Abstract
Purpose
Genitofemoral neuralgia (GFN) is a chronic pain condition that may be refractory to commonly employed treatment modalities. Implantation of a peripheral nerve stimulator (PNS) may provide significant pain relief; however, few reports have described placement of and response to a GFN PNS implant.
Clinical features
We implanted a StimRouter® PNS in a 42-yr-old male with severe GFN that did not respond to pharmacologic and interventional pain management modalities and impaired all aspects of his function and quality of life. The often-challenging sonographic visualization of the genitofemoral nerve was aided by intraprocedural sensory mapping using a stimulating probe. Preoperatively, the patient’s average pain was rated as 7 on a 0 to 10 numeric rating scale. Following the procedure, the patient experienced over 90% pain relief after one week. At one and five months post implantation, the patient’s average pain scores were 1 and 0.5, respectively. The patient also reported substantial improvement in the physical component scores on the 12-Item Short Form Survey (SF-12), which remained similar at the five-month follow-up (from 26.1 preop to 57.2 at one month and 49.7 at five months).
Conclusions
Peripheral nerve stimulator implantation may be a promising intervention when other analgesic modalities fail to manage refractory GFN. Further research to verify the effectiveness of this intervention and evaluate for appropriate integration in patient care is required.
Résumé
Objectif
La névralgie génito-crurale (NGC) est une douleur chronique pouvant être réfractaire aux modalités de traitement couramment utilisées. L’implantation d’un stimulateur nerveux périphérique (SNP) peut apporter un soulagement significatif de la douleur. Cependant, peu de présentations de cas ont décrit la mise en place et la réponse à l’implantation d’un SNP pour soulager une névralgie génito-crurale.
Caractéristiques cliniques
Nous avons implanté un SNP StimRouter® chez un homme de 42 ans atteint d’une NGC grave qui ne répondait pas aux modalités pharmacologiques et interventionnelles de prise en charge de la douleur et entravait tous les aspects fonctionnels et de qualité de vie. La visualisation échographique souvent difficile du nerf génito-crural a été facilitée grâce à une cartographie sensorielle intraprocédurale, réalisée à l’aide d’une sonde de stimulation. Avant la procédure, la douleur moyenne du patient a été évaluée à 7 sur une échelle d’évaluation numérique de 0 à 10. Suite à l’intervention, le patient a ressenti un soulagement de la douleur de plus de 90 % après une semaine. À un et à cinq mois suivant l’implantation, les scores moyens de douleur du patient étaient de 1 et 0,5, respectivement. Le patient a également rapporté une amélioration substantielle des scores de la composante physique du questionnaire SF-12, scores qui sont restés similaires au suivi à cinq mois (de 26,1 avant l’intervention à 57,2 à un mois et 49,7 à cinq mois).
Conclusion
L’implantation d’un stimulateur nerveux périphérique pourrait être une intervention prometteuse lorsque d’autres modalités analgésiques ne parviennent pas à prendre en charge une névralgie génito-crurale réfractaire. D’autres recherches sont nécessaires pour vérifier l’efficacité de cette intervention et évaluer son intégration appropriée dans les soins aux patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Chronic postsurgical pain (CPSP), defined as pain in a surgical incision or referred pattern of pain lasting more than three months after surgery,1 is a disabling complication after many surgical procedures. Urologic procedures appear to be at greater risk of chronic postsurgical pain compared with other noncardiac procedures.2 Unfortunately, CPSP is often refractory to various treatment modalities, posing a significant source of long-term physical, emotional, psychologic, and social distress.3
Varicocelectomy is a surgical procedure usually performed when varicocele causes infertility or persistent pain unresponsive to conservative treatment.4,5 Orchiectomy is performed to treat tumour, infection, torsion, and trauma. When testicular pain persists or develops after orchiectomy, or after other peritesticular procedures, it is imperative to consider surgically induced injuries to local peripheral nerves such as the genitofemoral nerve.6 Genitofemoral neuralgia is characterized by persistent pain and paresthesia in the distribution of the genitofemoral nerve—the femoral branch innervates the upper thigh region and the genital branch innervates the anterior scrotal skin in males and the mons pubis and labia majora in females.7
Peripheral nerve stimulation (PNS) is a neuromodulation technique used to treat peripheral neuropathic pain syndromes that are refractory to other treatment modalities. Patients who experience significant yet short-term pain relief from percutaneous PNS may benefit from a fully implantable system that may provide long-term pain relief.
Our case report presents the clinical course of a 42-yr-old male who underwent a genitofemoral PNS implantation for refractory postsurgical pain following varicocelectomy and orchiectomy. This is an unusual case describing the use of the novel, less-invasive StimRouter® (Bioventus, Durham, NC, USA) device for genitofemoral neuralgia.
