Abstract
Purpose
To compare the incidence and nature of secondary infections (SI) between critically ill patients with viral pneumonia due to COVID-19 and seasonal influenza and explore the association between SI and clinical outcomes.
Methods
We conducted a historical cohort study of patients admitted to the intensive care unit (ICU) at two tertiary care centers during the first wave of the COVID-19 pandemic and patients admitted with influenza during the 2018–2019 season. The primary outcome was the rate of SI. Secondary outcomes included rates of ICU and in-hospital mortality, organ-support-dependent disease, and length of ICU and hospital stay.
Results
Secondary infections developed in 55% of 95 COVID-19 patients and 51% of 47 influenza patients (unadjusted odds ratio [OR], 1.16; 95% confidence interval [CI], 0.57 to 2.33). After adjusting for baseline differences between cohorts, there were no significant differences between the COVID-19 cohort and the influenza cohort (adjusted OR, 1.00; 95% CI, 0.41 to 2.44). COVID-19 patients with SI had longer ICU and hospital stays and duration of mechanical ventilation. The SI incidence was higher in COVID-19 patients treated with steroids than in those not treated with steroids (15/20, 75% vs 37/75, 49%).
Conclusion
Secondary infections were common among critically ill patients with viral pneumonia including COVID-19. We found no difference in the incidence of SI between COVID-19 and influenza in our cohort study, but SI in patients with COVID-19 were associated with worse clinical outcomes and increased healthcare resource use. The small cohort size precludes any causal inferences but may provide a basis for future research.
Résumé
Objectif
Comparer l’incidence et la nature des infections secondaires entre les patients gravement malades atteints de pneumonie virale due à la COVID-19 et ceux atteints de la grippe saisonnière et explorer l’association entre les infections secondaires et les issues cliniques.
Méthode
Nous avons réalisé une étude de cohorte historique de patients admis à l’unité de soins intensifs (USI) dans deux centres de soins tertiaires pendant la première vague de la pandémie de COVID-19 et de patients admis pour la grippe au cours de la saison 2018-2019. Le critère d’évaluation principal était le taux d’infections secondaires. Les critères d’évaluation secondaires comprenaient les taux de mortalité à l’USI et à l’hôpital, les maladies nécessitant un support d’organes et la durée du séjour à l’USI et à l’hôpital.
Résultats
Des infections secondaires se sont développées chez 55 % des 95 patients atteints de COVID-19 et 51 % des 47 patients grippaux (rapport des cotes [RC] non ajusté, 1,16; intervalle de confiance [IC] à 95 %, 0,57 à 2,33). Après ajustement pour tenir compte des différences initiales entre les cohortes, aucune différence significative n’a été observée entre la cohorte de COVID-19 et la cohorte de grippe (RC ajusté, 1,00; IC 95 %, 0,41 à 2,44). Les patients atteints de COVID-19 atteints d’infections secondaires ont séjourné plus longtemps aux soins intensifs et à l’hôpital et la durée de la ventilation mécanique était plus longue pour ces patients. L’incidence d’infections secondaires était plus élevée chez les patients atteints de COVID-19 traités par stéroïdes que chez ceux non traités par stéroïdes (15/20, 75 % vs 37/75, 49 %).
Conclusion
Les infections secondaires étaient fréquentes chez les patients gravement malades atteints de pneumonie virale, y compris de COVID-19. Nous n’avons observé aucune différence dans l’incidence d’infections secondaires entre les patients atteints de COVID-19 et ceux atteints de grippe dans notre étude de cohorte, mais les infections secondaires chez les patients atteints de COVID-19 étaient associées à de moins bonnes issues cliniques et à une utilisation accrue des ressources de soins de santé. La petite taille de la cohorte exclut toute inférence causale, mais peut fournir une base pour les recherches futures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
SARS-CoV-2 is a novel coronavirus that causes severe respiratory illness (COVID-19) with high rates of intensive care unit (ICU) admission and significant morbidity and mortality.1,2 Complications necessitating ICU admission include acute respiratory distress syndrome, cardiac complications,3 thrombotic events,4 and secondary infections. While early reports found 50% of patients who died with COVID-19 had secondary infections (SI),5 the actual rate of SI in critically ill COVID-19 patients, and how this rate compares with that of other respiratory viruses, remains unclear.
