Abstract
Purpose
Erector spinae plane blocks (ESPB) and pectointercostal fascial (PIFB) plane blocks are novel interfascial blocks for which local anesthetic (LA) doses and concentrations necessary to achieve safe and effective analgesia are unknown. The goal of this prospective observational study was to provide the timing (Tmax) and concentration (Cmax) of maximum total and free plasma bupivacaine after ESPB in breast surgery and after PIFB in cardiac surgery patients.
Methods
Erector spinae plane blocks or PIFBs (18 patients per block; total, 36 patients) were performed with 2 mg⋅kg-1 of bupivacaine with epinephrine 5 μg⋅mL-1. Our principal outcomes were the mean or median Cmax of total and free plasma bupivacaine measured 10, 20, 30, 45, 60, 90, 180, and 240 min after LA injection using liquid chromatography with tandem mass spectrometry.
Results
For ESPB, the mean (standard deviation [SD]) total bupivacaine Cmax was 0.37 (0.12) μg⋅mL-1 (range, 0.19 to 0.64), and the median [interquartile range (IQR)] Tmax was 30 [50] min (range, 10–180). For ESPB, the mean (SD) free bupivacaine Cmax was 0.015 (0.017) μg⋅mL-1 (range, 0.003–0.067), and the median [IQR] Tmax was 30 [20] min (range, 10–120). After PIFB, mean plasma concentrations plateaued at 60–240 min. For PIFB, the mean (SD) total bupivacaine Cmax was 0.32 (0.21) μg⋅mL-1 (range, 0.14–0.95), with a median [IQR] Tmax of 120 [150] min (range, 30–240). For PIFB, the mean (SD) free bupivacaine Cmax was 0.019 (0.010) μg⋅mL-1 (range, 0.005–0.048), and the median [IQR] Tmax was 180 [120] min (range, 30–240). For both ESPB and PIFB, we observed no correlations between pharmacokinetic and demographic parameters.
Conclusion
Total and free bupivacaine Cmax observed after ESPB and PIFB with 2 mg⋅kg-1 of bupivacaine with epinephrine 5 μg⋅mL-1 were five to twenty times lower than levels considered toxic in the literature.
Résumé
Objectif
Les blocs des muscles érecteurs du rachis (ESP) et les blocs des plans fasciaux pecto-intercostaux (PIFB) sont de nouveaux blocs interfasciaux pour lesquels les doses et les concentrations d’anesthésique local (AL) nécessaires à obtenir une analgésie sécuritaire et efficace sont inconnues. L’objectif de cette étude observationnelle prospective était de déterminer le moment d’administration (Tmax) et la concentration (Cmax) de bupivacaïne plasmatique totale et plasmatique libre maximale après un bloc ESP pour chirurgie mammaire et après un PIFB chez les patients en chirurgie cardiaque.
Méthode
Des blocs ESP ou PIFB (18 patients par bloc; total, 36 patients) ont été réalisés avec 2 mg⋅kg-1 de bupivacaïne et 5 μg⋅mL-1 d’épinéphrine. Nos principaux critères d’évaluation étaient la Cmax moyenne ou médiane de bupivacaïne plasmatique totale et libre mesurée 10, 20, 30, 45, 60, 90, 180 et 240 min après l’injection d’AL par chromatographie liquide avec spectrométrie de masse en tandem.
Résultats
Pour le bloc ESP, la Cmax de bupivacaïne totale moyenne (écart type [ET]) était de 0,37 (0,12) μg⋅mL-1 (plage, 0,19 à 0,64), et le Tmax médian [écart interquartile (ÉIQ)] était de 30 [50] min (intervalle, 10–180). Pour le bloc ESP, la Cmax de bupivacaïne libre moyenne (ET) était de 0,015 (0,017) μg⋅mL-1 (plage, 0,003–0,067), et le Tmax médian [ÉIQ] était de 30 [20] min (intervalle, 10–120). Après un PIFB, les concentrations plasmatiques moyennes ont plafonné à 60–240 min. Pour le bloc PIFB, la Cmax de bupivacaïne totale moyenne (ET) était de 0,32 (0,21) μg⋅mL-1 (plage, 0,14–0,95), et le Tmax médian [ÉIQ] était de 120 [150] min (intervalle, 30–240). Pour le bloc PIFB, la Cmax de bupivacaïne libre moyenne (ET) était de 0,019 (0,010) μg⋅mL-1 (plage, 0,005–0,048), et le Tmax médian [ÉIQ] était de 180 [120] min (intervalle, 30–240). Pour le bloc ESP et le PIFB, nous n’avons observé aucune corrélation entre les paramètres pharmacocinétiques et démographiques.
