Abstract
Purpose
Recently, more attention has been given to the costoclavicular space (CCS) as an alternative pathway for ultrasound-guided brachial plexus block (BPB). While 0.5% ropivacaine was used in most related studies, research has shown effective ultrasound-guided supraclavicular BPB using lower local anesthetic concentrations, and our preliminary data have indicated that 0.375% ropivacaine may be effective when given in the CCS. Hence, we hypothesized that the efficacy of 0.375% ropivacaine would be noninferior compared with 0.5% in ultrasound-guided BPB via the CCS.
Methods
We conducted a randomized, double-blind, single-centre, noninferiority clinical trial. Seventy patients undergoing elective forearm or hand surgery were randomly assigned to receive either 20 mL of 0.375% ropivacaine (experimental group) or 0.5% ropivacaine (control group) in the CCS for BPB. We assessed sensory and motor blockade at five, ten, 15, 20, 25, and 30 min after the injection. The primary outcome was the rate of successful BPB. Secondary outcomes included onset time, duration of sensory and motor blockade, and adverse reactions. The depth from the skin to the CCS was also recorded during the procedure.
Results
A total of 69 patients were evaluable for block success. There was one failed block in both groups, yielding a BPB block success rate of 97% in both groups. 0.375% Ropivacaine was noninferior to 0.5% ropivacaine (P = 0.98). There was no significant difference in the median [interquartile range (IQR)] onset time of sensory-motor blockade in the experimental group (15 [15–20] min; N = 34) compared with the control group (15 [13–20] min; N = 33; Mann–Whitney test, P = 0.48). The median [IQR] duration of sensory blockade was significantly shorter in the experimental group (455 [398–490] min vs 610 [570–655] min in the control group; Hodges–Lehmann estimator of the difference, 165 min; 95.08% confidence interval (CI), 130 to 195; P < 0.001). Likewise, the median [IQR] duration of motor blockade was significantly shorter in the experimental group (470 [409–500] min vs 625 [578–665] min in the control group; Hodges–Lehmann estimator of the difference, 165 min; 95.08% CI, 130 to 195; P < 0.001). There were no adverse reactions directly related to the technique or the ropivacaine injection in either group.
Conclusions
0.375% Ropivacainewas noninferior to 0.5% ropivacaine with regard to rate of successful ultrasound-guided costoclavicular BPB.
Study registration
chictr.org.cn (ChiCTR20000306570); registered 8 March 2020.
Résumé
Objectif
L’espace costo-claviculaire (ECC) a récemment bénéficié d’un regain d’intérêt comme voie de substitution pour le bloc du plexus brachial (BPB) échoguidé. La ropivacaïne 0,5 % a été utilisée dans la majorité des études sur ce sujet, mais la recherche a montré un BPB supra-claviculaire échoguidé efficace en utilisant de plus faibles concentrations d’anesthésique local et nos données préliminaires ont indiqué que la ropivacaïne à 0,375 % pouvait être efficace en administration dans l’ECC. En conséquence, nous avons émis l’hypothèse selon laquelle l’efficacité de la ropivacaïne 0,375 % serait non inférieure à la ropivacaïne 0,5 % dans le BPB échoguidé via l’ECC.
Méthodes
Nous avons mené un essai clinique monocentrique de non-infériorité, randomisée en double insu. Soixante-dix patients subissant une chirurgie élective de l’avant-bras ou de la main ont été randomisés dans un groupe recevant 20 mL de ropivacaïne 0,375 % (groupe expérimental) ou de ropivacaïne 0,5 % (groupe contrôle) dans l’ECC pour un BPB. Nous avons évalué les blocs sensoriel et moteur à 5, 10, 15, 20, 25 et 30 minutes après l’injection. Le critère d’évaluation principal était le taux de succès du BPB. Les critères d’évaluation secondaires étaient, notamment, le délai d’action, la durée des blocs sensoriel et moteur, et les événements indésirables. La profondeur de la peau à l’ECC a aussi été consignée pendant la procédure.
