandemic], and history of immunosuppression) of patient oxygenation/ventilation impairment, or discuss with other members of the ECMO team when case complexity was encountered (which included one or two additional intensivists on service, a thoracic surgeon, and in selected cases the director of the ECMO program and/or the head of the department). Compared with the beginning of the study, eligibility criteria became more restrictive as the number of referrals rapidly increased because of the COVID-19 pandemic, reducing the age cut-off to > 60 yr, and assigning higher weight to comorbid conditions based on published literature.8 Referral decisions could be made in real time if enough information to support the decision was available, or after a consensus discussion with other members of the ECMO team. The ECMO team could also provide management recommendations to the referral centre, and then make referral decisions after obtaining a better understanding of the clinical trajectory of the patient via follow-up communication with the referring physician.
The intensivist taking the referral documented in a dedicated registry the characteristics of the referral (Table 1), the outcome of the referral (accepted/declined), and the reason for refusal. No patient identifying information was collected during the referral process, and neither was the cause of respiratory failure. Reasons for refusal were grouped in three mutually exclusive categories, selected a priori, and based on the expected probability of short-term survival: “too sick now,” “too sick before,” and “not sick enough” (Table 2). As the primary participants of the study were the referring physicians, no patients, family members, or referring hospitals were contacted to retrieve patient outcomes. Physicians making the referral were called by study personnel to invite them to participate in the study and collect patient outcome data on day 7 after the referral was made. We selected day 7 as the survival endpoint given the limitations produced by intensivist turnover at referring centres and the need for approval from individual institutional review boards to request outcome information from each referral institution.
Statistical analysis
The population studied represented all adult (18 yr and older) patients with severe acute respiratory failure refractory to conventional ventilatory management. Severe respiratory failure meeting criteria for referral to a centre providing ECMO is rare. The study aimed to capture all referrals for respiratory failure made within a calendar year, expecting at least one to three referrals per week, based on our experience from the year prior to starting the study. The number of referrals captured by our registry was collated with CritiCall ECMO referral registries.
We grouped data based on referral (accepted/declined) and patient (alive/deceased) outcomes. Baseline characteristics between groups were compared using t test, Chi square, and Fisher’s exact test. Unadjusted associations between variables and outcomes were calculated. Logistic regression exploring the association between age and the referral outcome (accepted/declined) adjusted for potential confounders (time of referral, and discussion with other members of the ECMO team) was used to obtain the effect estimate of age in the referral decision. Stratification by time of day of the referral was used to explore confounding in the association between referral outcome and involvement of other members of the ECMO team in the referral decision. Only available data were used for primary analyses. Missing patient outcomes were considered missing not at random. We performed a sensitivity analysis to provide a quantitative estimate of the direction, magnitude and uncertainty arising from the missing outcome values. The potential selection bias due to loss-to-follow-up was bounded by characterizing the relationship between missing outcomes and our estimates, as previously described by Lash and Smith.9,10 In our risk of bias analysis, we recalculated the estimates assuming that missing values are equal to their directional extremes (i.e., all missing patients had died vs all missing patients had survived), thereby allowing for the identification of a range of values for the effect that is very compatible with the data. For all analyses, P values < 0.05 were considered significant. Data were collected in Microsoft® Excel 2013 (Microsoft Corporation, Redmond, WA, USA) and analyzed using RStudio 1.4.1717 (Posit, PBC, Boston, MA, USA).
Results
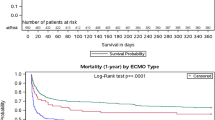
A total of 193 referrals were included in the registry (Figure). Seventy three percent of referrals were declined for transfer (Table 1). None of the declined referrals were related to insufficient hospital resources. Age (odds ratio [OR], 0.97; 95% confidence interval [CI], 0.95 to 0.99; P < 0.01) and involvement of the other members of the ECMO team in the referral decision (OR, 4.42; 95% CI, 1.28 to 15.23; P < 0.01) were significantly associated with being declined for transfer (Table 1). When stratifying by after-hour calls, the involvement of the other members of the ECMO team in the referral decision was still strongly associated with referral outcome (adjusted OR, 4.06; 95% CI, 1.14 to 14.5; P = 0.03).
