Abstract
Background
Freon™ is a halogenated hydrocarbon often used as a refrigerant. When inhaled recreationally, it has the desired effects of euphoria and intoxication. Toxic effects include cardiovascular and neurologic insults such as arrhythmias and seizures, and less well-described toxicities include airway and lung injury. The treatment in general is primarily supportive.
Clinical features
We present the case of a 42-yr-old previously healthy male who developed acute bronchiolitis and pneumonitis following inhalation of Freon leading to severe respiratory failure. He was supported by veno-venous extracorporeal membrane oxygenation and managed with high-dose corticosteroids.
Conclusion
To our knowledge, this is the first case report of an inhaled Freon exposure resulting in acute lung injury refractory to conventional therapy that was salvaged by vv-ECMO as a bridge towards a full recovery.
Résumé
Contexte
Le Fréon™ est un hydrocarbure halogéné souvent utilisé comme réfrigérant. Lorsqu’il est inhalé à des fins récréatives, il a les effets souhaités d’euphorie et d’intoxication. Les effets toxiques comprennent les lésions cardiovasculaires et neurologiques telles que les arythmies et les convulsions, et les toxicités moins bien décrites comprennent les lésions des voies aériennes et des poumons. En général, le traitement est principalement un traitement de soutien.
Caractéristiques cliniques
Nous présentons le cas d’un homme de 42 ans auparavant en bonne santé qui a développé une bronchiolite et une pneumonite aiguës après inhalation de Fréon, entraînant une insuffisance respiratoire sévère. Il a été supplémenté par oxygénation par membrane extracorporelle veino-veineuse et pris en charge avec des corticostéroïdes à forte dose.
Conclusion
À notre connaissance, il s’agit de la première présentation de cas d’exposition au Fréon inhalé entraînant une lésion pulmonaire aiguë réfractaire au traitement conventionnel et pour laquelle un sauvetage par ECMO-VV a favorisé un rétablissement complet.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Inhalants comprise a broad range of household and industrial chemicals, usually in the form of hydrocarbons, whose volatile vapours and pressurized gases are concentrated and breathed in via the nose or mouth to produce intoxication. The desired effects of these hydrocarbons include euphoria, lightheadedness, and a general state of intoxication similar to that of ingesting alcohol or marijuana.1
Freon™ (The Chemours Company, Wilmington, DE, USA), used as a refrigerant or for air conditioning, is a halogenated hydrocarbon that produces central nervous system (CNS) as well as cardiovascular toxicity.2 Less frequently, toxicity may include gastrointestinal, renal, hematologic, skin, and soft tissue, as well as respiratory untoward effects. While CNS effects of euphoria, hallucinations, and disorientation induced by Freon may be desirable for the intentional user, they can also cause agitation, seizures, and coma. Cardiovascular toxicity can be very serious; most notably arrhythmias leading to “sudden sniffing death,” with numerous case reports of fatality following inhalant exposure.3 Less frequently, inhalational exposure to hydrocarbons can produce pneumonitis, bronchospasm, and pulmonary edema.4
Treatment for such exposures is primarily supportive. In case of respiratory failure, supportive care including bronchodilators, supplemental oxygen, and mechanical ventilation are usually enough for recovery. We present the case of a 42-yr-old otherwise healthy male presenting with an acute bronchiolitis and pneumonitis leading to severe respiratory failure following an intentional inhalation of Freon. His respiratory failure was refractory to conventional treatment, thus requiring veno-venous extracorporeal membrane oxygenation (vv-ECMO). He made a full functional recovery. He has provided written and informed consent for publication of this report.
Case presentation
A 42-yr-old male presented to a community hospital with a chief complaint of dyspnea. He reported to have been unwell during the previous days with symptoms of subjective fever, cough, sore throat, and headache. He admitted to inhaling Freon a few days before his symptoms started and reported that he had been inhaling Freon a few times per year for several years. He denied any other drug exposures prior to presentation. His social history included being unemployed with no fixed address as well as prior polysubstance use of inhaled crack cocaine and fentanyl. His home medication was 1,200 mg of slow-release oral morphine daily.