Résultats
Un total de 69 patients était évaluable pour le succès du bloc. Il y a eu un échec du bloc dans chacun des deux groupes, ramenant le taux de succès du BPB à 97 % dans les deux groupes. La ropivacaïne 0,375 % a été non inférieure à la ropivacaïne 0,5 % (P = 0,98). Il n’y a pas eu de différence significative concernant le délai d’action médian (plage interquartile [PIQ]) du bloc sensori-moteur dans le groupe expérimental (15 [15 à 20] minutes; n = 34) comparativement au groupe contrôle (15 [13 à 20] minutes; n = 33; test de Mann–Whitney, P = 0,48). La durée médiane [PIQ] du bloc sensitif a été significativement plus courte dans le groupe expérimental (455 [398 à 490] minutes contre 610 [570 à 655] minutes dans le groupe contrôle; estimateur de la différence de Hodges–Lehmann, 165 minutes; intervalle de confiance [IC] à 95,08 % : 130 à 195; P < 0,001). De même, la durée médiane [PIQ] du bloc moteur a été significativement plus courte dans le groupe expérimental (470 [409 à 500] minutes contre 625 [578 à 665] minutes dans le groupe contrôle; estimateur de la différence de Hodges–Lehmann, 165 minutes; IC à 95,08 %, 130 à 195; P < 0,001). Il n’y a pas eu d’événement indésirable directement lié à la technique ou à l’injection de ropivacaïne dans l’un ou l’autre groupe.
Conclusions
La ropivacaïne 0,375 % a été non inférieure à la ropivacaïne 0,5 % en ce qui concerne le taux de succès du BPB costo-claviculaire échoguidé.
Enregistrement de l’étude
chictr.org.cn (ChiCTR20000306570); Enregistrée le 8 mars 2020.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Brachial plexus blockade (BPB) is widely used in upper limb surgery anesthesia. Perineural injections guided by ultrasound are more successful than injections guided by surface anatomy or electrical neurostimulation.1,2 The classical approaches to BPB include interscalene, supraclavicular, infraclavicular, and axillary approaches.
Recently, with the advances in ultrasonic guidance more attention has been given to the costoclavicular space (CCS) as an alternative access pathway to BPB. The CCS is located between the clavicle and the first rib, deep behind the middle of the clavicle.3 The CCS was introduced as a feasible pathway for BPB by Dahlstrom and Olinger in 2012 in their study of thoracic outlet syndrome,4 which was subsequently confirmed in a 2016 autopsy study by Sala–Blanch et al.5 Subsequent studies using the ultrasound-guided CCS approach to BPB,3,6,7,8,9 totalling 300 patients, found that the compact anatomy of the CCS had little interindividual variability and allowed for simple performance with few complications and a high success rate, all of which increases the feasibility for continuous nerve blockade.3,4,10 In recent years, some studies have applied this approach to special groups of individuals, such as pediatric and obese patients, and reported satisfactory results.11,12 In most of these studies, 0.5% ropivacaine was used.3,7,8,9 Recent research has shown effective ultrasound-guided supraclavicular BPB using lower local anesthetic concentrations,13 and preliminary data of our own provided an indication that 0.375% ropivacaine may be effective when given in the CCS. As the risk of systemic and direct neurotoxicity increases with higher concentrations, we felt it important to understand if a lower concentration of ropivacaine can be used for BPB in the CCS. Hence, the purpose of this study was to compare the efficacy of 0.375% ropivacaine with 0.5% ropivacaine in ultrasound-guided BPB in the CCS. Our hypothesis was that 0.375% ropivacaine would be noninferior to 0.5% ropivacaine in producing successful BPB when given in the CCS.
Methods
Enrollment
We conducted a single-centre, prospective, randomized, double-blind, noninferiority clinical trial. The study was approved by the Ethics Committee of Nanfang Hospital Affiliated to Southern Medical University (ethics number, NFEC-2020-088), and written informed consent was obtained from all individuals participating in the trial. Before patient enrollment, the trial was registered at the Chinese Clinical Trial Registry (chictr.org.cn; registry number: ChiCTR2000030657; date of registration: 8 March 2020; principal investigator: Shuang Wang).
Patients were enrolled between 9 May 2020 and 30 September 2020. Inclusion criteria were age 18 to 65 yr; American Society of Anesthesiologists Physical Status I to II; undergoing elective surgery of the forearm or hand under BPB; voluntary participation in the trial; and provision of written informed consent. Exclusion criteria were a history of allergy to local anesthetics; nerve injury or sensory abnormality of the affected limb; prior surgery on the infraclavicular fossa; bleeding tendency or evidence of coagulopathy; damage or infection at the site of needle insertion; a diagnosis of mental, language, or hearing impairment; or participation in another clinical trial within the last three months.