Conclusion
: Les Cmax de bupivacaïne totale et libre observées après un bloc ESP et PIFB avec 2 mg⋅kg-1 de bupivacaïne avec 5 μg⋅mL-1 d’épinéphrine étaient cinq à vingt fois plus faibles que les niveaux considérés comme toxiques dans la littérature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The last decade has witnessed the emergence of perioperative ultrasound-guided interfascial plane blocks,1,2 for which local anesthetic (LA) doses and concentrations necessary to achieve optimal analgesia are not known. The erector spinae plane block (ESPB) and the pectointercostal fascial plane block (PIFB) are among the novel interfascial plane blocks that aim to relieve acute pain after surgery.1,3,4,5,6,7,8 As in any block, ESPB and PIFB LA doses and concentrations must balance the need for profound conduction blockade and/or long-lasting analgesia9 with the potential for LA toxicity, which limits maximum doses to the lowest effective dose for each block. While the injection site has an important effect on LA pharmacokinetics, including peak plasma LA concentrations associated with potential toxicity,10,11 LA pharmacokinetic (PK) absorption data specific to ESPB and PIFB are lacking.
The goal of this prospective observational study was therefore to provide PK data including levels (principal outcome) and timing of peak bupivacaine plasma concentrations after ESPB and PIFB.
Methods
Study design and patient population
This manuscript adheres to the applicable STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines.Footnote 1 This single-center, prospective, descriptive, observational study was approved by the Institutional Ethics Board of the Centre hospitalier de l’Université de Montréal, and written informed consent was obtained from all participants. The ESPB study and PIFB study were both registered before patient enrollment at ClinicalTrials.gov (NCT03841409; principal investigator: S. W.; date of registration: 15 February 2019; NCT03920904; principal investigator: S. W.; date of registration: 19 April 2019). Results of the two studies are reported together as both the ESPB and PIFB studies were designed with similar objectives and methods, then performed simultaneously by the same research team.
To study the ESPB, patients with an American Society of Anesthesiologists (ASA) Physical Status classification less than IV, between 18 and 90 yr of age, and undergoing mastectomy with or without axillary lymph node dissection were consecutively screened and recruited from July 2019 to October 2019.
For the PIFB study, patients with an ASA classification less than V, between 18 and 90 yr of age, and undergoing full median sternotomy for elective coronary artery bypass graft surgery and/or valve replacement surgery were consecutively screened and recruited from August 2019 to September 2019. Patients were not recruited if they could not provide informed consent, were allergic to amide LA, had severe heart failure (ejection fraction < 30%), had severe liver disease (Child-Pugh score B and C), had severe renal insufficiency with a glomerular filtration rate12 less than 30 mL⋅min-1/1.73 m-2, had an infection in the designated block area, or refused to participate. Patients for whom the predetermined sampling regimen could not be observed or who required allogeneic blood transfusions during the sampling period were excluded.
Drug administration
A limited group of investigators (A. M., S. W., S. A., S. M.) with experience in ESPB and PIFB performed all blocks using the technique described below.
Erector spinae plane blocks were carried out preoperatively and before general anesthesia, with two intravenous accesses, in the antecubital fossa or proximal forearm contralateral to the ESPB, serving solely to draw blood for the study. Patients were monitored in a sitting position with an electrocardiogram, pulse oximeter, and noninvasive blood pressure cuff. Under sterile conditions, a 15–6 MHz linear ultrasound probe (Sonosite HFL50; FUJIFILM Sonosite, Inc., Bothell, WA, USA) placed parasagittally 3 cm lateral to the midline guided an 80-mm, 22G needle (SonoPlex® STIM NanoLine, PAJUNK® GmbH Medizintechnologie, Geisingen, Germany) inserted cephalically and in-plane into the interfascial plane below the erector spinae muscle group at the level of the fifth thoracic vertebra (see Electronic Supplementary Material [ESM] eFig. 1). A total dose of 2 mg⋅kg-1 (lowest of actual weight or Devine’s ideal body weight formula, 50 kg + (0.91 × [height in cm − 152.4]) for males and 45.5 kg + (0.91 x [height in cm − 152.4]) for females,13 maximum 150 mg) bupivacaine 0.5% with epinephrine 5 μg⋅mL-1 was delivered, with negative aspiration between 5-mL aliquots and echographic confirmation of injection in the targeted location. Surgery was performed under general anesthesia, and any use of additional bupivacaine by the anesthesiologist or surgeon was prohibited.