Respiratory viral infections are commonly complicated by SI, which can worsen disease severity and mortality in the host.5 These are best described in the context of influenza, where a recent meta-analysis of 27 studies showed an overall incidence of bacterial coinfection of 23%, most commonly with S. pneumoniae (35% of coinfections) and S. aureus (28% of coinfections) with substantial study-to-study variability.6
Described rates of SI in COVID-19 vary from 5%7 to 100%,8 and most are in the 10–50% range.9,10,11,12,13,14,15,16 Respiratory infections and bloodstream infections were the most common sites of infection reported in critically ill patients.7,13 Similar to influenza, common pathogens identified in early (< 48 hr post hospital admission) respiratory infections were S. aureus, S. pneumoniae, and H. influenzae9,11 with increasing prevalence of gram-negative bacilli as length of stay in ICU increases.7,8,13 Common causal organisms of bloodstream infections were gram-positive cocci (Enterococcus, coagulase-negative staphylococci, S. aureus), and gram-negative bacilli (Klebsiella, Pseudomonas, Acinetobacter).7,12,15 Two recent meta-analyses have reported higher rates of SI in critically ill vs ward patients (8% vs 6%17 and 14% vs 4%18). A recent review reported that early coinfections are rare (occurring in < 4% of admitted patients) with SI occurring one to two weeks post admission being more common (39% and 48% in two studies).19
We undertook this historical cohort study to comprehensively describe SI in critically ill COVID-19 patients and explore if there were any differences between the two cohorts. Specifically, we sought to characterize the incidence of SI, risk factors for SI, and the impact of SI on outcomes. Our hope is that the findings from this work would help guide future diagnosis and management of SI in critically ill patients with COVID-19 and stimulate additional research in this area.
Methods
We conducted a historical cohort study through electronic chart review. We included two groups: 1) all consecutive patients admitted to the ICU at Toronto General Hospital (TGH) and Toronto Western Hospital (TWH) (both, Toronto, ON, Canada) between March 1, 2020 and May 31, 2020 with a diagnosis of COVID-19 proven by polymerase chain reaction (PCR) identification of SARS CoV-2 virus on a respiratory tract sample; 2) all consecutive patients admitted to ICU at TGH and TWH between December 1, 2018 and March 31, 2019 with diagnoses of influenza A or B proven by PCR on respiratory tract sample. These time periods were chosen to reflect the first wave of the COVID-19 pandemic and the most recent preceding peak influenza season, respectively. Given the descriptive nature of the study, there was no predetermined sample size. We did not match the two cohorts for the purpose of comparison.
The study population is unique: it includes patients at two distinct tertiary centers. The TGH is the largest extracorporeal membrane oxygenation (ECMO) referral center in Canada and one of the largest solid organ transplant centers in the world. The TWH is a tertiary academic center with a diverse ICU.
Ethics approval was granted by University Health Network ethics board (Toronto, ON, Canada; REB 20-5590.1). The need for consent was waived. Data were collected by two independent clinicians (A. B. and B. J.) reviewing individual patients’ electronic hospital records. A standardized data collection form was used (Electronic Supplementary Material [ESM] eAppendix 1).
We extracted data for both COVID-19 and influenza cohorts including demographics, outcome data, and SI specifics (ESM Appendix 1). The primary outcome, presence of SI, was identified using Centers for Disease Control and Prevention (CDC) definitions of nosocomial infections (ESM Appendix 2). In the two cases without consensus on SI, discussion between A. B. and B. J. yielded a final unanimous decision. Secondary outcomes included ICU length of stay, length of mechanical ventilation, and mortality rate. A very small amount of data was missing, therefore observations with missing data were deleted in statistical models.