Randomization and blinding
Patients were randomly allocated 1:1 to receive either 0.375% ropivacaine (experimental group; N = 35) or 0.5% ropivacaine (control group; N = 35) for ultrasound-guided BPB. A computer-generated randomization list (1 = experimental group, 2 = control group) was prepared by a third party not associated with the study. The randomization list was contained in a sealed, opaque envelope. This was available only to the staff, also not involved in the study, who prepared the appropriate concentration of local anesthetic according to the random allocation. The investigator, anesthesiologists, outcome assessor, patients, and statistician were all blinded to group allocation.
Nerve block procedure
On arrival at the surgical suite, intravenous access was established (20 or 22G catheter) in the upper limb contralateral to the surgical site, and standard monitoring (electrocardiogram, noninvasive blood pressure, heart rate, and pulse oximetry) was instituted.
Each patient was placed in a supine position with the arm abducted 90 degrees on the affected side, and the head tilted contralaterally (without a pillow) for the BPB. A Venue 50 portable ultrasound system (GE Healthcare, Chicago, IL, USA) with a 5–12 MHz broadband linear array transducer was used for the scan. The transducer was placed directly over the midpoint of the clavicle in the transverse orientation with its orientation marker directed laterally (outward). The transducer was gently moved caudally until it slipped off the inferior border of the clavicle and the axillary artery and vein were visualized. The ultrasound image was optimized, and an effort was made to visualize all three cords of the brachial plexus.7 We confirmed that the brachial plexus nerve image was centred and that there were no blood vessels along the path of needle insertion. After strict aseptic precautions, an 80-mm nerve block needle (Stimuplex® D, B. Braun Melsungen AG, Melsungen, Germany) connected to the extension tube with the syringe was inserted in-plane and from a lateral to medial direction. With the position of the needle tip adjusted according to ultrasonic imaging, we aimed to place the needle tip at the centre of the nerve cluster by advancing the needle through the gap between the lateral and posterior cord, and then advancing it toward the medial cord. After negative aspiration of blood, a test bolus injection of 1–2 mL of 0.9% normal saline was given to confirm the needle tip was in the correct position. A single 20 mL dose of either 0.375% or 0.5% ropivacaine (i.e., 75 mg in the experimental group and 100 mg in the control group; AstraZeneca AB, Södertälje, Sweden) was slowly injected over two to three minutes at a single site, without any needle redirection. All nerve block operations were performed by one of two anesthesiologists, both of whom were experienced with this technique of ultrasound-guided costoclavicular BPB. Both anesthesiologists were familiar with various nerve block operations and had performed at least 50 cases of CCS BPB before this trial.
Observational index
After local anesthetic injection, BPB was assessed every five minutes for 30 min by a blinded observer. Sensory blockade was graded on the following 3-point scale using a cold test: 0 = no block; 1 = analgesia (patient can feel touch, not cold); and 2 = anesthesia (patient cannot feel touch). Sensory blockade of the following nerves and locations was assessed: musculocutaneous nerve (MCN) on the lateral aspect of the forearm, median nerve (MN) on the volar aspect of the thumb, radial nerve (RN) on the lateral aspect of the dorsum of the hand, ulnar nerve (UN) on the volar aspect of the fifth finger, and medial cutaneous nerve of the ulnar aspect of the forearm. Motor blockade was graded with the following 3-point scale: 0 = no block; 1 = paresis (slight or partial paralysis); and 2 = complete paralysis. Motor blockade was assessed for the following nerves and movements: MCN by elbow flexion, MN by thumb apposition, RN by thumb abduction, and UN by thumb adduction. The maximum score for nerve block performance was 18 points, and a successful nerve block was defined as a total score ≥ 16 points.6,7,14
If, after 30 min, the composite sensory and motor blockade score was less than 16 points, the BPB was considered a failure and the observations were stopped. The clinical protocol in such cases was to initiate iv opioid analgesia (sufentanil 7.5 or 15 μg) and/or local anesthesia, and if necessary, change to general anesthesia to allow the operation to be completed safely and comfortably. This was left to the discretion of the treating anesthesiologist and not recorded for the current study. Participants were hospitalized for at least 24 hr. During that period, the investigator (blinded to group assignment) assessed the patients every 15 min until complete resolution. Duration of sensory block was defined as time from the end of the injection to the recovery of pain senses that is subjectively similar to the untreated side. Duration of motor block was defined as time from the end of the injection to normal movement of the hand, elbow, and wrist. During these assessments, the investigator also recorded any adverse reactions or complications such as persistent numbness and paresthesia or motor deficits, vascular injury, local anesthetic toxicity, pneumothorax, and nerve injury. If any adverse reactions persisted at discharge, the investigator followed up via telephone as needed.
