Abstract
Purpose
Peripheral nerve blocks improve analgesia following hip fracture; however, there are little published data on safety and outcomes of continuous regional anesthetic techniques. Our institution offers pre- and perioperative, anesthesiologist-delivered ultrasound-guided suprainguinal fascia iliaca catheters (FICs) to patients with hip fracture. We aimed to document the safety profile of this technique and establish whether there are any significant clinical benefits in outcomes measured by the UK National Hip Fracture Database.
Methods
We performed a single-centre historical cohort study of 2,187 patients admitted to our institution with hip fracture over a 5.75-year period. Of these, 915 were treated with FIC and 1,272 received standard care (single-shot block). To control for baseline differences between these two cohorts, we used propensity score matching and exact matching, resulting in two well-matched groups of 728 patients treated with an FIC and standard care.
Results
No serious complications were observed as a result of an FIC. Unplanned removal occurred in 146/852 (17.1%) patients with documented data. No differences in 30-day mortality, pressure ulcer rates, or hospital length of stay were observed between the matched groups. The percentage of patients who were discharged to their usual residence was 79.3% in the FIC cohort vs 75.1% in the standard care cohort (difference, 4.2%; 95% confidence interval, -0.1 to 8.4; P = 0.06).
Discussion
Our single-centre propensity-matched historical cohort study suggests that ultrasound-guided suprainguinal fascia iliaca catheterization is a safe technique for patients with hip fracture and that our service is deliverable and sustainable within the UK’s National Health Service. This study did not show statistically significant differences in outcomes between patients treated with FIC and standard care. An adequately powered multicentre randomized controlled trial comparing these approaches is warranted.
Résumé
Objectif
Les blocs nerveux périphériques améliorent l’analgésie après une fracture de la hanche; cependant, il existe peu de données publiées sur l’innocuité et les devenirs des techniques d’anesthésie régionale continue. Notre établissement propose des cathéters iliofasciaux suprainguinaux échoguidés pré- et périopératoires aux patients souffrant d’une fracture de la hanche. Notre objectif était de documenter le profil d’innocuité de cette technique et de déterminer s’il existe des avantages cliniques significatifs au niveau des devenirs tels que mesurés par la Base de données nationale sur les fractures de la hanche du Royaume-Uni.
Méthode
Nous avons réalisé une étude de cohorte historique monocentrique portant sur 2187 patients admis dans notre établissement avec une fracture de la hanche sur une période de 5,75 ans. De ce nombre, 915 ont été traités avec un cathéter iliofascial et 1272 ont reçu des soins standard (bloc à injection unique). Pour tenir compte des différences initiales entre ces deux cohortes, nous avons utilisé l’appariement par score de propension et l’appariement exact, ce qui a donné deux groupes bien appariés de 728 patients chaque, les patients étant traités par cathéter ilio-fascial ou soins standard.
Résultats
Aucune complication grave n’a été observée à la suite de l’utilisation d’un cathéter iliofascial. Un retrait imprévu est survenu chez 146/852 (17,1 %) patients dont les données ont été documentées. Aucune différence dans la mortalité à 30 jours, les taux d’escarres ou la durée de séjour à l’hôpital n’a été observée entre les groupes appariés. Le pourcentage de patients qui ont reçu leur congé à leur résidence habituelle était de 79,3 % dans la cohorte cathéter iliofascial vs 75,1 % dans la cohorte de soins standard (différence, 4,2 %; intervalle de confiance à 95 %, -0,1 à 8,4; P = 0,06).
Discussion
Notre étude de cohorte historique monocentrique et appariée par propension suggère que le cathétérisme iliofascial suprainguinal échoguidé est une technique sécuritaire pour les patients atteints de fracture de la hanche et que notre service est utilisable et durable au sein du National Health Service du Royaume-Uni. Cette étude n’a pas montré de différences statistiquement significatives dans les devenirs entre les patients traités par cathéter iliofascial ou par soins standard. Une étude randomisée contrôlée multicentrique suffisamment puissante comparant ces approches est justifiée.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Hip fracture causes a significant burden on healthcare services worldwide.1 Pain is at its worst in the preoperative period when the fracture is unstable; however, traditional analgesic options are constrained because of a high incidence of renal, cardiovascular, and gastrointestinal pathologies in this patient cohort.