Patient characteristics and variables describing hospital course were summarized using means and standard deviations (SDs) or medians and interquartile ranges [IQRs] for continuous data, and counts and percentages for categorical data. Comparisons between cohorts and between those with and without SI were made using Fisher’s exact test (when expected counts were low) or the Chi-square test for binary outcomes, and with the Wilcoxon rank-sum test for continuous outcomes. We used univariable and multivariable logistic regression models to estimate the association of viral infection type with risk of SI. The multivariable model included baseline variables that differed between the two cohorts, and which could be related to SI risk and two variables specified a priori: patient age and source of admission (home, long-term care, another acute healthcare facility). Prior studies have shown worse outcomes in older patients20 and many patient transfers to TGH were for ECMO assessment, indicating a more critically ill group of patients. In the COVID-19 cohort, unadjusted odds ratios (ORs) relating to steroid use and SI, and ORs adjusted for age and admission source were both estimated. All statistical analyses were performed using R version 4.0.5 (R Foundation for Statistical Computing, Vienna, Austria).21
We followed the STROBE checklistFootnote 1 in the writing up of this manuscript and reporting of statistical methods and results.
Results
Patient characteristics
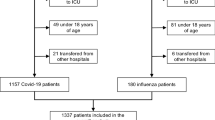
Between March 1 and 31 May 2020, we identified 104 COVID-19 patient admissions to TGH and TWH ICUs. Nine admissions were excluded as they represented readmissions to the ICU. Therefore, a total of 95 patients were included in the COVID-19 cohort. We identified 104 patients who were admitted to the TGH and TWH ICUs between 1 December 2018 and 31 March 2019 (2018–2019 influenza season), and these were labeled as influenza in the patient database. We excluded 34 patients who had acute respiratory distress syndrome but not influenza, 23 patients representing same patient readmissions, and one patient in whom influenza was deemed noncontributory to routine postoperative admission. The remaining 47 patients were included in the influenza ICU cohort. Complete data sets were available for the majority of patients (15 patients in the COVID-19 group and eight patients in the influenza group had missing data relating to components of the sequential organ failure score [SOFA] score).
Baseline characteristics of both cohorts are presented in Table 1. A majority of the COVID-19 cohort (62%) were admitted from other acute healthcare facilities for consideration of ECMO with only 36% admitted directly from home. Although not all patients received ECMO, this was the chief reason for transfer to TGH. In comparison, the majority of influenza patients (57%) were admitted from home with 40% being admitted from another institution. Influenza patients were also more likely to have chronic lung disease and be considered immunocompromised than COVID-19 patients were (38% vs 12%; P = 0.001; and 30% vs 7%; P = 0.001). These differences are likely explained by the subspecialty care offered at TGH, namely ECMO and organ transplantation services. Influenza patients were transferred to TGH for either ECMO consideration or specialized organ transplantation care. The COVID-19 cohort largely consisted of patients being referred for ECMO consideration, which excluded patients with significant comorbidities or immunosuppression.
The COVID-19 group had a median [IQR] age of 55 [49–66] years and was predominantly male (70%). The group that developed SI had higher illness severity scores than those who did not develop SI (median [IQR] SOFA score, 12 [11–14] vs 11 [9–12]; P = 0.03) (Table 2). The median [IQR] age of the influenza group was 56 [46–68] and was predominantly male (60%). There were no significant differences in baseline characteristics between those who did and did not develop SI (Table 3).
Nature of secondary infection
While there was no statistically significant difference in the risk of SI between the two cohorts, there were wide confidence intervals (CIs) in our analyses (univariate OR, 1.16; 95% CI, 0.57 to 2.33) and multivariable logistic regression model (adjusted OR, 1.00; 95% CI, 0.41 to 2.44) (Table 4).
