Abstract
Purpose
Patients with neuromuscular disorders (NMDs) are at increased risk of perioperative complications. The objective of this scoping review was to examine emerging evidence from published studies, case reports, and review articles on anesthetic management of patients with NMDs, following the methodological frame for scoping reviews.
Sources
We searched PubMed and EMBASE for articles published between 1 January 2000 and 14 July 2021.
Principal findings
Three prospective and 21 retrospective studies on altered pharmacokinetics and pharmacodynamics of neuromuscular blocking agents (NMBA) in NMD patients were included. Furthermore, 168 case reports/series reporting 212 anesthetics in 197 patients were included. These studies showed that preanesthetic neuromuscular monitoring can be used for precise NMBA dosing in myasthenia gravis patients. Sugammadex was associated with fewer postoperative myasthenic crises. Perioperative complications were not associated with specific anesthetic agents. Case reports/series showed that in 32% (67/212) of anesthetics, at least one complication was reported. Unexpected intensive care unit admission was a frequently reported complication. Patients with a complicated disease course may have had a higher use of succinylcholine (unadjusted relative risk, 0.13; 95% confidence interval [CI], 0.20 to 0.86) and volatile anesthetics (adjusted odds ratio [OR], 0.38; 95% CI, 0.20 to 0.73; P = 0.004).
Conclusion
Evidence on the anesthetic management and perioperative complications of patients with NMDs is mainly based on small retrospective studies and case reports. Further clinical trials or large retrospective studies are required to investigate the choice of safe anesthetic agents. Main areas of interest are the potential benefits of neuromuscular monitoring and sugammadex and the risks possibly associated with volatile anesthetics and succinylcholine.
Résumé
Objectif
Les patients atteints de maladies neuromusculaires (MNM) courent un risque accru de développer des complications périopératoires. L'objectif de cette étude de portée est de résumer les connaissances émergentes tirées des études, présentations de cas et comptes rendus publiés portant sur l’anesthésie des patients atteints de MNM, tout en suivant le cadre méthodologique d’une étude de portée.
Constatations principales
ont été incluses trois études prospectives et 21 études rétrospectives comprenant des patients atteints de MNM chez lesquels les myorelaxants ont eu des propriétés pharmacocinétiques et pharmacodynamiques modifiées. En outre, 168 présentations / séries de cas portant sur 212 gestes d'anesthésie chez 197 patients ont été incluses. Ces études ont démontré qu'un suivi neuromusculaire peut être utilisé en pré-anesthésie pour ajuster les doses de myorelaxant chez les patients atteints de myasthénie grave. En postopératoire, un taux plus faible de crises de myasthénie grave a été observé avec le sugammadex. Aucune relation entre les anesthésiques et les complications périopératoires n’a été détectée. Dans les présentations / séries de cas, les patients ayant eu au moins une complication représentaient 67 (32 %) des cas. L’admission non programmée en réanimation est une complication fréquemment rapportée. Les patients dont la maladie s’est dégradée plus rapidement ont possiblement reçu des doses plus fortes de succinylcholine (risque relatif non ajusté 0,13, intervalle de confiance [IC] 95 %, 0,20 à 0,86) et d’agents volatils (rapport de cotes [RC] ajusté, 0,38 (IC 95 %, 0,20 à 0,73), P = 0.004).
Sources
Les articles sont issus des bases de données PubMed et EMBASE (articles publiés entre le 1er janvier 2000 et le 14 juillet 2021).
Conclusion
Les données probantes sur la prise en charge anesthésique et les complications périopératoires affectant les patients atteints de MNM sont principalement fondées sur de petites études rétrospectives et des cas cliniques. Des études cliniques ou rétrospectives d’envergure sont nécessaires pour orienter le choix de la technique d’anesthésie optimale. Les principaux domaines d’intérêt sont les bienfaits potentiels du monitorage neuromusculaire et de l'utilisation de sugammadex ainsi que les effets indésirables possibles des anesthésiques volatils et de la succinylcholine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Perioperative care of patients with neuromuscular disorders (NMDs) is challenging. Although not uncommon as a group, most NMDs are rare and the experience with specific conditions can be limited among anesthesiologists.1 NMDs are highly diverse clinically and genetically, with over 500 different diagnoses and an even wider range of disease manifestations and comorbidities.2 Despite the differences between specific disorders, anesthetic risks and considerations have a significant overlap, as, for example, neuromuscular transmission defects or cardiorespiratory involvement are common to many NMDs and have important implications for perioperative care.3,4,5,6 Other major concerns are the association of some NMDs with impaired thermoregulation due to a reduced muscle mass, malignant hyperthermia (MH), and anesthesia-induced rhabdomyolysis (AIR).7 In addition, side effects of frequently used anesthetic agents are more pronounced in neuromuscular patients because of upregulation of α7 nicotinic acetylcholine and possibly endorphin receptors.8,9,10,11
Because most NMDs are rare, there are only few prospective clinical studies concerning the anesthetic management of these patients. Current knowledge regarding this topic is based on retrospective studies, small case series, case reports, consensus statements, and expert opinion-based reviews.11,12,13 We recently organized the 259th European Neuromuscular Centre (ENMC) international workshop on anesthesia and NMDs. The main aims of the workshop were to work on a consensus statement among international experts on the anesthetic management of patients with NMDs and to explore future international collaborative research possibilities.14 The major goal of this scoping review was to examine and to summarize the nature and extent of the current evidence on the anesthetic management of patients with motor neuron diseases, poly(radiculo)neuropathies, neuromuscular junction disorders, and muscle disorders including acquired and inherited myopathies, muscular dystrophies, and (non)dystrophic myotonias. Another goal of this scoping review was to focus on the nature and extent of perioperative complications in patients with NMDs and the association of volatile anesthetics, neuromuscular monitoring, neuromuscular blocking agents (NMBAs), and antagonists with well-known complications in NMD patients such as MH, AIR, residual muscle relaxation, and pulmonary complications. This scoping review also evaluated knowledge gaps that can guide future research and may contribute to the education of relevant healthcare professionals.