The primary outcome was the success rate of blockade, defined as the number (%) of successful blocks (total blockade score ≥ 16 within 30 min) divided by the number of patients in each group. Onset time for blockade (in minutes) was assessed as a secondary outcome. Overall onset time was calculated from the minimum time to reach a score of 16. Onset times for sensory and motor blockade were calculated separately (9 out of 10 points for sensory blockade and 7 out of 8 points for motor blockade). The times for recovery of sensory function and motor function (in minutes) were also assessed as a secondary outcome. The duration of sensory block was defined as the time from the end of the injection to the recovery of pain senses that was subjectively similar to the untreated side; duration of motor block was defined as time from the end of the injection to normal movement of the hand, elbow and wrist. The depth from the skin to the CCS was also recorded during the performance of the block
Statistical analysis and sample size calculation
We used IBM SPSS Statistics for Windows, version 20.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism, version 8.4.3 (GraphPad, San Diego, CA, USA) for statistical analysis. Normally distributed continuous variables are presented as mean (standard deviation [SD]) and categorical variables are presented as n/total N (%). A noninferiority study design was used to assess the primary endpoint. The primary outcome is presented as percent with 95% confidence interval (CI) and was analyzed with a noninferiority test of rates (from the normal approximation interval). Normality of the data was first assessed with the Lilliefors test. Normally distributed continuous data were analyzed with Student’s t test. Categorical data were analyzed with the Chi square test. The secondary outcomes of sensory and motor blockade onset and recovery times are presented as median [interquartile range (IQR)] and were analyzed with the Mann–Whitney test; differences are reported as the Hodges–Lehmann estimator and its 95.08% CI. Blockade of each nerve at the various times after injection is presented as n/total N (%) and were analyzed with Fisher’s exact test. Comparisons were two-sided and differences were considered statistically significant at P < 0.05.
A previous study reported a 97% success rate for ultrasound-guided BPB in the CCS with 20 mL of 0.5% ropivacaine.7 In our preliminary studies, we found a similar success rate with 20 mL of 0.375% ropivacaine. For this study, a noninferiority margin was set at δ = 15%, with a unilateral α = 0.025 and β = 0.2 (power = 0.8), using a ratio of 1:1 for study and control groups. Based on these assumptions, a sample size calculation (PASS 15.0 software; NCSS, LLC; Kaysville, UT, USA) estimated that 64 patients (32 per group) were needed to show the noninferiority of 0.375% ropivacaine to 0.5% ropivacaine. A total of 70 patients were planned for recruitment, with an estimated 10% dropout rate.
Results
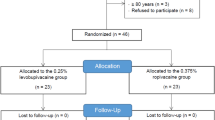
Of the 70 patients who were randomized (Fig. 1), one patient in the control group and one patient in the experimental group did not have a successful BPB block (composite score < 16 at 30 min) and were converted to general anesthesia. These patients were included in the data for calculation of block success rate, but were not included in the secondary analyses. It is of note that the ultrasound image of the patient in the control group was challenging to interpret because of excessive adipose tissue, which may have contributed to the failed block. A second patient in the control group had a successful BPB block but was discharged early and was withdrawn from the study because of loss to follow-up. Thus, there were 69 evaluable patients for the primary outcome (35 in the experimental group and 34 in the control group), and 67 for secondary outcomes and follow-up analyses (34 in the experimental group and 33 in the control group).
The Table shows baseline patient characteristics and clinical parameters.
The CCS BPB was successfully performed on 67 patients. All optimized ultrasound images were captured as a video file for subsequent review; all confirmed correct positioning of the needle tip before ropivacaine injection.