Compared with systemic analgesia, peripheral nerve blocks provide better pain relief for hip fracture, facilitate easier nursing care, reduce the time taken to perform spinal anesthesia, and improve patient satisfaction.2 They reduce the requirement for systemic opioids, the risk of pneumonia, the time to first mobilization, and the cost of analgesic regimen (for single-shot blocks).2 Despite being a standard of care in the UK,1 only half of patients receive a nerve block before surgery.3
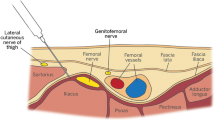
The fascia iliaca compartment block is commonly used to provide hip fracture analgesia. Ultrasound-guidance increases its success rate compared with the traditional landmark approach.4 The suprainguinal approach5 is relatively novel and further improves the original infrainguinal technique. It produces more consistent spread of local anesthetic to the lateral femoral cutaneous and obturator nerves as well as the femoral nerve, achieving complete sensory block of the thigh more frequently (80%) than the infrainguinal technique (30%) does.6 Furthermore, this approach allows easy insertion of an infusion catheter and is further away from the groin and its associated hygiene concerns.
Complications of ultrasound-guided suprainguinal fascia iliaca catheters (FICs) include hematoma, neuropraxia, local anesthetic systemic toxicity, perforation of peritoneal cavity contents, and bladder puncture.7
Time from admission to operative hip fracture fixation affects mortality and is recommended to be less than 36 hr.8 The duration of action of a single-shot fascia iliaca block of around eight hours9 is of inadequate duration for most patients. Our institution therefore offers patients admitted with hip fracture an FIC in the preoperative period. A randomized controlled trial of FICs compared with systemic analgesia showed improved function at six weeks as well as improved early postoperative pain and mobilization.10
The primary aim of this study was to review the safety profile of FICs in hip fracture patients. The secondary aim was to evaluate whether their use was associated with any differences in outcomes measured by the National Hip Fracture Database (NHFD). This is a UK database that allows institutions to benchmark their performance against national data and to track progress of quality improvement initiatives. Data are collected prospectively at each institution by specialist nurses.
Methods
Service evaluations such as this are exempt from National Health Service (NHS) Research Ethics Committee approval11 as all the data used were collected for normal care of patients. The project was registered with our clinical governance department and approval gained from the institutional data protection guardian to publish the data.
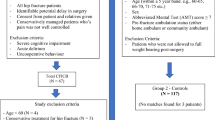
We performed a single-centre retrospective historical cohort study of hip fracture patients at the Royal Victoria Infirmary, Newcastle upon Tyne, UK. All patients with hip fracture treated in our institution between 1 September 2013 and 31 May 2019 were identified from the NHFD. Over the same time period, patients treated with FICs were identified from our electronic pain database. This database includes information on indication, reason for removal, and complications, all of which are collected prospectively.
Hospital record number and admission date were used to cross-reference the NHFD data set with the patients from the pain database. This allowed comparison between two patient cohorts: those who received standard care and those treated with FICs. Treatment in the standard care cohort was broadly representative of usual care for hip fracture across the UK,8 namely multimodal intraoperative analgesia with or without preoperative single-shot nerve block. Patients with hip fracture who are managed nonoperatively are known to have significantly worse outcomes than those treated with surgical fixation12 and were therefore excluded from analysis to minimize the effects of confounding variables. All NHFD data pertaining to demographics, preinjury health, and functional status, and details of hospital management were included in the analysis. We evaluated outcomes using the following measures, which were consistently present on the NHFD during this period: 30-day mortality, final discharge destination, acute hospital ward length of stay (excluding rehabilitation unit), and pressure ulcer incidence.
Patients with hip fracture were managed according to an institutional protocol. This included prioritization of initial assessment, fast-track admission to a hip fracture ward, and referral by emergency department (ED) physicians to the anesthesia team for consideration of regional analgesia. Initial pain management in the ED was with intravenous acetaminophen and morphine.