Patient outcomes (deceased/alive) were missing in 46 (24%) declined referral because the referring physician could not be located or could not recall the outcome. Data from 147 referrals (95 declined and 52 accepted referrals) were used to investigate the effects of referral decision on outcome (Table 3, Figure). Survival to day 7 was 49% in declined referrals (33% for patients deemed “too sick now,” 53% for patients deemed “too sick before,” 100% for patients deemed “not sick enough,” and 50% for patients with a non-reported reason for refusal) and 98% in transferred patients. Six patients who were declined but later transferred were considered alive for the purpose of the analysis. In an exploratory analysis on factors influencing survival, being declined for transfer (probability of surviving to day 7: accepted −98%; 95% CI, 89 to 99 vs declined −49%; 95% CI, 39 to 59; P < 0.001) and reason for refusal (probability of surviving to day 7: too sick now −35%; 95% CI, 23 to 48 vs too sick before −53%; 95% CI, 26 to 78; P = 0.32) (too sick now −35%; 95% CI, 23 to 48 vs not sick enough −100%; 95% CI, 79 to 100; P < 0.001) (too sick before −53%; 95% CI, 26 to 78 vs not sick enough −100%; 95% CI, 79 to 100; P = 0.007) were independently associated with the outcome. Table 2 shows the distribution of survival for individual components of reasons for refusal using available data.
In a sensitivity analysis, in which all missing outcomes were set to directional extreme values, the survival rate to day 7 after being declined ranged between 33% (95% CI, 26 to 42) (all missing died) and 66% (95% CI, 58 to 74) (all missing survived) (Table 4).
Fifty-five percent of accepted referrals received ECMO. There were no differences in survival to day 7 between transferred patients supported with or without ECMO (93% vs 100%, P = 0.49). Survival to discharge from the TGH intensive care unit was 51% in patients supported with ECMO. As the majority of patients who did not receive ECMO and survived to day 7 were repatriated to the referring centre, longer-term outcomes in this group were unavailable.
Discussion
The results of this study show that nearly half of the patients declined for ECMO referral for whom outcome data were available were still alive on day 7. These data suggest that being declined for ECMO consideration should not equal presumption of certain short-term death. A deeper understanding of the clinical trajectory and disease severity of patients with severe acute respiratory failure is needed to improve the definition of ECMO eligibility and resource use.11 Nevertheless, the fact that one third of patients deemed too sick to benefit from ECMO were still alive on day 7 does not imply that the decision to decline for transfer was wrong, and highlights the need to weigh expected risks vs benefits and patient preferences during the decision process. Moreover, 45% of transferred patients meeting ECMO criteria at the time of referral were not cannulated because of clinical improvement at the ECMO centre, underlining either the positive impact of referrals to specialized centres for respiratory support, as shown in previous landmark studies,12 or the risk of premature referrals to an ECMO centre based on arbitrary time cut-offs for ventilatory support.
To date, only one single-centre prospective observational study has described outcomes of patients with respiratory failure denied ECMO during the COVID-19 pandemic.13 The results showed that 90-day survival in patients declined because strict ECMO selection criteria were not met (“not yet”) was 46%, and 14% in patients declined because of anticipated poor prognosis despite ECMO (“never”). Our results are, overall, consistent with these data. Survival to day 10 of patients in the group declined for poor anticipated prognosis, or for not meeting ECMO criteria, closely matches survival in patients considered “too sick” and “not sick enough” in our study (36% vs 34% and 70% vs 100%). Nevertheless, our study analyzed referrals with more diverse causes of acute hypoxemic respiratory failure, not only COVID-19 acute respiratory distress syndrome, used less defined selection criteria to determine acceptance to transfer to an ECMO centre, and included pandemic and prepandemic referrals, reflecting different geographical and logistic conditions.