For the PIFB study, anesthesia included invasive arterial pressure monitoring, and the use of bupivacaine during surgery or anesthesia was prohibited except for the PIFB. At the end of surgery, after sternal closure, under general anesthesia, bilateral PIFB was performed at the third and sixth intercostal spaces in the sterile surgical field. A 15–6 MHz linear ultrasound probe (Sonosite HFL50) placed parasagittaly 3 cm lateral to the midline guided an 80-mm, 22G needle (SonoPlex® STIM NanoLine) inserted cephalically and in-plane into the interfascial space between the pectoralis major and intercostal muscles (see ESM eFig. 2). A total dose of 2 mg⋅kg-1 (lowest of actual or ideal body weight,13 maximum 150 mg) bupivacaine 0.25% with epinephrine 5 μg⋅mL-1 was delivered with negative aspiration between aliquots of maximum 5 mL and echographic confirmation of injection in the targeted location.
Blood sampling, handling, processing
The designated intravenous access (ESPB) and arterial line (PIFB) were first cleared by drawing 3 and 9 mL of discard volume, respectively. Blood samples (4.5 mL) were withdrawn 10, 20, 30, 45, 60, 90, 120, 180, and 240 min after completing bupivacaine injection and placed in lithium heparinized tubes (BD Vacutainer® Barricor™, Becton, Dickinson and Company, Franklin Lakes, NJ, USA) on ice, then centrifuged (5 min at 10,000 × g) with the supernatant plasma aliquots stored at −20°C. Once all samples had been obtained, 1 mL of the supernatant plasma aliquot was collected from each sample and centrifuged through a filter in a Centrifree ultrafiltration device (Millipore, Fisher Scientific, Ottawa, ON, Canada) at 1,000 × g for ten minutes to separate free bupivacaine from protein-bound bupivacaine. Liquid chromatography with tandem mass spectrometry analysis with a 1,200 series high-performance liquid chromatography system coupled to 6,410 electrospray tandem mass spectrometer (Agilent Technologies, Montréal, QC, Canada) was used to measure total and free bupivacaine concentrations in each sample.
Assessment of sensory block
Though postoperative assessment of sensory block was severely limited by the wound dressing for ESPB and level of consciousness for PIFB, an attempt was made in all patients to assess the extent of sensory block using a 6.1 g von Frey filament. Assessments of ESPB patients were made in the postanesthetic care unit (PACU), while PIFB patients were assessed 240 min after injection in the intensive care unit.
Pharmacokinetic analysis
Our principal outcomes of interest were the level (Cmax) as well as the timing (Tmax) of peak plasma concentration of total and free bupivacaine after PIFB and ESPB. A standard noncompartmental PK data analysis was performed with Phoenix® NLME version 8.3 (Certara USA, Inc., Princeton, NJ, USA). Peak plasma concentration (Cmax) and time to Cmax (Tmax) were identified for each patient. The slope of the terminal phase was computed when a downward trend of the PK profile was present and the terminal half-life was reported as \(T_{{{\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 2}}\right.\kern-\nulldelimiterspace} \!\lower0.7ex\hbox{$2$}}}} = ~\frac{{Log~\left( 2 \right)}}{{terminal~\,slope}}\). We used R software (R Foundation for Statistical Computing, Vienna, Austria) to generate model diagnostic figures and tables of descriptive statistics.14
Statistical analysis
For both ESPB and PIFB, the sample size estimation was based on reported Cmax in patients after intercostal blockade with bupivacaine 0.25%.15 Considering an alpha error of 0.05 and desired precision of ± 0.1 μg⋅mL-1, 15 patients were required for each block,16 to which we added three patients for each of the two blocks to compensate for sampling or other problems, giving a total of 18 patients per block.
Descriptive and PK data were verified for normality by the Shapiro–Wilk test and presented as means (standard deviation [SD]) or medians [interquartile range (IQR)], as appropriate. A relation between patient age, weight, ideal weight or body mass index (BMI), and Cmax was evaluated by computing the Spearman’s rank-order correlation coefficients and associated P values.