Regarding the primary outcome, the BPB block success rate was 97% in the experimental group and 97% in the control group, with a difference between the two groups of 0.1% (95% CI, -7.8 to 8.0; P = 0.98). The lower interval was greater than -0.15%, so noninferiority of 0.375% ropivacaine was established. There were no significant differences between the control and experimental groups in sensory or motor blockade of the major branches of the brachial plexus at any time up to 30 minutes after injection (see Electronic Supplementary Materials [ESM], eTables 1 and 2; Figs. 2 and 3).
There were no significant differences between the control and experimental groups in onset time for BPB blockade, sensory blockade, or motor blockade. Overall median [IQR] onset time in the experimental group was 15 [15–20] min (N = 34) compared with 15 [13–20] min (N = 33) in the control group (Mann–Whitney test, P = 0.48). Median [IQR] sensory and motor blockade onset in the experimental group was 15 [15–20] min and 10 [10–15] min, respectively, compared with 15 [13–20] min and 10 [10–15] min in the control group (Mann–Whitney test, P = 0.47 and P = 0.61, respectively). The median [IQR] duration of sensory blockade was significantly shorter in the experimental group (455 [398–490] min vs 610 [570–655] min in the control group; Hodges–Lehmann estimator of the difference, 165 min; 95.08% CI, 130 to 195; Mann–Whitney test, P < 0.001). Similarly, the median [IQR] duration of motor blockade was significantly shorter in the experimental group (470 [409–500] min vs 625 [578–665] min in the control group; Hodges–Lehmann estimator of the difference, 165 min; 95.08% CI, 130 to 195; Mann–Whitney test, P < 0.001).
The mean (SD) depth of the CCS to the skin surface for male and female participants was 2.5 (0.4) cm and 2.6 (0.4) cm, respectively. No vascular injury, nerve injury, local anesthetic toxicity, pneumothorax, or other complications were observed in either group. Three patients in the control group (0.5% ropivacaine) reported complications during the follow-up period. These patients felt numbness and paralysis in their upper limb and were unable to move it for up to ten hours after the procedure, gradually returning to normal as the effect of local anesthetics faded. None of the patients reported persistent neurologic signs or symptoms in the ipsilateral upper extremity at the 24 hr postoperative follow-up.
Discussion
In this study, we found that 0.375% ropivacaine was noninferior to 0.5% ropivacaine in producing successful ultrasound-guided costoclavicular BPB; the rate of successful sensory-motor blockade was equally high (97%) in both groups.
Our observed rates of successful blockade were similar to that reported by Li et al., who used 20 mL of 0.5% ropivacaine.7 Under the conditions of the current study, the costoclavicular BPB produced rapid onset of sensory-motor blockade that was effective for surgical anesthesia. There was no difference in the time to onset of sensory blockade or motor blockade between the two groups. The compact neural topography at the CCS, where the cords are separated from one another,3,5 is purported to provide a swift and reliable sensory-motor block when local anesthetic is deposited between the three cords of the brachial plexus.5 Ming et al. showed in a dose-finding study that the minimal effective volume 90 for CCS BPB with 0.5% ropivacaine was 20.9 mL (95% CI, 20.7 to 21.8).15 The volume of local anesthetic in our trial (20 mL) may be sufficient for diffusion throughout the CCS, which may explain the similar onset times with the two different concentrations of ropivacaine. With similar efficacy (successful block and onset time to sensory-motor block) in the two groups, an advantage of the 0.375% concentration is a 25% lower total dose of ropivacaine, which reduces the risk of systemic and local toxicity of the ropivacaine.
The recovery time for sensory-motor function was significantly longer in the control (0.5% ropivacaine) group. Although there were no complications directly related to the technique or the local anesthetic injection, three patients in this group reported complications during the follow-up period. These patients felt numbness and paralysis in their upper limb and were unable to move it for up to ten hours after the procedure. This was associated with discomfort, tension, and anxiety/fear. Although a long period of nerve block at a higher concentration provides extended postoperative analgesia, it also increases the potential risk of postoperative skin compression necrosis, nerve injury, and other complications. A higher concentration may not be necessary since the time to recovery of both sensory and motor function at the lower concentration was over 7.5 hours, which provides sufficient time to complete surgical procedures on the upper limb.