Our centre, as most ECMO centres, use a combination of the clinical criteria of the Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome trial,14 and case-specific risk vs benefit assessments to select eligible patients. These risk vs benefit assessments retain subjectivity and limit consistency in decision-making, resulting in heterogeneity in clinical practice. Nevertheless, criteria to decline or accept ECMO referrals from remote centres need to consider specific local logistics and geographical context to reduce inappropriate deployment of resource intensive interventions (interhospital transfers, inhaled pulmonary vasodilators, ECMO), or premature limitation of treatment based on anticipated poor prognosis.
Our study has a number of limitations. First, it is a single-centre study. The TGH is the largest adult ECMO referral centre in Canada, and generalizability of the data may be limited by provincial guidelines and local standards of practice. In addition, referral patterns are subject to the number of referral centres providing ECMO within a geographical area. As the number of ECMO centres within these geographical limits increase or decrease, external validity of our data will change. Second, information on the outcome on day 7 was missing in 24% of declined referrals. Nevertheless, sensitivity analyses on the influence of missing outcomes supported the results of the primary analysis. Third, the surge in cases of severe respiratory failure due to the COVID-19 pandemic meant that a number of accepted ECMO referrals were not captured by our registry, as physicians taking the referrals were unable to fill the referral log. Fourth, because of design, patient outcomes were limited to 7-day survival, which may not represent true patient-centred outcomes. Given we only limited the analyses to 7-day survival, conclusions about the duration and intensity of somatic support for patients declined for transfer cannot be provided without exploring mid-to-long-term (14–60-day) outcomes. Fifth, we do not have enough data to understand the relationship between involving other members of the ECMO team in the referral decision. At the moment, we can only speculate physicians receiving the referral are more likely to involve other members of the ECMO team when referrals are complex, and there is an expected benefit from transfer.
Information related to ECMO referrals, clinical characteristics, and clinical follow-up is not routinely collected. The results of our study strongly suggest that routine monitoring of the outcome of declined referrals for ECMO consideration may be one key factor for improving ECMO eligibility criteria and resource use. As we navigate through the resource-intensive setting of the COVID-19 pandemic, it might be worth establishing systems to capture these data as a part of standard practice, to better characterize ECMO referrals, to understand the impact ECMO centres have in the management of patients with respiratory failure, and to further refine referral criteria and clinical decision-making.
In conclusion, despite the limitations given by short-term outcomes and missing data, nearly half of the patients declined for ECMO consideration were alive on day 7 after the referral. A deeper understanding of the clinical trajectory and disease severity is needed to improve the definition of ECMO eligibility, criteria for referral to specialized centres for respiratory support, and resource use. Current criteria to decline patients for transfer to specialized centres providing advanced mechanical ventilation and/or ECMO are based on limited evidence and should be adjusted to context and frequently revised. Patients declined for not meeting criteria should be actively followed, long-term outcomes need to be collected, and early treatment limitation on the basis of being declined for ECMO alone should not be standard practice.