Results
Erector spinae plane block
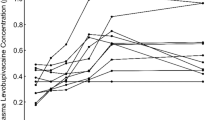
Figure 1 shows the ESPB recruitment flowchart, while Table 1 shows patient demographic, morphologic, and surgical characteristics. Deposition of LA into the interfascial space between the pectoralis major and intercostal muscles and into the interfascial plane below the erector spinae muscle group at the level of the fifth thoracic vertebra was successfully visualized in all patients. The mean (SD) dose and volume of bupivacaine injected were 108 (10) mg and 22 (2) mL. The mean (SD) total bupivacaine Cmax was 0.37 (0.12) μg⋅mL-1, with a median [IQR] Tmax of 30 [50] min. The mean (SD) free bupivacaine Cmax was 0.015 (0.017) μg⋅mL-1, with a median [IQR] Tmax of 30 [20] min. Figure 2 shows mean total and free plasma concentrations over time (see ESM eTable 1 for individual Cmax/Tmax values). The highest single value for total bupivacaine Cmax was 0.64 μg⋅mL-1 at 10 min, and for free bupivacaine Cmax was 0.067 μg⋅mL-1 at 20 min (in two different patients). Free bupivacaine plasma concentrations represented on average 4% of their respective total bupivacaine plasma concentrations. Sample mishandling made measurement impossible for total bupivacaine in nine samples (four in patient #10 and three in patient #17) and for free bupivacaine in patient #9. Fifty-eight percent of the 153 free bupivacaine measurements were under the lower limit of calibration of 0.01 μg⋅mL-1. We observed no significant correlation between total or free Cmax and age (P = 0.09 and 0.29, respectively), weight (P = 0.60 and 0.54, respectively), or BMI (P = 0.83 and 0.64, respectively). Assessment of sensory blockade levels in the PACU was severely limited by the wound dressing: three patients had a sensory block between T1 and T2, one between T1 and T3, two between T2 and T3, two between T3 and T5, two between T3 and T6, two between T4 and T5, and two between T5 and T6, while in four patients sensory blockade could not be determined.
Pectointercostal fascial plane block
Figure 3 shows the PIFB recruitment flowchart. Notably, ten patients were excluded because their surgery coincided with that of another recruited patient (simultaneous collection of blood samples according to the predetermined schedule for more than one patient was not possible). Table 2 shows the demographic and surgical characteristics of the study population. Deposition of LA into the interfascial space between the pectoralis major and intercostal muscles at the four injection points was successfully achieved in all patients. The mean (SD) dose and volume of bupivacaine injected were 127 (16) mg and 51 (6) mL. The mean (SD) total bupivacaine Cmax was 0.37 (0.12) μg⋅mL-1, with a median [IQR] Tmax of 120 [150] min. The mean (SD) free bupivacaine Cmax was 0.019 (0.010) μg⋅mL-1 with a median [IQR] Tmax of 180 [120] min. Figure 4 shows mean total and free plasma concentrations over time. Mean total bupivacaine concentrations formed a broad plateau of values between 0.30 and 0.34 μg⋅mL-1 over the last three hours of the measurement period (see ESM eTable 2 for individual Cmax/Tmax values). The highest single value for total bupivacaine Cmax was 0.95 μg⋅mL-1 at 45 min, and the highest free bupivacaine Cmax was 0.048 μg⋅mL-1 at 60 min (in the same patient). Free bupivacaine plasma concentrations represented on average 5% of their respective total bupivacaine plasma concentrations. Forty-two percent of 162 free bupivacaine measurements were under the lower calibration limit of 0.01 μg⋅mL-1. We observed no significant correlation between total or free Cmax and age (P = 0.41 and 0.86, respectively), weight (P = 0.37 and 0.42, respectively), and BMI (P = 0.65 and 0.90, respectively). The results of sensory block assessment in the nine extubated patients at 240 min were as follows: three patients had a sensory block between T3 and T6, two between T3 and T7, one between T4 and T6, and one between T4 and T7. All sensory blockade territories were bilateral, asymmetrical at the edges, and none extended lateral to the midclavicular line. One patient also had a sensory block between T3 and T6, but very limited to the incision line. Finally, despite a numerical rating scale pain score at rest of 0, one patient did not exhibit sensory blockade.