When expertise and availability of the anesthesia team allowed, patients were offered an FIC. Ideally, this was performed immediately after discharge from the ED on the way to the hip fracture ward, but could be following ward admission if theater workload precluded timely insertion. If nerve catheter experience was not available, a single-shot technique was offered: infrainguinal or suprainguinal fascia iliaca or femoral nerve block, depending on the preference of the practitioner. For patients who did not receive a preoperative FIC, standard practice was to perform a single-shot ultrasound-guided suprainguinal fascia iliaca block as part of their anesthetic for surgical fixation.
Suprainguinal FIC was performed using an ultrasound-guided in-plane catheter-through-needle technique.5 Catheters were threaded 5–10 cm beyond the end of the ultrasound-visible Tuohy needle (Pajunk Medical Produkte GmbH, Geisingen, Germany). Catheters were fixed to the skin using skin glue, fixation dressings (LOCKIT plus; Smiths Medical, Minneapolis, MN, USA), clear adhesive dressings, and flexible adhesive fabric dressings around the edges of the clear dressing. Patients received 40 mL of levobupivacaine 2.5 mg⋅mL-1 as a bolus before starting an infusion of levobupivacaine 1.25 mg⋅mL-1 at 8 mL⋅hr-1. The bolus and infusion doses were reduced in patients weighing less than 50 kg. At the time of operative fixation, a bolus of 20 mL levobupivacaine 2.5 mg⋅mL-1 was administered through the FIC alongside spinal or general anesthesia. The infusion was continued for approximately 24 hr postoperatively. Removal intraoperatively was sometimes necessary if the catheter was too close to the planned operation site because of anesthesiologist or surgeon preference.
An analysis of risk and benefit was made in circumstances of anticoagulation or reduced mental capacity. We did not consider anticoagulation in the therapeutic range to be a contraindication to FIC insertion, but measures were taken to reduce the risk of harm, including provision by a more experienced operator. In patients lacking the mental capacity to provide informed consent, a best-interests decision was made—if possible after discussion with the patient’s next of kin. Insertion was attempted with conservative measures to keep the patient calm (distraction, gentle handholding to prevent desterilization of the field) unless it was considered unsafe to do so.
Details of all patients treated with an FIC were entered into our pain database; these data included indications, insertion details, complications, and reasons for catheter removal. Patients were reviewed daily by a specialist pain nurse or anesthesiologist to assess analgesic efficacy and to check for any catheter-related complications. Serious complications were considered to be nerve injury, hematoma or bleeding issues, infection, and local anesthetic systemic toxicity (see Table 1). In addition, we used our institution’s nerve injury referral pathway and clinical incident reporting systems to identify any complications missed by our pain system.
We analyzed the data using R software version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria). Missing data were assessed using the “finalfit” package (version 1.0.1) as described in the package vignette.13 We calculated confidence intervals (CIs) for complications using the Clopper–Pearson method.14 Differences between the two treatment cohorts were compared with the Chi square test for categorical data, Student’s t test for normally distributed numerical data, and the Mann–Whitney U test for non-normally distributed numerical data. Disparities in time to operation across different years of study were analyzed using the Kruskal–Wallis test. We performed an iterative matching process15 to correct imbalance in baseline variables between the FIC and standard care cohorts. Optimal matching and propensity matching with various calipers16 resulted in residual imbalance, particularly in time to operation, abbreviated mental test score (AMTS), and total hip replacement. We therefore performed exact matching followed by propensity score matching (see Electronic Supplementary Material eAppendix for further details). We first calculated propensity scores from all patients using binomial logistic regression. We then performed exact matching using the previously imbalanced baseline variables (year; time to operation; AMTS 9–10; total hip replacement). Finally, we performed optimal pair matching using the propensity score within the exact matching strata. We considered a standardized mean difference (SMD) < 0.1 of baseline variables to be adequately balanced.17 Significance testing is not recommended to check for balance between matched groups18 but is reported for readers not familiar with SMDs. Following matching, the statistical significance of treatment was calculated using paired statistical tests to take account of the matched nature of the groups:19 we used conditional logistic regression for categorical data and the Wilcoxon signed-rank test for continuous data. We considered P < 0.05 as statistically significant.
Results
We identified 2,187 patients in the 5.75-year period of study treated for hip fracture within our hospital. Of these, 915 (41.8%) were treated with an FIC and 1,272 (58.2%) with standard care. No other regional anesthetic catheter techniques were used. In the standard care cohort, 1,237/1,272 (97.3%) patients received a single-shot block during their admission, 2/1,272 (0.2%) received no nerve block, and 33/1,272 (2.6%) had missing data.