References
Combes A, Peek GJ, Hajage D, et al. ECMO for severe ARDS: systematic review and individual patient data meta-analysis. Intensive Care Med 2020; 46: 2048–57. https://doi.org/10.1007/s00134-020-06248-3
Munshi L, Walkey A, Goligher E, Pham T, Uleryk EM, Fan E. Venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: a systematic review and meta-analysis. Lancet Respir Med 2019; 7: 163–72. https://doi.org/10.1016/s2213-2600(18)30452-1
Combes A, Schmidt M, Hodgson CL, et al. Extracorporeal life support for adults with acute respiratory distress syndrome. Intensive Care Med 2020; 46: 2464–76. https://doi.org/10.1007/s00134-020-06290-1
Fan E, Beitler JR, Brochard L, et al. COVID-19-associated acute respiratory distress syndrome: is a different approach to management warranted? Lancet Respir Med 2020; 8: 816–21. https://doi.org/10.1016/s2213-2600(20)30304-0
Bullen EC, Teijeiro-Paradis R, Fan E. How I select which patients with ARDS should be treated with venovenous extracorporeal membrane oxygenation. Chest 2020; 158: 1036–45. https://doi.org/10.1016/j.chest.2020.04.016
Tonna JE, Abrams D, Brodie D, et al. Management of adult patients supported with venovenous extracorporeal membrane oxygenation (VV ECMO): guideline from the Extracorporeal Life Support Organization (ELSO). ASAIO J 2021; 67: 601–10. https://doi.org/10.1097/mat.0000000000001432
Gillon SA, Rowland K, Shankar-Hari M, et al. Acceptance and transfer to a regional severe respiratory failure and veno-venous extracorporeal membrane oxygenation (ECMO) service: predictors and outcomes. Anaesthesia 2018; 73: 177–86. https://doi.org/10.1111/anae.14083
Lebreton G, Schmidt M, Ponnaiah M, et al. Extracorporeal membrane oxygenation network organisation and clinical outcomes during the COVID-19 pandemic in Greater Paris, France: a multicentre cohort study. Lancet Respir Med 2021; 9: 851–62. https://doi.org/10.1016/s2213-2600(21)00096-5
Lash TL, Fox MP, MacLehose RF, Maldonado G, McCandless LC, Greenland S. Good practices for quantitative bias analysis. Int J Epidemiol 2014; 43: 1969–85. https://doi.org/10.1093/ije/dyu149
Smith LH, VanderWeele TJ. Bounding bias due to selection. Epidemiology 2019; 30: 509–16. https://doi.org/10.1097/ede.0000000000001032
Rocker G, Cook D, Sjokvist P, et al. Clinician predictions of intensive care unit mortality. Crit Care Med 2004; 32: 1149–54. https://doi.org/10.1097/01.ccm.0000126402.51524.52
Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009; 374: 1351–63. https://doi.org/10.1016/s0140-6736(09)61069-2
Levy D, Lebreton G, Pineton de Chambrun M, et al. Outcomes of Patients denied extracorporeal membrane oxygenation during the COVID-19 pandemic in Greater Paris, France. Am J Respir Crit Care Med 2021; 204: 994–7. https://doi.org/10.1164/rccm.202105-1312le
Combes A, Hajage D, Capellier G, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med 2018; 378: 1965–75. https://doi.org/10.1056/nejmoa1800385
Author contributions
Ricardo Teijeiro-Paradis, Eddy Fan, and Lorenzo Del Sorbo contributed to all aspects of this manuscript, including conception and design; acquisition, analysis, and interpretation of data; and drafting the article. Jasmine Grenier contributed to the conception and design of the study, interpretation and review of data, and drafting the article. Martin Urner contributed to interpretation and review of data, statistical analysis, and drafting the article. Ghislaine Douflé, Andrew Steel, Ewan Goligher, and Niall Ferguson contributed to the conception and design of the manuscript, data acquisition, and reviewed and authorized the drafted article. Marcelo Cypel and Shaf Keshavjee contributed to the conception and design of the manuscript and reviewed and authorized the drafted article. Margaret Herridge and John Granton contributed to data acquisition and reviewed and authorized the drafted article.
Acknowledgements
We would like to acknowledge the support and contributions provided by CritiCall Ontario in the development and execution of this research project. We thank all referring physicians who generously participated in this study.
Disclosures
None.
Funding statement
Dr. Urner is supported by a Vanier Canada Graduate Scholarship from the Canadian Institutes of Health Research. Dr. Fan reports personal fees from ALung Technologies, Boehringer-Ingelheim, GE Healthcare, and Vasomune outside the submitted work. Dr. Ferguson reports personal fees from Baxter and Xenios, outside the submitted work.
Editorial responsibility
This submission was handled by Dr. Alexis F. Turgeon, Associate Editor, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Teijeiro-Paradis, R., Grenier, J., Urner, M. et al. Outcomes of patients with respiratory failure declined for extracorporeal membrane oxygenation: a prospective observational study. Can J Anesth/J Can Anesth 70, 1226–1233 (2023). https://doi.org/10.1007/s12630-023-02501-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-023-02501-7