Discussion
When new peripheral nerve blocks are introduced, PK data specific to these new LA injection sites are needed to ensure safety while optimizing the LA dose to provide maximal analgesia with minimal toxicity.17 The present study shows low peak bupivacaine serum concentrations after injection of 2 mg⋅kg-1 of adrenalized solution for both ESPB and PIFB.
After ESPB and PIFB, average total and free plasma bupivacaine levels were more than five to twenty times lower than levels associated with central nervous or cardiovascular systemic toxicity (total arterial plasma bupivacaine 4 μg⋅mL-1,18 free plasma arterial bupivacaine 0.3 μg⋅mL-1,18 total venous plasma bupivacaine 2.118 or 2.2519 μg⋅mL-1, and free venous bupivacaine 0.11 μg⋅mL-1).18 Erector spinae plane block and PIFB bupivacaine Cmax values observed in the present study were two to four times lower than after a bilateral intercostal block with a total of 140 mg of bupivacaine without epinephrine,20 three times lower than after a transversus abdominis plane block (TAPB) and rectus sheath block with 130 mg of levobupivacaine without epinephrine,21 and about one third lower than following epidural administration of 100 mg of bupivacaine with epinephrine.22 As for free bupivacaine plasma concentration after ESPB and PIFB, no previous study has compared Cmax after fascial blocks. It is unknown whether the plasma levels of bupivacaine reported in this study have a systemic analgesic effect but if so, it may be lower than with the other blocks described above.
Reported bupivacaine Tmax values are 30 min after intercostal block,20 32 min after TAPB,21 61 min after rectus sheath block,21 and 21 min after epidural block.22 The present ESPB Tmax exhibited similarities with those of epidural, intercostal, and TAPB blocks. For PIFB, contrary to most other reported LA PK values for regional blocks,20,23,24,25,26,27,28,29 bupivacaine plasma concentrations seemed to plateau over 60–240 min, without a clearly defined peak. The use of a moderate concentration of bupivacaine with epinephrine, limiting bupivacaine absorption,30 has been shown after a TAPB not only to decrease the total bupivacaine Cmax by almost half but also to produce a plateau-shaped concentration–time curve.31 A delayed PIFB Tmax could also be the result of poor vascularization of the pectointercostal fascial plane, or altered perfusion combined with a change in LA disposition following cardiac surgery.
For both PIFB and ESPB, we observed no significant correlation between Cmax and demographic or morphologic parameters. The American Society of Regional Anesthesia and Pain Medicine recommends using lean body weight for dose adjustment in truncal blocks.32 Dose adjustment to ideal body weight coupled with adjunctive epinephrine may have reduced variation related to demographic and morphologic parameters in the present study.30,31
Limitations
A first limitation of the present study is that the ESPB study population was composed only of females undergoing breast surgery, while PIFB was performed only in cardiac surgery patients, most being post-cardiopulmonary bypass; therefore, our results may not be generalizable to other surgical populations. Variations in volume of distribution, alterations in drug binding and metabolism, and instability of renal function have been described postoperatively in patients who undergo cardiopulmonary bypass.33 Another limitation is that, unlike most other blocks,20,23,27,28,34 240 min was insufficient to confirm the Cmax and Tmax values needed to create a PK model for bupivacaine after PIFB. Further studies examining PIFB using bupivacaine with epinephrine could sample plasma levels for more than 240 min. Nevertheless, given the shape of the curve and the fact that the Cmax was not observed more than four hours after LA injection in other blocks, it is likely that the maximum concentrations observed are similar to the Cmax that would be observed over a longer period of time. The effect of different volumes and concentrations of LA on Cmax and Tmax values for both the PIFB and the ESPB should be the object of further study. In addition, this study was not designed to evaluate clinical “success” of the blocks, which may have affected plasma LA concentrations despite achieving a consistent echographic endpoint for all blocks. Finally, the limited number of participants in the study may have limited the ability to relate variability in plasmatic concentrations with specific cohorts such as the elderly, the morbidly obese, or those with renal or hepatic insufficiency. Further studies of ESPB and PIFB pharmacokinetics should consider using a larger sample size to lower variance.
Conclusion
Bupivacaine with epinephrine pharmacokinetics after ESPB and PIFB with 2 mg⋅kg-1 produced maximum total and free plasma concentrations much lower than those considered toxic. It is hoped the data presented in this study will be useful when choosing LA doses in further studies of ESPB and PIFB pharmacokinetics and clinical efficacy.