Secondary infections developed in 55% of COVID-19 patients and 51% of influenza patients at a median of 10.0 and 10.5 days following initial laboratory diagnosis of viral infection respectively. Sites of SI in the COVID-19 and influenza groups were predominantly lung (55% and 58%) and blood (38% and 29%), followed by urinary (4% and 8%) and intra-abdominal infections (2% and 0%). Clostridioides difficile colitis occurred in one patient in each group (3% and 4%).
The most common pathogens causing SI were gram-positive bacteria (43% and 50%) followed by gram-negative bacteria (36% and 33%), fungi (6% and 4%), and Clostridioides difficile (2% and 4%) in the COVID-19 and influenza groups, respectively. S. aureus was the most common pathogen causing SI in the COVID-19 group (28% of all SI). Although the primary goal was to quantify bacterial infections, infections due to Candida species were also quantified in cases where it was thought to be pathogenic (3/53 SI in the COVID-19 group; 6% of all infections). There were no infections secondary to Aspergillus species detected in the COVID-19 cohort and one in the influenza group (Table 5).
Impact of secondary infection
COVID-19 group
Among patients who developed SI, there was greater use of vasopressors (21 vs 14; P = 0.04) and a longer median [IQR] duration of mechanical ventilation (21.5 [15.0–31.5] days vs 7 [2.0–13.5] days, P < 0.001). They also had longer median [IQR] ICU admission (542 [376–910] hr vs 195 [80–369] hr, P < 0.001) and hospital admission (647 [516–1,171] hr vs 315 [180–469] hr, P < 0.001) (Table 6).
Twenty-one percent of patients in the COVID-19 group received steroids compared with 36.2% in the influenza group.
Influenza group
Nine patients with SI and two patients without SI were ventilated in the prone position as part of their care (P = 0.05). The median [IQR] duration of mechanical ventilation was longer in those who developed SI (15 [2–31] days vs 5 [0.5–13] days, P = 0.02), and SI were also associated with longer median [IQR] ICU stays (477 [108–678] hr vs 174 [71–395] hr, P < 0.006) and hospital admissions (653 [483–1,300] hr vs 304 [186–510] hr, P < 0.005) (Table 6).
Antimicrobial use
Antibiotic use was higher in the influenza cohort than in the COVID-19 cohort (30 vs 25 antibiotic days per patient). Patients with documented SI received antibiotics for more days than those without in both cohorts (COVID-19, 19 vs 6 days; influenza, 20 vs 10 days).
Discussion
Major findings
Our findings show that development of SI in critically ill patients with a viral pneumonia was not different between our COVID-19 and influenza cohorts. In our cohort, the development of SI in COVID-19 patients was associated with significant morbidity and healthcare resource use.
The incidence of SI was greater than 50% in both the COVID-19 and influenza cohorts. The adjusted OR relating the risk of SI to viral infection type was 1.0, but the 95% CI for the OR included values consistent with both a two-fold increase and a 50% decrease with COVID-19 compared with influenza. The sites of infection and causative pathogens were not different between groups. The SI rate that we describe is higher than that reported in the previous influenza cohort studies,6,22 which may relate to the critically ill nature of our cohort. In our COVID-19 cohort, the rate of SI (55%) is also higher than previously reported, even in the critically ill population,17,18,19 although admittedly the range previously reported is broad. We speculate this could be due to the extremely sick nature of our cohort, including patients who either received or were assessed for ECMO. The pathogens responsible for SI in the COVID-19 cohort were typical of organisms commonly associated with SI in other viral pneumonias or hospital-acquired infections.
These findings highlight the magnitude of SI among patients with viral pneumonia and associated significant implications at both patient and healthcare system levels. As the global pandemic continues to place enormous strain on local and national healthcare resources, any modifiable factors that may reduce the burden of COVID-19 should be explored. Our study supports the implementation and continuation of systems of care directed at preventing nosocomial infection.