There were no complications identified in any of the 915 patients treated with FICs (95% CI for complications, 0 to 0.004, or 0 to 1 in 249), either at the time of catheter insertion or from subsequent infusion of local anesthetic. Specifically, there were no incidences of nerve damage, bleeding complications, infection, or local anesthetic toxicity requiring lipid emulsion treatment.
The reasons for catheter removal were documented in 852 cases—46/852 (5.4%) were planned intraoperative removal, 660/852 (77.5%) were planned postoperative removal, 108/852 (12.7%) were unplanned removal by patient, 34/852 (4.0%) were unplanned removal by staff, 2/852 (0.2%) were ineffective analgesia, 1/852 (0.1%) was removal at patient request, and 1 (0.1%) was removal to facilitate MRI. Fascia iliaca catheters were resited after unplanned removal in 79 patients—one patient had three resites, four patients had two resites, and the remainder one resite. The individual who inserted the FIC was recorded for 693 insertions. Ninety-two different individuals were identified with a median [IQR] of 2 [1–5] insertions. Four anesthesiologists inserted over 50 catheters each, the maximum being 76 (8.3% of all catheter insertions).
Missing data analysis revealed higher levels of missing data for American Society of Anesthesiologists (ASA) Physical Status classification (8% missing) and AMTS on admission (2%) than for other variables, with interactions between missingness and other variables including mortality. Missing values for ASA Physical Status classification and AMTS were therefore treated as a separate category for each variable for propensity score matching.20 Baseline characteristics of the cohorts are outlined in Table 2. Patients treated with an FIC mostly had lower risk factors, including lower age, lower ASA Physical Status classification level, higher AMTS on admission, better pre-fracture mobility, and a higher proportion admitted from their own home or sheltered accommodation. These patients were more frequently treated with a total hip replacement. Nevertheless, patients treated with an FIC had a longer time from admission to surgery.
Outcome data are shown in Table 3. In these raw data, the FIC cohort had a higher incidence of pressure ulcers but more frequent discharge to their usual place of residence. Pressure ulcers were more common in patients waiting > 48 hr for their operation, but this difference did not reach statistical significance (Table 4). There was a year-on-year increase in the proportion of fractured neck of femur patients managed with an FIC, from 21% in 2013 to 67% in the first five months of 2019 (Table 5). Time to operation and mortality remained static during this period, but the incidence of pressure ulcers increased from 6% to 17%. To control for the baseline imbalance between the FIC and standard care cohorts, we performed an iterative matching process. This resulted in two groups of 728 each, all SMDs for baseline variables < 0.1 (mean SMD, 0.019; maximum, 0.073), suggesting good balance21 between the groups (see Figure 1). Baseline data for the matched treatment groups are shown in Table 6.
Outcome data for the matched groups are shown in Table 7. There were no statistically significant differences in outcome measures between the patients treated with an FIC and those without. Nevertheless, discharge to patients’ usual place of residence neared statistical significance: 79.3% in the FIC cohort vs 75.1% in the standard care cohort; difference, 4.2%; 95% CI, -0.1 to 8.4; P = 0.06.
Discussion
In this single-centre propensity-matched historical cohort study, we observed no significant complications after insertion of 915 consecutive suprainguinal fascia iliaca catheters in hip fracture patients. The most frequently observed problem was unplanned removal (17.1%) by the patient or by the healthcare team (e.g., leaking catheters, disconnection). This incidence of unplanned catheter removal was similar to our experience with paravertebral catheters (15%).22 Our observed low complication rate is reflected across the published literature.23,24 We found an increasing incidence of pressure ulcers across the years of study. Previous (unpublished) local investigation concluded this was due to better reporting of pressure ulcers and inclusion of lower stage pressure ulcers. There was no difference in pressure ulcer incidence between the FIC and standard care cohorts.