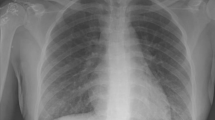
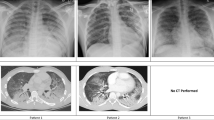
His vital signs on presentation were a temperature of 38°C, heart rate of 104 beats per min in sinus rhythm, and blood pressure of 147/88 mm Hg. His initial oxygen saturation (SpO2) was 90% on room air but he quickly progressed to needing a fraction of inspired oxygen of 80% via high flow nasal cannula to attain a SpO2 of 90–94%. A chest radiograph showed diffuse reticular nodular opacities throughout both lungs (Fig. 1). His initial bloodwork showed signs of elevated inflammatory markers with mild leukocytosis at 11.1 × 109·L-1 (normal, 4.0–10.5 × 109·L-1) and C-reactive protein of 218.4 mg·L-1 (normal, 0.0–2.0 mg·L-1) but other results were noncontributory. His urine drug screen was positive for amphetamines, cocaine, fentanyl, and opioids. Due to progressive hypoxemic respiratory failure, he was intubated and admitted to the intensive care unit (ICU). Three nasopharyngeal swabs and a tracheal aspirate sample were all negative for SARS-CoV-2. He was treated with intravenous piperacillin-tazobactam, azithromycin, and vancomycin as well as methylprednisolone 40 mg twice daily. A computed tomography (CT) scan of the chest was performed on day 3 of illness and revealed extensive bilateral ground-glass and centrilobular nodular opacities without evidence of pulmonary embolism (Fig. 2).
Four days after his admission to hospital, he was transferred to the regional referral centre for higher level of care because of the progressive nature of his respiratory failure refractory to prone positioning and use of inhaled epoprostenol. Upon arrival, he was severely hypercapnic and hypoxemic. He was unable to ventilate effectively at maximal ventilatory settings with full sedation and paralysis and was extremely unstable requiring bag mask ventilation. His arterial blood gas on admission was pH 6.82, PaCO2 202 mm Hg, PaO2 91 mm Hg, and bicarbonate 31 mmol·L-1 via bag mask ventilation on 100% O2. A chest radiograph revealed near complete bilateral opacification of the lungs (Fig. 1). He was crashed onto vv-ECMO via femoral-femoral configuration with 23 Fr return and 25 Fr access cannulas. Upon cannulation to extracorporeal support, his oxygenation and ventilation significantly improved. A bronchoscopy was subsequently performed and revealed that his airways were filled with fluid. Approximately 1 L of frothy secretions were suctioned with minimal instillment of saline or irrigation (Fig. 3). There was no evidence of alveolar hemorrhage on bronchoalveolar lavage, and samples obtained from subsequent bronchoscopies were negative for bacterial and fungal cultures, viral respiratory panel, SARS-CoV-2, Pneumocystis jirovecii, acid-fast bacilli, Leigonella, Cryptococcal antigen, Galactomann, Nocardia, and Acinobacter. He was negative for HIV and hepatitis A, B, and C. Antineutrophil cytoplasmic antibodies, cyclic citrullinated peptide antibodies, and rheumatoid factor were also negative. With a presumptive diagnosis of severe bronchiolitis and pneumonitis secondary to inhalation of Freon, he was treated with 500 mg iv methylprednisolone daily for three days and stepped down to 1 mg·kg–1 of prednisone for 4–6 weeks.