Our findings are consistent with the previous reports20 indicating that SI are likely to happen upwards of a week after hospital admission. These results should be used to inform decision-making when initiating empiric antibiotherapy when SI is clinically suspected. The ability to minimize unnecessary antibiotic exposures is required to prevent development of antibiotic-resistant organisms and promote antibiotic stewardship.
In the COVID-19 cohort, patients with SI showed significantly longer hospital and ICU stays and greater duration of mechanical ventilation compared with patients who did not develop SI (Table 6). Increased morbidity and mortality in ICU patients with COVID-19 and SI have also been shown in other studies focused on this population.12,13
There are two plausible mechanisms explaining the association of increased length of stay of critically ill patients with COVID-19 and SI in both the hospital and ICU: SI extends recovery from COVID-19, but also an extended hospital and ICU course predisposes to higher frequency of SI. The observational design of our study means we cannot differentiate between these mechanisms.
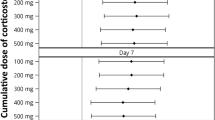
Steroid use was associated with SI in the COVID-19 cohort. Our study occurred before the results of RECOVERY were published,23 when the use of steroid therapy in COVID-19 patients was not standard of care. Steroids were prescribed at the discretion of the treating clinician and possibly for various indications—viral pneumonia, shock, and treatment of cryptogenic organizing pneumonia. Although we have showed an association of steroids and SI, we cannot infer causality because of the observational nature of the study. In addition, the relatively low use of steroids in our study (21% of all COVID-19 patients; 20 patients total) makes it challenging to associate steroid use with specific infections. Nevertheless, a significant strength of our study is the possibility (because of the timing of data collection) to compare the incidence of SI in those receiving steroids with that of a control group who did not receive steroids—this quickly became impossible with publication of the RECOVERY trial. Therefore, despite the small sample size, this is a unique and interesting finding (Table 7).
Limitations and weaknesses
In this historical cohort study, we are unable to differentiate between association and causation of SI and increased morbidity among our COVID-19 cohort. It may be that SI did not cause an increase in morbidity and that this subset of COVID-19 patients were more critically ill from the outset and therefore more likely to have longer hospital and ICU stays and extended mechanical ventilation. Interestingly, there was a higher prevalence of chronic lung disease and immunosuppression among the influenza cohort, which would be expected to predispose to worse clinical outcomes if they had developed a SI, but this was not seen. This speaks to COVID-19’s broad mechanisms in causing severe illness and thus, despite the rate of SI not being statistically different, clinical outcomes in this population were worse.
Another limitation of this study is the subjective nature of diagnosing bacterial infections and potential difficulty differentiating infection from colonization (in pneumonia for example). Nevertheless, we used CDC definitions for bacterial infections and if there was any disagreement between the authors a full discussion was had and consensus reached.
While the relatively smaller size of our comparison cohort is a drawback, we feel that the 2018–2019 influenza season was the best control group to use considering the severity and high level of critical illness locally during this influenza season. The 47 patients with influenza were all the patients admitted to the TGH and TWH ICUs that season, so it was not possible to increase this sample size. A consequence is that the CI for the OR relating risk of SI to COVID-19 is wide and cannot rule out what may be important increases or decreases in risk. A case-matched retrospective study may have addressed this limitation although would mean not all available COVID-19 patient data would be used. We do, however, feel that the comparison of the COVID-19 with the influenza cohort in an identical clinical setting is a unique strength of this study. The diversity of our population is also a unique strength of this study.