Analysis of the matched groups showed that use of an FIC was not associated with any statistically significant outcome differences. The only outcome which approached statistical significance was return to original place of residence, with an absolute increase of 4.2% in the FIC group (95% CI, -0.1 to 8.4; P = 0.06), which would give a number needed to treat of 24. Given the significant long-term morbidity following hip fracture,25 this finding is of interest, and if demonstrated with statistical significance, we believe this difference would represent a clinically relevant improvement to these patients and therefore the most promising outcome for future research. Using our data to calculate the sample size for a randomized controlled trial, 1,531 patients would be required in each treatment group to achieve 80% power with 5% alpha error to show an improvement in discharge home from 75.8% to 80.0%. This outcome measure represents the overall quality of hip fracture care26 and has been adopted by the NHFD as one of its key performance indicators.8 Our retrospective data can, at best, only highlight associations between treatment and outcome, and is unable to account for unmeasured confounding factors. Nevertheless, a systematic comparison of observational studies using propensity scores with randomized clinical trials in the high-impact critical care literature found that results of the former generally agreed with the results of the latter.27 The only published randomized controlled trial investigating FICs showed improved mobility six weeks after hip fracture in the intervention group.10
Very few catheters in our cohort were removed for being ineffective (0.2%), although this is likely to be an underestimate of inadequate analgesia. Prospective studies have used visual analog or verbal rating pain scores, opioid consumption, or time to first analgesic request to measure analgesic efficacy,23 none of which were documented adequately enough to be included in this study. Many patients with hip fracture have concurrent cognitive impairment, and although appropriate assessment tools exist, there is no consensus on how pain should be assessed in this group.23 Development of an injury-specific functional assessment tool, analogous to Pain, Inspiratory capacity, and Cough (PIC) scoring for rib fracture,28 may better identify inadequate analgesia in patients with hip fracture.
There are several different models to provide nerve blocks for hip fracture patients. Most commonly in the UK, fascia iliaca blocks are performed by ED physicians after diagnosis, using an infrainguinal single-shot landmark approach.29 Roughly, one third are provided by anesthesiologists, and less commonly orthopedic surgeons are responsible.29 There are examples of nurses being trained to provide fascia iliaca blocks,30 and even blocks performed before arriving at the hospital by first attenders.31
We chose an anesthesiologist-delivered service, believing this brings benefits foremost in safety and quality. Team familiarity with performing regional anesthesia, appropriate monitoring, safety checks, and adequate postprocedure observation are already routine. Anesthesiologists are familiar with ultrasound guidance, which is known to improve the quality, onset time, and extent of fascia iliaca blocks,4,32 and can perform a catheter technique to prolong the duration of analgesia.
The primary problems with an anesthesiologist-delivered service are delays and missed patients. Today, roughly one third of our patients do not receive an FIC, as expertise is not always available, or the team may be busy, especially out of hours when staff are stretched thinly. Even when an anesthesiologist and assistant are available, time to block will always be longer than ED-delivered blocks. The combination of an ultrasound-guided block by a trained ED physician followed by an FIC by an anesthesiologist when possible10 might balance benefit to the patient while minimizing delays.
The NHFD does not record the use of FICs or other continuous techniques, but we believe the use of catheters is not widespread. If proven to be beneficial to patient recovery, the technique would have the potential to improve outcomes on a national scale. We have shown that the service is deliverable within the constraints of the NHS, and have increased the proportion of patients receiving FICs through regular training of anesthesiologists using cadaveric sessions and e-learning.33
Limitations of our study largely stem from its retrospective design. Several important baseline characteristics differed in the two groups though this was addressed by statistical matching to leave good balance of measured baseline variables between the two groups. Nevertheless, there are many other variables not measured by the NHFD that could have caused confounding. Only an adequately powered randomized trial can ensure balance in both measured and unmeasured variables. In addition, we have discovered some errors in the recording of original data. For example, 15 patients (four received an FIC) were added to the NHFD despite being aged under 60 yr. Such errors are common in retrospective analyses, and we have included these patients in our analysis. The data collected by the NHFD have evolved with time, so some variables of interest such as delirium incidence, postoperative mobilization, and preoperative nerve block incidence were not available for this analysis. Other risk-stratification tools such as the Nottingham Hip Fracture Score were also not available for the full data set.