During the first two weeks of vv-ECMO treatment, he required a high level of ECMO support with blood and sweep gas flows of 6 and 11 L·min–1, respectively. He was deeply sedated to attain adequate oxygenation. He had near complete consolidation of his lungs and severely poor lung compliance. He was mechanically ventilated using pressure-controlled mode with a positive end-expiratory pressure of 12 cm H2O and inspiratory pressure of 12 cm H2O, yielding only tidal volumes of approximately 50 mL per breath. He required daily therapeutic bronchoscopies for bronchial toilet, and his respiratory secretions were noted to be progressively thicker and tenacious. A lengthy bronchoscopy on day 12 of illness yielded multiple large tracheobronchial casts (Fig. 3). On microscopic examination, these casts had acute inflammatory cells but were negative for malignant cells, pneumocystis jerovici pneumonia, and fungal and viral inclusions.
His respiratory function improved over time. By day 24, his tidal volumes were up to 450 ml/breath while the same ventilatory settings, and ECMO support was weaned. He showed radiographic improvement (Fig. 1). He was decannulated from vv-ECMO on day 26 of illness and liberated from the ventilator support and discharged from the ICU on day 28 of illness. He was discharged from ambulatory hospital care on day 50 of illness, requiring no oxygen and tolerating a full diet.
Discussion
Here, we present an unusual case of pulmonary toxicity from intentional inhalation of Freon, leading to severe and refractory respiratory failure requiring salvage institution of vv-ECMO support and high doses of corticosteroids to attain full recovery. We hypothesize that inhalation of Freon caused severe acute bronchiolitis and subsequent pneumonitis leading to respiratory failure.
Bronchiolitis is often used to describe nonspecific inflammatory injury to primarily the small airways, often sparing a considerable portion of the interstitium.5 Although tissue biopsies were not taken for histopathology, there were bronchoscopic, clinical, radiographic, and microscopic features that supported the diagnosis of acute bronchiolitis. There is no direct laboratory test for inhalants available clinically, but our patient did admit to inhaling Freon a few days before his acute respiratory failure. Diffuse bronchial injury is supported by the bronchoscopic findings of copious bronchial secretions and later thick tracheobronchial casts. The obstruction of the airways from these secretions and casts led to increased airway resistance and worsening lung compliance shown by extremely low tidal volumes on pressure control ventilation, and near complete lung consolidation on chest x-ray. The casts examined under microscopy were mostly of inflammatory cells with no evidence of malignancy or infection. Moreover, the diffuse distribution of centrilobular nodules shown on CT further supported the nature of inhalational injury. His acute recovery and single system involvement make other inflammatory disorders much less likely.
Inhaled hydrocarbons are known to produce pulmonary toxicity but their CNS and cardiac effects are not well described.6 An acute lung injury known as hydrocarbon pneumonitis has been described in the pediatric literature following unintentional ingestion and aspiration of liquid hydrocarbons by young children. Hydrocarbon pneumonitis is similar to aspiration pneumonitis, leading to inflammation and consolidation of dependent lobes such has the middle and lower lobes of the lung.7 There have been pediatric case reports of hydrocarbon pneumonitis being successfully treated with ECMO.8 Our patient’s presentation is different from hydrocarbon pneumonitis as he intentionally rather than accidentally inhaled Freon and showed a diffuse centrilobular distribution of lung injury. Furthermore, he did not appear to suffer from nonpulmonary manifestations such as cardiac arrhythmias or neurologic disturbances. These features are more prominent during acute intoxication and his lung injury developed a few days after the intentional exposure to Freon.
The treatment of pulmonary toxicity following hydrocarbon exposure is primarily supportive. There is scarce literature to guide us on how to manage such patients, mainly case reports from the pediatric literature describing accidental ingestion and hydrocarbon pneumonitis.9 In addition to lung protective strategies during mechanical ventilation for acute lung injury,10 other described treatment modalities include ECMO and the administration of glucocorticoids, antibiotics, and exogenous surfactants.11 Our patient received all of these except for exogenous surfactant. In our case, vv-ECMO was a lifesaving strategy. Given the initial blood gases and clinical deterioration on presentation to our ECMO referral centre, he would not have survived without extracorporeal support. The toxicological exposure leading to rapid progressive respiratory failure in an otherwise healthy man provided a good indication for ECMO to recover lung function. While exogenous surfactant use has been well-studied in both adult and pediatric patients with acute lung injuries, its therapeutic benefit in the adult population has unfortunately not been shown.12 Supportive management such as lung protective ventilator strategies along with frequent bronchoscopy for bronchial toileting allowed for enough time for lung recovery.