Finally, we recognize that much has changed since the initial wave of the pandemic in terms of therapeutic options. In particular, the use of steroids and other immunomodulators (e.g., IL6 receptor antagonist, tocilizumab) is now standard of care in critically ill patients.23,24 The impact of these therapies on SI has not been extensively studied, but mechanistically, they should increase rates of SI because of their immunomodulatory role. The multivariate analysis of our COVID-19 cohort receiving steroids showed significantly higher rates of SI, although number of patients analyzed was small. It is also feasible that effective therapeutic treatments may shorten the course of critical illness making patients less prone to nosocomial illness.23,24,25 Our study, because of its timing in early 2020, provides a unique comparison between critically ill patients who received steroids and those who did not.
Research context and contribution to current knowledge
Many previous studies of SI lacked sufficient detail to allow application to specific patient groups with major limitations being failure to differentiate between healthcare settings (critically ill vs not), lack of granular detail on SI, and their risk factors.26
We addressed gaps in the literature by focusing on ICU patients, extracting extensive detail from available data, and having a control group—a critically ill influenza cohort—with influenza having a known association with SI.
Finally, our study includes a clinically diverse critically ill population with both patients referred to TGH from across the province and patients directly admitted via the emergency department to TWH and TGH.
Conclusion
We found no statistically significant difference in the incidence of SI among COVID-19 ICU patients compared with critically ill patients with influenza. Nevertheless, the development of an SI in this cohort remains an important clinical issue, since SI was associated with longer ICU and hospital admission, and with a greater duration of mechanical ventilation.
Notes
The STROBE Initiative: Strengthening the Reporting of Observational Studies in Epidemiology. Available at URL: https://www.strobe-statement.org/checklists/ (accessed October 2022)
References
Phua J, Weng L, Ling L, et al. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med 2020; 8: 506–17. https://doi.org/10.1016/s2213-2600(20)30161-2
Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA 2020; 323: 1545–6. https://doi.org/10.1001/jama.2020.4031
Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061–9. https://doi.org/10.1001/jama.2020.1585
Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicentre prospective cohort study. Intensive Care Med 2020; 46: 1089–98. https://doi.org/10.1007/s00134-020-06062-x
Smith AM, McCullers JA. Secondary bacterial infections in influenza virus infection pathogenesis. Curr Top Microbiol Immunol 2014; 385: 327–56. https://doi.org/10.1007/82_2014_394
Klein EY, Monteforte B, Gupta A, et al. The frequency of influenza and bacterial coinfection: a systematic review and meta-analysis. Influenza Other Respir Viruses 2016; 10: 394–403. https://doi.org/10.1111/irv.12398
Cataldo MA, Tetaj N, Selleri M, et al. Incidence of bacterial and fungal bloodstream infections in COVID-19 patients in intensive care: alarming "collateral effect". J Glob Antimicrob Resist 2020; 23: 290–1. https://doi.org/10.1016/j.jgar.2020.10.004
Sharifipour E, Shams S, Esmkhani M, et al. Evaluation of bacterial co-infections of the respiratory tract in COVID-19 patients admitted to ICU. BMC Infect Dis 2020; 20: 646. https://doi.org/10.1186/s12879-020-05374-z
Kreitmann L, Monard C, Dauwalder O, Simon M, Argaud L. Early bacterial co-infection in ARDS related to COVID-19. Intensive Care Med 2020; 46: 1787–9. https://doi.org/10.1007/s00134-020-06165-5
Mendes Neto AG, Lo KB, Wattoo A, et al. Bacterial infections and patterns of antibiotic use in patients with COVID-19. J Med Virol 2021; 93: 1489–95. https://doi.org/10.1002/jmv.26441
Contou D, Claudinon A, Pajot O, et al. Bacterial and viral co-infections in patients with severe SARS-CoV-2 pneumonia admitted to a French ICU. Ann Intensive Care 2020; 10: 119. https://doi.org/10.1186/s13613-020-00736-x