This study supports the safety of FICs and investigates their effects on key outcomes in hip fracture patients. Consecutive sampling represents authentic experience from our centre. We have shown that it is possible to deliver this service within the NHS without additional funding, and have found ultrasound-guided fascia iliaca catheterization to be a safe technique for these patients. We echo the call from the National Institute for Health and Care Excellence1 for a definitive randomized controlled trial comparing nerve blocks with opioid use, but would propose that continuous catheter techniques may convey further, as yet unproven, benefit to patients.
References
National Institute For Health And Care Excellence. Hip fracture: management. Available from URL: https://www.nice.org.uk/guidance/cg124/resources/hip-fracture-management-pdf-35109449902789 (accessed February 2022).
Guay J, Parker MJ, Griffiths R, Kopp SL. Peripheral nerve blocks for hip fractures: a Cochrane review. Anesth Analg 2018; 126: 1695–704.
Royal College of Physicians. National hip fracture database. Availabe from URL: https://www.nhfd.co.uk/20/NHFDcharts.nsf/vwCharts/Anaesthesia?open&org= (accessed February 2022).
Dolan J, Williams A, Murney E, Smith M, Kenny GN. Ultrasound guided fascia iliaca block: a comparison with the loss of resistance technique. Reg Anesth Pain Med 2008; 33: 526–31.
Hebbard P, Ivanusic J, Sha S. Ultrasound-guided supra-inguinal fascia iliaca block: a cadaveric evaluation of a novel approach. Anaesthesia 2011; 66: 300–5.
Vermeylen K, Desmet M, Leunen I, et al. Supra-inguinal injection for fascia iliaca compartment block results in more consistent spread towards the lumbar plexus than an infra-inguinal injection: a volunteer study. Reg Anesth Pain Med 2019; https://doi.org/10.1136/rapm-2018-100092.
O’Reilly N, Desmet M, Kearns R. Fascia iliaca compartment block. BJA Educ 2019; 19: 191–7.
Royal College of Physicians, National Hip Fracture Database. Annual report 2019. Available from URL: https://www.nhfd.co.uk/files/2019ReportFiles/NHFD_2019_Annual_Report_v101.pdf (accessed February 2022).
Pinson S. Fascia Iliaca (FICB) block in the emergency department for adults with neck of femur fractures: a review of the literature. Int Emerg Nurs 2015; 23: 323–8.
Morrison RS, Dickman E, Hwang U, et al. Regional nerve blocks improve pain and functional outcomes in hip fracture: a randomized controlled trial. J Am Geriatr Soc 2016; 64: 2433–9.
Health Research Authority. Decision tools: defining research table. Available from URL: http://www.hra-decisiontools.org.uk/research/docs/DefiningResearchTable_Oct2017-1.pdf (accessed February 2022).
Kim SJ, Park HS, Lee DW. Outcome of nonoperative treatment for hip fractures in elderly patients: a systematic review of recent literature. J Orthop Surg (Hong Kong) 2020; https://doi.org/10.1177/2309499020936848.
Harrison E. Missing data. Available from URL: https://cran.r-project.org/web/packages/finalfit/vignettes/missing.html (accessed February 2022).
Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 1934; 26: 404–13.
Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci 2010; 25: 1–21.
Rosenbaum P. Modern algorithms for matching in observational studies. Annu Rev Stat Appl 2020; 7: 143–76.
Nguyen TL, Collins GS, Spence J, et al. Double-adjustment in propensity score matching analysis: choosing a threshold for considering residual imbalance. BMC Med Res Methodol 2017; https://doi.org/10.1186/s12874-017-0338-0.
Imai K, King G, Stuart EA. Misunderstandings between experimentalists and observationalists about causal inference. J R Statis Soc A 2008; 171: 481–502.
Austin PC. Comparing paired vs non‐paired statistical methods of analyses when making inferences about absolute risk reductions in propensity‐score matched samples. Stat Med 2011; 30: 1292–301.
Twala BE, Jones MC, Hand DJ. Good methods for coping with missing data in decision trees. Pattern Recognit Lett 2008; 29: 950–6.
Austin PC. A tutorial and case study in propensity score analysis: an application to estimating the effect of in-hospital smoking cessation counseling on mortality. Multivariate Behav Res 2011; 46: 119–51.