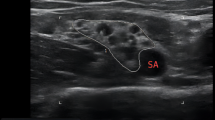
Complete sensory and motor blockade of major branches of the brachial plexus was also achieved quickly. The sequence of blockade was first the MCN followed by the RN, with the UN the slowest. Another study with 20 mL of 0.5% ropivacaine found that the RN was the first to show blockade, and the MN was the slowest.7 The fact that the MCN originates from the lateral cords of the brachial plexus and the UN originates from the medial cords may explain these differences and may also provide an opportunity for selective nerve blockade. By observing the anatomical structure and ultrasonic imaging of CCS (Fig. 4), it can be seen that the posterior and medial cords are very closely apposed, bound together by a common connective tissue, and they also run separate from the lateral cord. We suggest that the sequence of nerve block may be related to primary diffusion location of the local anesthetic, which in turn is related to the injection location. Therefore, in clinical practice, it might be possible to customize the location of injection and drug administration, such that the target nerve bundle could be blocked more quickly, and nerves outside of the surgical site blocked less or not at all.
One male patient in the 0.5% ropivacaine group with a weight of 43 kg and height of 167 cm (body mass index [BMI], 15.4 kg·m-2), required only 15 mL of drug to fully cover the three nerve bundles of the brachial plexus (but as per protocol, the full 20 mL was administered). Thus, it is possible that dosing may be optimized according to each patient’s BMI or from the ultrasound visualization of the nerve structure. Further study on this topic would be beneficial.
In a cadaver study, Dahlstrom and Olinger observed no significant difference in mean (SD) depth of the CCS between men (1.36 [0.40] cm) and women (1.35 [0.55] cm).4 We similarly found no difference between men and women but deeper mean (SD) CCS measurements of 2.6 (0.4) cm and 2.5 (0.4) cm, respectively. We suggest that our measurements in live individuals are likely to be more accurate.
In addition, the unique anatomy of CCS is suitable for continuous analgesia. This is something that is worth further study and improvement.
This study had numerous limitations. First, BMI was not restricted by protocol, and this may have been the reason that two participants had to be converted to general anesthesia. In the one patient in the 0.5% ropivacaine group, ultrasound imaging of the nerve bundles was obscured by excessive adipose tissue. The incomplete blockade 30 min after drug administration in the one patient in the 0.375% ropivacaine group was thought to be related to the patient’s weight (95 kg; height, 185 cm; BMI, 27.8 kg·m-2). Second, we only used a single in-plane needle injection approach. According to previous studies,16,17,18,19,20 there may be differences in the effective time and success rate of single-point and multiple-point injection, and of medial approach and lateral approach injection. Third, for BPB, we only identified three bundles of the brachial plexus under the guidance of ultrasound and did not combine it with nerve stimulation. In some patients with an unclear display of the brachial plexus, nerve stimulation combined with ultrasound guidance may have been more advantageous. Fourth, the recovery time of motor function is typically shorter than that of sensory function. While the recovery time of sensory function and motor function outcomes in this study were similar, we speculate that this may be related to limb numbness and hypoesthesia affecting patients’ subjective control of movement when the sensation had not recovered. During postoperative follow-up, we found that fixation with bandages, plaster, stents, and other tools impacted the evaluation of sensory and motor function recovery, as these rendered the patients’ movement slower and stiffer. To examine the impact of this factor, we performed a post hoc analysis of patients who underwent finger surgery only (n = 9) and found that the recovery of motor function was earlier than that of sensory function in all participants (P = 0.004 in the experimental group and P < 0.001 in the control group). Finally, we must acknowledge that our study involved a relatively limited sample size and was conducted within a single institutional setting. Therefore, the findings require validation in the multicentre context and larger sample sizes are required to better support the secondary and safety outcomes, which are currently underpowered.