Kokkoris S, Papachatzakis I, Gavrielatou E, et al. ICU-acquired bloodstream infections in critically ill patients with COVID-19. J Hosp Infect 2021; 107: 95–7. https://doi.org/10.1016/j.jhin.2020.11.009
Bardi T, Pintado V, Gomez-Rojo M, et al. Nosocomial infections associated to COVID-19 in the intensive care unit: clinical characteristics and outcome. Eur J Clin Microbiol Infect Dis 2021; 40: 495–502. https://doi.org/10.1007/s10096-020-04142-w
Maes M, Higginson E, Pereira-Dias J, et al. Ventilator-associated pneumonia in critically ill patients with COVID-19. Crit Care 2021; 25: 25. https://doi.org/10.1186/s13054-021-03460-5
Giacobbe DR, Battaglini D, Ball L, et al. Bloodstream infections in critically ill patients with COVID-19. Eur J Clin Invest 2020; 50: e13319. https://doi.org/10.1111/eci.13319
Dudoignon E, Caméléna F, Deniau B, et al. Bacterial pneumonia in COVID-19 critical ill patients: a case series. Clin Infect Dis 2021; 72: 905–6. https://doi.org/10.1093/cid/ciaa762
Langford BJ, So M, Raybardhan S, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect 2020; 26: 1622–9. https://doi.org/10.1016/j.cmi.2020.07.016
Lansbury L, Lim B, Baskaran V, Lim WS. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect 2020; 81: 266–75. https://doi.org/10.1016/j.jinf.2020.05.046
Westblade LF, Simon MS, Satlin MJ. Bacterial coinfections in coronavirus disease 2019. Trends Microbiol 2021; 29: 930–41. https://doi.org/10.1016/j.tim.2021.03.018
Palaiodimos L, Kokkinidis DG, Li W, et al. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism 2020; 108: 154262. https://doi.org/10.1016/j.metabol.2020.154262
The R Foundation. The R Project for statistical computing. Available from URL: https://www.r-project.org/index.html (accessed October 2022).
Morris DE, Cleary DW, Clarke SC. Secondary bacterial infections associated with influenza pandemics. Front Microbiol 2017; 8: 1041. https://doi.org/10.3389/fmicb.2017.01041
Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021; 384: 693–704. https://doi.org/10.1056/nejmoa2021436
Gordon AC, Mouncey PR, Al-Beidh F, et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med 2021; 384: 1491–502. https://doi.org/10.1056/nejmoa2100433
RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2021; 397: 1637–45. https://doi.org/10.1016/s0140-6736(21)00676-0
Rawson TM, Moore LS, Zhu N, et al. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis 2020; 71: 2459–68. https://doi.org/10.1093/cid/ciaa530
Author contributions
Alina Beliavsky and Barry Johnston came up with the idea for the research project, performed chart review and data extraction, and wrote and edited the manuscript. Qixuan Li and George Tomlinson helped to perform the statistical analysis for the research project and helped to write the Methods section of the manuscript. Rupert Kaul and John Granton provided direction for the research project, reviewed the written manuscript in detail, and assisted with interpreting statistical analysis in detail.
Disclosures
There are no financial disclosures (with respect to funding for this study) and no conflict of interest disclosures from any of the authors.
Funding statement
Rupert Kaul received peer-reviewed funding from the Canadian Institutes of Health Research (#MM1-174919). This study was also supported by the Toronto General Hospital and Toronto Western Hospital foundation.
Editorial responsibility
This submission was handled by Dr. Stephan K. W. Schwarz, Editor-in-Chief, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Prior conference presentations
American Thoracic Society, San Francisco, California 2022: Secondary infections in critically ill patients during the first wave of COVID-19. Poster presentation by A. Beliavsky.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Beliavsky, A., Johnston, B., Li, Q. et al. Secondary infections in critically ill patients with viral pneumonia due to COVID-19 and influenza: a historical cohort study. Can J Anesth/J Can Anesth 70, 374–383 (2023). https://doi.org/10.1007/s12630-022-02376-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-022-02376-0