Notes
The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement. Available from URL: https://www.strobe-statement.org (accessed February 2022).
References
Kelava M, Alfirevic A, Bustamante S, Hargrave J, Marciniak D. Regional anesthesia in cardiac surgery: an overview of fascial plane chest wall blocks. Anesth analg 2020; 131: 127–35.
Marhofer P, Feigl GC, Hopkins PM. Fascial plane blocks in regional anaesthesia: how problematic is simplification? Br J Anaesth 2020; 125: 649–51.
Liu H, Emelife PI, Prabhakar A, et al. Regional anesthesia considerations for cardiac surgery. Best Pract Res Clin Anaesthesiol 2019; 33: 387–406.
Forero M, Adhikary SD, Lopez H, Tsui C, Chin KJ. The erector spinae plane block: a novel analgesic technique in thoracic neuropathic pain. Reg Anesth Pain Med 2016; 41: 621–7.
Krishna SN, Chauhan S, Bhoi D, et al. Bilateral erector spinae plane block for acute post-surgical pain in adult cardiac surgical patients: a randomized controlled trial. J Cardiothorac Vasc Anesth 2019; 33: 368–75.
Macaire P, Ho N, Nguyen T, et al. Ultrasound-guided continuous thoracic erector spinae plane block within an enhanced recovery program is associated with decreased opioid consumption and improved patient postoperative rehabilitation after open cardiac surgery-a patient-matched, controlled before-and-after study. Cardiothorac Vasc Anesth 2019; 33: 1659–67.
Nagaraja PS, Ragavendran S, Singh NG, et al. Comparison of continuous thoracic epidural analgesia with bilateral erector spinae plane block for perioperative pain management in cardiac surgery. Ann Card Anaesth 2018; 21: 323–7.
Leyva FM, Mendiola WE, Bonilla AJ, Cubillos J, Moreno DA, Chin KJ. Continuous erector spinae plane (esp) block for postoperative analgesia after minimally invasive mitral valve surgery. J Cardiothorac Vasc Anesth 2018; 32: 2271–4.
Gropper MA, Cohen NH, Eriksson LI, Fleisher LA, Leslie K, Wiener-Kronish JP. Miller's Anesthesia, 9th ed. Philadelphia: Elsevier; 2019.
Covino BG, Vassallo HG. Local Anesthetics: Mechanisms of Action and Clinical Use. New York: Grune and Stratton; 1976.
Butterworth IV J. Clinical pharmacology of local anesthetics. Available from URL: https://www.nysora.com/foundations-of-regional-anesthesia/pharmacology/clinical-pharmacology-local-anesthetics/ (accessed February 2022).
Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–12.
McCarron MM, Devine BJ. Clinical pharmacy: case studies: case number 25: gentamicin therapy. Drug Intell Clin Pharm 1974; 8: 650–5.
R Core Team. R: a language and environment for statistical computing. Available from URL: https://www.r-project.org (accessed February 2022).
Ferraro LHC, Takeda A, Barreto CN, Faria B, Assunção NA. Pharmacokinetic and clinical effects of two bupivacaine concentrations on axillary brachial plexus block (Portuguese). Braz J Anesthesiol 2018; 68: 115–21.
Charan J, Biswas T. How to calculate sample size for different study designs in medical research? Indian J Pyschol Med 2013; 35: 121–6.
Denny NM, Harrop-Griffiths W. Location, location, location! Ultrasound imaging in regional anaesthesia. Br J Anaesth 2005; 94: 1–3.
Knudsen K, Beckman Suurküla M, Blomberg S, Sjövall J, Edvardsson N. Central nervous and cardiovascular effects of i.v. infusions of ropivacaine, bupivacaine and placebo in volunteers. Br J Anaesth 1997; 78: 507–14.
Bardsley H, Gristwood R, Baker H, Watson N, Nimmo W. A comparison of the cardiovascular effects of levobupivacaine and rac-bupivacaine following intravenous administration to healthy volunteers. Br J Clin Pharmacol 1998; 46: 245–9.
Kopacz DJ, Emanuelsson BM, Thompson GE, Carpenter RL, Stephenson CA. Pharmacokinetics of ropivacaine and bupivacaine for bilateral intercostal blockade in healthy male volunteers. Anesthesiology 1994; 81: 1139–48.