Case description
We obtained written informed patient consent for this report. A 42-year-old previously healthy male presented at our pain medicine outpatient clinic for evaluation of a left groin and testicular pain lasting for the past three years. The pain started following varicocelectomy complicated by inadvertent ligation of a branch of the left testicular artery, with subsequent orchiectomy that did not alleviate the pain. Upon presentation at our clinic, pain was constant with a score of 4 out of 10 on the best day and 9 out of 10 on the worst day, and was characterized by a sharp and stabbing sensation, as if “there is a knife inside the pocket that keeps poking the area.” Pain interfered with sleep and daily activities such as driving and walking, and was exacerbated by short walks, cold weather, bending, lifting, or during Valsalva maneuvers. Due to these severe limitations, he was no longer able to work at his marketing office job. Rest and medications alleviated the pain slightly.
Prior to presenting at our clinic, the patient’s pain was already refractory to various medications, including opioids (e.g., hydromorphone), gabapentin, duloxetine, nonsteroidal anti-inflammatory drugs, cannabidiol oil, lidocaine, and ketamine infusions. A trial of tricyclic antidepressants was refused by the patient because of the inefficacy and side-effects of other medications. All medications were discontinued prior to any diagnostic intervention. The patient also underwent several ilioinguinal steroid injections, along with pulsed radiofrequency. Of all interventions, the ilioinguinal steroid injections were most helpful, with up to 50% pain relief.
Although initially considered for further interventions to the ilioinguinal nerve, physical examination revealed a pain distribution more compatible with genitofemoral neuralgia (GFN). Following a diagnostic block of the left genitofemoral (GF) nerve, which provided 60% pain relief for 24 hr, the patient underwent GF pulsed radiofrequency with steroid injection, which provided 75% pain relief for three to four weeks. For several months, the patient found great benefit with treatments to the GF nerve; however, given the short duration of effect, PNS implantation was considered and discussed with the patient. Emphasis was placed on possible technical challenges of placing a PNS device in close proximity to the GF nerve given its poor visualization on ultrasound. After consideration of the risks and benefits of the procedure, the patient provided written informed consent. Preoperatively, the patient was asked to complete the Brief Pain Inventory (BPI) and the 12-Item Short Form Survey (SF-12). Pre- and postoperative scores are outlined in the Table 1.
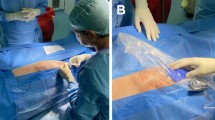
Peripheral nerve stimulation implantation was performed under sterile conditions using previously published approaches for performing an ultrasound-guided GF nerve block.8 Nevertheless, as described in prior reports, it is challenging to visualize the GF nerve and it could reside within or outside the spermatic cord. Given the need for a PNS implant to be in close approximation to the nerve, we used the stimulating stylet to probe around and within the spermatic cord under ultrasound guidance to identify the location of the GF nerve. Using an in-plane approach, we identified the GF nerve at approximately the 6 to 7 o’clock position within the spermatic cord (12 o’clock is the most superficial aspect and 9 o’clock was cephalad). The patient described stimulation distribution concordant with his area of pain radiating to his groin and scrotum. We then advanced an assembled StimRouter introducer sheath over the stimulating stylet to this location within the spermatic cord, and removed the dilator and slowly advanced the StimRouter lead within the lead loader (Fig. 1). Once we confirmed stimulation again with the lead loader, lead anchors were deployed. We then tunnelled the remaining exposed end of the lead using a second incision. Both incisions were dressed and the patient underwent uneventful recovery. He met with the representative from Bioventus who programmed his PNS device and gave recommendations on using the device.
One week after the procedure, the patient reported that titrating up his PNS device usage provided a greater than 90% reduction in pain, which significantly improved his function and quality of life. This was sustained at the one-month follow-up, with an average pain score of 1/10 on the BPI and an improvement of 31.11 and 1.93 on the physical component score (PCS) and mental component score (MCS) of the SF-12 questionnaire, respectively (minimal clinically important differences in the SF-12 PCS and MCS scores were 3.29 and 3.77, respectively, in low back pain patients).9
The patient was also followed-up at five months post implantation, and continued to benefit significantly from the device. On average, he scored his pain intensity as 1 (Table), least pain as 0, and worst pain as 1. Pain-related interference with his daily living was also substantially decreased. His SF-12 physical and mental component scores continued to improve since the preoperative assessment (five-month SF-12 PCS and MCS, 49.7 and 60.6, respectively). The patient also had a good “carry-over” effect, with sustained pain relief for hours even after the device was turned off. Qualitatively, the patient was able to achieve his preoperative goals and specifically emphasized his regained ability of walking his kids home after school. He also plans to return to work, but initially on a part-time basis and was finally able to start golfing again.