All local anesthetics exert a dose-dependent depression of cardiac contractility and cardiac conduction. Increasing the total dose increases the concentration gradient between tissue depot and blood, thereby increasing the rate of rise in plasma concentration and thus the risk of local anesthetic systemic toxicity (LAST).21,22 Nevertheless, the doses of both the high-concentration group and the low-concentration group were within the recommended safe range. Nevertheless, we should be aware that individual patient sensitivities, comorbidities, and physician or practice deficiencies may predispose to catastrophic outcomes. Therefore, as ample research has pointed out, untoward systemic uptake of local anesthetic is best mitigated by using the lowest effective local anesthetic dose, which can be facilitated by low-volume ultrasound-guided techniques; the American Society of Regional Anesthesia and Pain Medicine’s recommendations for preventing LAST mention using the lowest effective dose of local anesthetic (dose = product of volume × concentration).23
In conclusion, in this study, 0.375% ropivacaine was noninferior to 0.5% ropivacaine with regard to the rates of successful ultrasound-guided BPB in the CCS. The time to onset of blockade was also not significantly different between the two concentrations. The duration of blockade with 0.375% ropivacaine (~7.5 hours) was sufficient in terms of the requirements for these types of limb surgeries. The lower concentration allowed for faster recovery of sensory and motor function and was not associated with any patient anxiety; collectively, this may allow a faster to return to normal activities. 20 ml of 0.375% ropivacaine was effective and devoid of apparent threats to safety, suggesting that this concentration can be considered for routine use for BPB in the CCS.
References
Sites BD, Beach ML, Spence BC, et al. Ultrasound guidance improves the success rate of a perivascular axillary plexus block. Acta Anaesthesiol Scand 2006; 50: 678–84. https://doi.org/10.1111/j.1399-6576.2006.01042.x
Abrahams MS, Aziz MF, Fu RF, Horn JL. Ultrasound guidance compared with electrical neurostimulation for peripheral nerve block: a systematic review and meta-analysis of randomized controlled trials. Br J Anaesth 2009; 102: 408–17. https://doi.org/10.1093/bja/aen384
Karmakar MK, Sala-Blanch X, Songthamwat B, Tsui BC. Benefits of the costoclavicular space for ultrasound-guided infraclavicular brachial plexus block: description of a costoclavicular approach. Reg Anesth Pain Med 2015; 40: 287–8. https://doi.org/10.1097/aap.0000000000000232
Dahlstrom KA, Olinger AB. Descriptive anatomy of the interscalene triangle and the costoclavicular space and their relationship to thoracic outlet syndrome: a study of 60 cadavers. J Manipulative Physiol Ther 2012; 35: 396–401. https://doi.org/10.1016/j.jmpt.2012.04.017
Sala-Blanch X, Reina MA, Pangthipampai P, Karmakar MK. Anatomic basis for brachial plexus block at the costoclavicular space: a cadaver anatomic study. Reg Anesth Pain Med 2016; 41: 387–91. https://doi.org/10.1097/aap.0000000000000393
Sotthisopha T, Elgueta MF, Samerchua A, et al. Minimum effective volume of lidocaine for ultrasound-guided costoclavicular block. Reg Anesth Pain Med 2017; 42: 571–4. https://doi.org/10.1097/aap.0000000000000629
Li JW, Songthamwat B, Samy W, Sala-Blanch X, Karmakar MK. Ultrasound-guided costoclavicular brachial plexus block: sonoanatomy, technique, and block dynamics. Reg Anesth Pain Med 2017; 42: 233–40. https://doi.org/10.1097/aap.0000000000000566
Beh ZY, Hasan MS. Ultrasound-guided costoclavicular approach infraclavicular brachial plexus block for vascular access surgery. J Vasc Access 2017; 18: e57–61. https://doi.org/10.5301/jva.5000720
Songthamwat B, Karmakar MK, Li JW, Samy W, Mok LY. Ultrasound-guided infraclavicular brachial plexus block: prospective randomized comparison of the lateral sagittal and costoclavicular approach. Reg Anesth Pain Med 2018; 43: 825–31. https://doi.org/10.1097/aap.0000000000000822
García-Vitoria C, Vizuete J, López Navarro AM, Bosch M. Costoclavicular space: a reliable gate for continuous regional anesthesia catheter insertion. Anesthesiology 2017; 127: 712. https://doi.org/10.1097/aln.0000000000001724