In summary, we report the first case of pulmonary toxicity from recreational inhalation of Freon, leading to severe and refractory hypoxemic respiratory failure requiring salvage vv-ECMO and high doses of corticosteroids to attain full recovery.
References
Webb M, Jentzen J. A case of difluoroethane toxicity—sudden sniffing death syndrome. In: Ketha H, Garg U (Eds.). Toxicology Cases for the Clinical and Forensic Laboratory. London: Academic Press; 2020: 405–7.
Flanagan RJ, Ruprah M, Meredith TJ, Ramsey JD. An introduction to the clinical toxicology of volatile substances. Drug Saf 1990; 5: 359–83. https://doi.org/10.2165/00002018-199005050-00005
Tormoehlen LM, Tekulve KJ, Nañagas KA. Hydrocarbon toxicity: a review. Clin Toxicol (Phila) 2014; 52: 479–89. https://doi.org/10.3109/15563650.2014.923904
Gerhardt RT. Acute Halon (bromochlorodifluoromethane) toxicity by accidental and recreational inhalation. Am J Emerg Med 1996; 14: 675–7. https://doi.org/10.1016/s0735-6757(96)90087-x
Swaminathan AC, Carney JM, Tailor TD, Palmer SM. Overview and challenges of bronchiolar disorders. Ann Am Thorac Soc 2020; 17: 253–63. https://doi.org/10.1513/annalsats.201907-569cme
Sabik LM, Abbas RA, Ismail MM, El-Refaei S. Cardiotoxicity of Freon among refrigeration services workers: comparative cross-sectional study. Environ Health 2009; 8: 31. https://doi.org/10.1186/1476-069x-8-31
Makrygianni EA, Palamidou F, Kaditis AG. Respiratory complications following hydrocarbon aspiration in children. Pediatr Pulmonol 2016; 51: 560–9. https://doi.org/10.1002/ppul.23392
Chyka PA. Benefits of extracorporeal membrane oxygenation for hydrocarbon pneumonitis. J Toxicol Clin Toxicol 1996; 34: 357–63. https://doi.org/10.3109/15563659609013804
Jolliff HA, Fletcher E, Roberts KJ, Baker SD, McKenzie LB. Pediatric hydrocarbon-related injuries in the United States: 2000-2009. Pediatrics 2013; 131: 1139–47. https://doi.org/10.1542/peds.2012-3913
Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342: 1301–8. https://doi.org/10.1056/nejm200005043421801
Sommer C, Johnson AB, Sam Wang G, Poel K. Surfactant for the management of pediatric hydrocarbon ingestion. Am J Emerg Med 2018; 36: 2260–2. https://doi.org/10.1016/j.ajem.2018.09.016
Raghavendran K, Willson D, Notter R. Surfactant therapy for acute lung injury and acute respiratory distress syndrome. Crit Care Clin 2011; 27: 525–59. https://doi.org/10.1016/j.ccc.2011.04.005
Author contributions
Chung-chi Jennifer Chao contributed to all aspects of the manuscript. Juan Ronco contributed to editing the manuscript.
Disclosures
None.
Funding statement
None.
Editorial responsibility
This submission was handled by Dr. Philip M. Jones, Deputy Editor-in-Chief, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chao, Cc.J., Ronco, J. A case report of recreational use of inhaled Freon leading to acute hypoxemic respiratory failure salvaged by veno-venous extracorporeal membrane oxygenation. Can J Anesth/J Can Anesth 69, 1300–1304 (2022). https://doi.org/10.1007/s12630-022-02296-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-022-02296-z