Discussion
Our report shows that the implantation of a GF PNS may be an effective treatment for refractory GFN. Genitofemoral neuralgia describes the condition of pain and paresthesias in the distribution of the GF nerve.10 This condition is most commonly reported as an iatrogenic injury, usually developing as a result of surgery in the inguinal region. Figure 2 shows the considerable overlap in the areas supplied by inguinal, iliohypogastric, GF, and lateral cutaneous femoral nerves,8 illustrating the potential challenge in the precise identification of the origin of a neuropathic insult in this area.
Anatomy of nerves around the inguinal region used with permission by Shanthanna H. Successful treatment of genitofemoral neuralgia using ultrasound guided injection: a case report and short review of literature. Case Reports in Anesthesiology 2014; 371703. https://doi.org/10.1155/2014/371703
Peripheral nerve stimulators use electrical neuromodulation to provide analgesia and reduce pain medication requirements.11 The PNS device in this case uses platinum-iridium insulated leads that can be introduced through an 18G needle under ultrasound guidance, enabling relatively rapid percutaneous insertion and subsequent withdrawal.12 The implanted leads are activated using an external pulse transmitter that can be activated using a wireless patient remote. The externalized battery avoids several downsides associated with traditional implantable neuromodulation devices (e.g., spinal cord stimulators) such as the need for a larger incision for an internal pulse generator and need for repeat procedures for battery changes.
To accurately implant the leads to specifically target the GF nerve, its exact identification must be accomplished, which is often a challenging task. Anatomically, the GF nerve is formed from the L1 and L2 nerve roots, pierces the psoas muscle, and bifurcates into the genital and femoral branches. The genital branch travels along with the ilioinguinal nerve through the inguinal canal with some sensory overlap within one another along the medial aspect of the thigh and scrotum. The femoral branch follows a more lateral pathway, piercing the fascia lata to travel within the femoral sheath, supplying cutaneous sensation to the anterior aspect of the upper thigh, along the region of the femoral triangle.7 In light of the often-challenging visualization, we used a stimulating needle to identify the GF nerve, which was identified within the posterior-inferior aspect of the spermatic cord. The lead was implanted within the spermatic cord, with no motor consequences arising from cremasteric proximity.
To our knowledge, only two case reports have been published on PNS implantation for resembling indications: a young male underwent genitofemoral and ilioinguinal PNS implantation for refractory chronic testicular pain with good results (pain intensity declined from 9 out of 10 to 2 out of 10 at seven months). The PNS was implanted using anatomical landmarks with x-ray confirmation, as opposed to the intraprocedural mapping as used in our case.13 In another report, a 46-yr-old female was implanted with two four-contact leads close to the right ilioinguinal and genitofemoral nerves for right groin pain, with a moderate 50% reduction in pain intensity at four months.14 Nevertheless, our case presents a unique ultrasound-guided GF implantation of the StimRouter device, a PNS device in which the pulse generator is not implanted but remains external, requiring less surgical exposure and obviates the need for battery replacement procedures. This is particularly advantageous in areas where placement of bulky hardware (e.g., the battery) may limit the patient’s range of motion or result in discomfort.
While PNS devices have been increasing in popularity in the USA over the past few years, their availability and use in Canada has been limited. Bioventus is the only PNS vendor that is Health Canada approved, and this approval was only obtained in 2018. Also, provincial health insurance coverages do not cover the PNS device costs, which needs to be purchased through hospital or program budgets. The device used in this case report was purchased using philanthropy donations.
While this novel modality can provide significant benefit in the appropriate patient, it is not without potential risks and complications. Despite a minimally invasive approach, implantation of a PNS device requires at least one incision, which can predispose the patient to risks of bleeding and infection. Specifically, an infection of the hardware is possible, which would necessitate an urgent explant. Other specific risks associated with PNS implantation include lead migration or damage, hardware erosion into vessels or through the skin, nerve injury, postoperative pain flare, and skin irritation/hypersensitivity to stimulation patches.12 Further, patients with a PNS implant should be made aware that the device is only magnetic resonance imaging (MRI) conditional, and that a radiologist should be made aware of the device and modifications needed for future MRI. As such, it is imperative to have strict patient selection criteria to maximize the benefit to risk ratio. Selected candidates for PNS should be patients with pain that significantly affects their quality of life, is refractory to noninvasive (e.g., medications, physical therapy) and interventional (e.g., nerve blocks) techniques, and follows a sensory distribution concordant with a specific nerve innervation, confirmed with a diagnostic peripheral nerve block.15
This report highlights two important aspects. First, it reports the advantage obtained from intraprocedural mapping for the identification of the GF nerve prior to implantation. Secondly, it reports the successful management of refractory GFN using an implantable PNS device. Further clinical research is necessary to verify that this modality is effective in longer-term treatment of refractory GFN and to identify its appropriate integration into the management of chronic neuropathic pain in terms of timing and indication.