Silva GR, Borges DG, Lopes IF, et al. Ultrasound-guided costoclavicular block as an alternative for upper limb anesthesia in obese patients [Portuguese]. Braz J Anesthesiol 2019; 69: 510–3. https://doi.org/10.1016/j.bjan.2019.01.004
Yayik AM, Cesur S, Öztürk F, Celik EC, Ahiskalioglu A. Ultrasound guided costoclavicular approach to brachial plexus: first pediatric report. J Clin Anesth 2019; 55: 136–7. https://doi.org/10.1016/j.jclinane.2019.01.008
Fang G, Wan L, Mei W, Yu HH, Luo AL. The minimum effective concentration (MEC90) of ropivacaine for ultrasound-guided supraclavicular brachial plexus block. Anaesthesia 2016; 71: 700–5. https://doi.org/10.1111/anae.13445
Leurcharusmee P, Elgueta MF, Tiyaprasertkul W, et al. A randomized comparison between costoclavicular and paracoracoid ultrasound-guided infraclavicular block for upper limb surgery. Can J Anesth 2017; 64: 617–25. https://doi.org/10.1007/s12630-017-0842-z
Wong MH, Karmakar MK, Mok LY, Songthamwat B, Samy W. Minimum effective volume of 0.5% ropivacaine for ultrasound-guided costoclavicular brachial plexus block: a dose finding study. Eur J Anaesthesiol 2020; 37: 780–6. https://doi.org/10.1097/eja.0000000000001287
Arab SA, Alharbi MK, Nada EM, Alrefai DA, Mowafi HA. Ultrasound-guided supraclavicular brachial plexus block: single versus triple injection technique for upper limb arteriovenous access surgery. Anesth Analg 2014; 118: 1120–5. https://doi.org/10.1213/ane.0000000000000155
Roy M, Nadeau MJ, Côté D, et al. Comparison of a single- or double-injection technique for ultrasound-guided supraclavicular block: a prospective, randomized, blinded controlled study. Reg Anesth Pain Med 2012; 37: 55–9. https://doi.org/10.1097/aap.0b013e3182367b97
Tran DQ, Muñoz L, Zaouter C, Russo G, Finlayson RJ. A prospective, randomized comparison between single- and double-injection, ultrasound-guided supraclavicular brachial plexus block. Reg Anesth Pain Med 2009; 34: 420–4. https://doi.org/10.1097/aap.0b013e3181ae733a
Layera S, Aliste J, Bravo D, et al. Single- versus double-injection costoclavicular block: a randomized comparison. Reg Anesth Pain Med 2020; 45: 209–13. https://doi.org/10.1136/rapm-2019-101167
Nieuwveld D, Mojica V, Herrera AE, Pomés J, Prats A, Sala-Blanch X. Medial approach of ultrasound-guided costoclavicular plexus block and its effects on regional perfussion. Rev Esp Anestesiol Reanim 2017; 64: 198–205. https://doi.org/10.1016/j.redar.2016.09.010
Vasques F, Behr AU, Weinberg G, Ori C, Di Gregorio G. A review of local anesthetic systemic toxicity cases since publication of the American Society of Regional Anesthesia recommendations: to whom it may concern. Reg Anesth Pain Med 2015; 40: 698–705. https://doi.org/10.1097/aap.0000000000000320
Neal JM. Ultrasound-guided regional anesthesia and patient safety: update of an evidence-based analysis. Reg Anesth Pain Med 2016; 41: 195–204. https://doi.org/10.1097/aap.0000000000000295
Neal JM, Barrington MJ, Fettiplace MR, et al. The Third American Society of Regional Anesthesia and Pain Medicine practice advisory on local anesthetic systemic toxicity: executive summary 2017. Reg Anesth Pain Med 2018; 43: 113–23. https://doi.org/10.1097/aap.0000000000000720
Author contributions
Shuang Wang and Haihong Fang contributed to all aspects of this manuscript, including study conception and design; acquisition, analysis, and interpretation of data; and drafting the article. Jun Qin and Weifeng Liu contributed to the conception and design of the study. Wei Wang, Youming Pei, and Ying Chen contributed to analysis and interpretation of the data. Chunshui Lin contributed to study conception and design and reviewing and editing of the manuscript.
Disclosures
None.
Funding statement
None.
Editorial responsibility
This submission was handled by Dr. Stephan K. W. Schwarz, Editor-in-Chief, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, S., Fang, H., Qin, J. et al. Comparison of the efficacy of costoclavicular space brachial plexus blockade with 0.5% versus 0.375% ropivacaine: a randomized, double-blind, single-centre, noninferiority clinical trial. Can J Anesth/J Can Anesth 70, 106–115 (2023). https://doi.org/10.1007/s12630-022-02327-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-022-02327-9