Yasumura R, Kobayashi Y, Ochiai R. A comparison of plasma levobupivacaine concentrations following transversus abdominis plane block and rectus sheath block. Anaesthesia 2016; 71: 544–9.
Burm AG, van Kleef JW, Gladines MP, Olthof G, Spierdijk J. Epidural anesthesia with lidocaine and bupivacaine: effects of epinephrine on the plasma concentration profiles. Anesth Analg 1986; 65: 1281–4.
Wildsmith JA, Tucker GT, Cooper S, Scott DB, Covino BG. Plasma concentrations of local anaesthetics after interscalene brachial plexus block. Br J Anaesth 1977; 49: 461–6.
Wulf H, Löwe J, Gnutzmann KH, Steinfeldt T. Femoral nerve block with ropivacaine or bupivacaine in day case anterior crucial ligament reconstruction. Acta Anaesthesiol Scand 2010; 54: 414–20.
Ala-Kokko TI, Karinen J, Räihä E, Kiviluoma K, Alahuhta S. Pharmacokinetics of 0.75% ropivacaine and 0.5% bupivacaine after ilioinguinal-iliohypogastric nerve block in children. Br J Anaesth 2002; 89: 438–41.
Flack SH, Martin LD, Walker BJ, et al. Ultrasound-guided rectus sheath block or wound infiltration in children: a randomized blinded study of analgesia and bupivacaine absorption. Paediatr Anaesth 2014; 24: 968–73.
Moore DC, Mather LE, Bridenbaugh LD, Balfour RI, Lysons DF, Horton WG. Arterial and venous plasma levels of bupivacaine following peripheral nerve blocks. Anesth Analg 1976; 55: 763–8.
Moore DC, Mather LE, Bridenbaugh PO, et al. Arterial and venous plasma levels of bupivacaine following epidural and intercostal nerve blocks. Anesthesiology 1976; 45: 39–45.
Junca A, Marret E, Goursot G, Mazoit X, Bonnet F. A comparison of ropivacaine and bupivacaine for cervical plexus block. Anesth Analg 2001; 92: 720–4.
Rosenberg PH, Veering BT, Urmey WF. Maximum recommended doses of local anesthetics: a multifactorial concept. Reg Anesth Pain Med 2004; 29: 564–75.
Corvetto MA, Echevarría GC, De La Fuente N, Mosqueira L, Solari S, Altermatt FR. Comparison of plasma concentrations of levobupivacaine with and without epinephrine for transversus abdominis plane block. Reg Anesth Pain Med 2012; 37: 633–7.
Neal JM, Barrington MJ, Fettiplace MR, et al. The Third American Society of Regional Anesthesia and Pain Medicine Practice Advisory on local anesthetic systemic toxicity: executive summary 2017. Reg Anesth Pain Med 2018; 43: 113–23.
Pea F, Pavan F, Furlanut M. Clinical relevance of pharmacokinetics and pharmacodynamics in cardiac critical care patients. Clin Pharmacokinet 2008; 47: 449–62.
McDonald SB, Jacobsohn E, Kopacz DJ, et al. Parasternal block and local anesthetic infiltration with levobupivacaine after cardiac surgery with desflurane: the effect on postoperative pain, pulmonary function, and tracheal extubation times. Anesth Analg 2005; 100: 25–32.
Author contributions
Sarah Maximos, Alex Moore, and Stephan Williams contributed to all aspects of this manuscript, including study conception and design; acquisition, analysis, and interpretation of the data; and drafting the article. Sophie Ayoub, Monique Ruel, and Julie Desroches contributed to the acquisition of the data. Pierre-Olivier Hétu, Alessandro De Cassai, Éric Vaillancourt-Jean, and Samer Mouksassi contributed to the conception and design of the study and interpretation of the data.
Disclosures
None.
Funding statement
Internal.
Editorial responsibility
This submission was handled by Dr. Stephan K. W. Schwarz, Editor-in-Chief, Canadian Journal of Anesthesia/ Journal canadien d’anesthésie.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Maximos, S., Vaillancourt-Jean, É., Mouksassi, S. et al. Peak plasma concentration of total and free bupivacaine after erector spinae plane and pectointercostal fascial plane blocks. Can J Anesth/J Can Anesth 69, 1151–1159 (2022). https://doi.org/10.1007/s12630-022-02260-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-022-02260-x