Womack J, Pearson JD, Walker IA, Stephens NM, Goodman BA. Safety, complications and clinical outcome after ultrasound‐guided paravertebral catheter insertion for rib fracture analgesia: a single‐centre retrospective observational study. Anaesthesia 2019; 74: 594–601.
Scurrah A, Shiner CT, Stevens JA, Faux SG. Regional nerve blockade for early analgesic management of elderly patients with hip fracture – a narrative review. Anaesthesia 2018; 73: 769–83.
Steenberg J, Møller AM. Systematic review of the effects of fascia iliaca compartment block on hip fracture patients before operation. Br J Anaesth 2018; 120: 1368–80.
Dyer SM, Crotty M, Fairhall N, et al. A critical review of the long-term disability outcomes following hip fracture. BMC Geriatr 2016; https://doi.org/10.1186/s12877-016-0332-0.
Voeten SC, Krijnen P, Voeten DM, Hegeman JH, Wouters MW, Schipper IB. Quality indicators for hip fracture care, a systematic review. Osteoporos Int 2018; 29: 1963–85.
Kitsios GD, Dahabreh IJ, Callahan S, Paulus JK, Campagna AC, Dargin JM. Can we trust observational studies using propensity scores in the critical care literature? a systematic comparison with randomized clinical trials. Crit Care Med 2015; 43: 1870–9.
Witt CE, Bulger EM. Comprehensive approach to the management of the patient with multiple rib fractures: a review and introduction of a bundled rib fracture management protocol. Trauma Surg Acute Care Open 2017; https://doi.org/10.1136/tsaco-2016-000064.
Miller GW, Godrey JJ, Sagmeister ML, Lewis TL. Provision of fascia iliaca compartment block in the acute management of proximal femoral fractures: a national observational study of UK hospitals. Injury 2016; 47: 2490–4.
Randall A, Grigg L, Obideyi A, Srikantharajah I. Fascia iliaca compartment block: a nurse-led initiative for preoperative pain management in patients with a fractured neck of femur. J Orthop Nurs 2008; 12: 69–74.
Dochez E, van Geffen GJ, Bruhn J, Hoogerwerf N, van de Pas H, Scheffer G. Prehospital administered fascia iliaca compartment block by emergency medical service nurses, a feasibility study. Scand J Trauma Resusc Emerg Med 2014; https://doi.org/10.1186/1757-7241-22-38.
Tran DQ, Salinas FV, Benzon HT, Neal JM. Lower extremity regional anesthesia: essentials of our current understanding. Reg Anesth Pain Med 2019; 44: 143–80.
Anonymous. Abstracts and highlight papers of the 37th Annual European Society of Regional Anesthesia & Pain Therapy (ESRA) Congress 2018: E-Poster Viewing Abstracts. Reg Anesth Pain Med 2018; 43: e97–199.
Barrington MJ, Brull R, Reina MA, Hadzic A. Complications and prevention of neurological injury with peripheral nerve blocks. Available from URL: https://www.nysora.com/foundations-of-regional-anaesthesia/complications/ (accessed February 2022).
Author contributions
Michael James, Richard A. Bentley, Jonathan Womack, and Ben A. Goodman contributed to all aspects of this manuscript, including conception and design; acquisition, analysis, and interpretation of data; and drafting the article.
Acknowledgements
The authors dedicate this manuscript to the memory of Dr. M. K. Varma, Consultant Anesthesiologist, Royal Victoria Infirmary, without whose pioneering and inspirational approach this service would not exist. The authors thank the acute pain nurses and nurse specialists in orthogeriatrics at the Royal Victoria Infirmary for their hard work following these patients up and compiling the databases. The authors thank Drs H. Dawson and K. Simpson, Consultant Anesthesiologists, Royal Victoria infirmary, for their considerable contribution to the development of this service and Professor R. Griffiths for reviewing the manuscript before submission.
Disclosures
None.
Funding statement
No external funding. This submission was handled by Dr. Stephan K. W. Schwarz, Editor-in-Chief, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
James, M., Bentley, R.A., Womack, J. et al. Safety profile and outcome after ultrasound-guided suprainguinal fascia iliaca catheters for hip fracture: a single-centre propensity-matched historical cohort study. Can J Anesth/J Can Anesth 69, 1139–1150 (2022). https://doi.org/10.1007/s12630-022-02279-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-022-02279-0