References
Schug SA, Lavand’homme P, Barke A, et al. The IASP classification of chronic pain for ICD-11: chronic postsurgical or posttraumatic pain. Pain 2019; 160: 45–52. https://doi.org/10.1097/j.pain.0000000000001413
Khan JS, Sessler DI, Chan MT, et al. Persistent incisional pain after noncardiac surgery: an international prospective cohort study. Anesthesiology 2021; 135: 711–23. https://doi.org/10.1097/aln.0000000000003951
Penny KI, Purves AM, Smith BH, Chambers WA, Smith WC. Relationship between the chronic pain grade and measures of physical, social and psychological well-being. Pain 1999; 79: 275–9. https://doi.org/10.1016/s0304-3959(98)00166-3
Lissoos I, Spiro FI. Non-operative treatment of varicocele. S Afr Med J 1986; 70: 805–6.
Park HJ, Lee SS, Park NC. Predictors of pain resolution after varicocelectomy for painful varicocele. Asian J Androl 2011; 13: 754–8. https://doi.org/10.1038/aja.2010.87
Dellon AL, Hashemi SS, Tollstrupt TH. Orchialgia after orchiectomy. Plast Reconstr Surg 2014; 134: 998e–9. https://doi.org/10.1097/prs.0000000000000726
Cesmebasi A, Yadav A, Gielecki J, Tubbs RS, Loukas M. Genitofemoral neuralgia: a review. Clin Anat 2015; 28: 128–35. https://doi.org/10.1002/ca.22481
Shanthanna H. Successful treatment of genitofemoral neuralgia using ultrasound guided injection: a case report and short review of literature. Case Rep Anesthesiol 2014; 2014: 1–4. https://doi.org/10.1155/2014/371703
Díaz-Arribas MJ, Fernández-Serrano M, Royuela A, et al. Minimal clinically important difference in quality of life for patients with low back pain. Spine 2017; 42: 1908–16. https://doi.org/10.1097/brs.0000000000002298
Magee RK. Genitofemoral causalgia: (a new syndrome). Can Med Assoc J 1942; 46: 326–9.
Ilfeld BM, Finneran JJ. Cryoneurolysis and percutaneous peripheral nerve stimulation to treat acute pain. Anesthesiology 2020; 133: 1127–49. https://doi.org/10.1097/aln.0000000000003532
Ilfeld BM, Gabriel RA, Trescot AM. Ultrasound-guided percutaneous cryoneurolysis for treatment of acute pain: could cryoanalgesia replace continuous peripheral nerve blocks? Br J Anaesth 2017; 119: 703–6. https://doi.org/10.1093/bja/aex142
Rosendal F, Moir L, de Pennington N, Green AL, Aziz TZ. Successful treatment of testicular pain with peripheral nerve stimulation of the cutaneous branch of the ilioinguinal and genital branch of the genitofemoral nerves. Neuromodulation 2013; 16: 121–4. https://doi.org/10.1111/j.1525-1403.2011.00421.x
Abd-Elsayed A. Wireless peripheral nerve stimulation for treatment of peripheral neuralgias. Neuromodulation 2020; 23: 827–30. https://doi.org/10.1111/ner.13131
Slavin KV. Peripheral nerve stimulation for neuropathic pain. Neurotherapeutics 2008; 5: 100–6. https://doi.org/10.1016/j.nurt.2007.11.005
Author contributions
James Khan contributed to all aspects of this manuscript, including the study conception and design; acquisition, analysis, and interpretation of data; and revising the article. Elad Dana contributed to study conception and design; acquisition, analysis, and interpretation of data; and drafting the article. Himanshu Gupta contributed to the analysis of data and drafting and revising the article. Rahul Pathak contributed to the acquisition and interpretation of data.
Disclosures
The authors report no conflicts of interest relevant to this case, including with the device manufacturer. The senior author and primary implanter (J. S. K.) has only a working relationship for patient care with Bioventus.
Funding statement
None.
Editorial responsibility
This submission was handled by Dr. Philippe Richebé, Associate Editor, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dana, E., Gupta, H., Pathak, R. et al. Genitofemoral peripheral nerve stimulator implantation for refractory groin pain: a case report. Can J Anesth/J Can Anesth 70, 163–168 (2023). https://doi.org/10.1007/s12630-022-02348-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-022-02348-4