Methods
This review was conducted using the methodological framework for scoping reviews as reported by Levac et al.15 and is reported according to the PRISMA extension reporting guideline for scoping reviews.16 Methods of the search strategy, inclusion and exclusion criteria, and data analysis were specified and documented in advance.
Stage 1: Identifying the research question
Our study population comprised adult patients with an NMD, who needed sedation or anesthesia for an operation or a nonsurgical intervention. Neuromuscular disorders included motor neuron diseases, poly(radiculo)neuropathies, neuromuscular junction disorders, and muscle disorders including acquired and inherited myopathies, muscular dystrophies, and (non)dystrophic myotonias. This study population represented a broad spectrum of NMDs, with one general research question: what is the current evidence on the anesthetic management of patients with an NMD?
Stage 2: Identifying relevant studies
We searched PubMed and EMBASE for clinical studies, retrospective studies, case series/reports, and systematic/narrative reviews on the anesthetic management of patients with NMDs.
We searched the following databases on 14 July 2021:
-
PubMed, The United States National Library of Medicine at the National Institutes of Health, from 1 January 2000.
-
EMBASE, Elsevier, from 1 January 2000.
The search was developed in collaboration with the information specialist from the medical library at the Radboud University in consultation with the authors. We used a broad search strategy, comprising three main categories: neuromuscular disorders, anesthesia, and perioperative complications. For each main category, relevant Medical Subject Headings terms, keywords, and synonyms were combined using “OR” within the categories. These three separate main categories were combined using “AND.” No limitation, except for the date, was applied. We did not search other databases or gray literature.
Details of the search strategy can be found in the Electronic Supplementary Material (ESM), eAppendix 1.
Stage 3: Study selection
Eligibility assessment was performed by two authors (L. B. and M. G.) in a blinded and standardized manner using a two-step model. For the first step, all studies were reviewed for eligibility based on title and abstract. In case of discrepancy between the reviewing authors, consensus was reached by discussion. When no consensus was reached, a third author (N. V.) was asked to make the final decision.
We included articles on anesthetic management of NMDs from four different levels of evidence criteria:
-
Prospective clinical studies on the anesthetic management of patients with an NMD.
-
Retrospective studies on the effect of anesthetic management of patients with an NMD.
-
Case reports or case series reporting the anesthetic management and perioperative outcome of patients with an NMD with need for anesthesia or procedural sedation for surgery or invasive diagnostic procedures.
-
Review articles or consensus statements on the anesthetic management of patients with an NMD. Although it is uncommon to include review articles, the level of evidence on this topic is extraordinarily low and sometimes even fully based on expert opinion. We are aware that the level of evidence of these review articles is low, but believe that a summary of expert opinion in the field of anesthesia and NMDs might help map current evidence and guide future research.
Articles matching the following criteria were excluded:
-
Studies reporting perioperative risks or complications with a focus on surgical techniques/complications.
-
Studies reporting patients with NMD predominantly located above the motor neuron (e.g., primary lateral sclerosis).
-
Studies reporting pediatric cases (age < 18 yr) because anesthetic management, indication for surgery, and the neuromuscular diagnosis of these patients differ significantly from adult patients with NMD and would have resulted in having a very heterogeneous group.
-
Responses to published articles.
-
Nonclinical studies (e.g., laboratory studies).
-
Animal studies.
For the second step, we reviewed the full texts of the studies considered eligible to make a final decision about inclusion. After exploring the available literature, inclusion and exclusion criteria for the second step were tightened for the review articles and clinical studies as outlined in detail below.
All case reports and case series reporting the anesthetic management and outcome of patients with NMDs matching the inclusion criteria were included. Since most literature focused on the association of perioperative complications in patients with an NMD with AIR, MH, and pharmacological strategies, we included studies and reviews if least one of the following topics was studied:
-
The perioperative complications of specific NMDs or NMDs in general.
-
The association of MH or AIR with specific NMDs.
-
The use of NMBAs and their antagonists in specific NMDs or NMDs in general.
-
The perioperative use of cholinesterase inhibitors in patients with myasthenia gravis (MG) in relation to perioperative complications.
During the second step of study selection, the following articles were excluded:
-
Reviews published before 2015 since these articles are mainly based on literature published between 2000 and 2015. Inclusion of review articles published before 2015 might have led to recommendations based on outdated literature.
-
Review articles written by one author since these articles lack a multidisciplinary approach.
-
Articles in any language other than English.
Stage 4: Charting the data
We developed a separate data extraction sheet for clinical/retrospective studies, case studies, and review articles. The data extraction sheets were pilot tested on five randomly selected clinical/retrospective studies, case studies, and review articles and subsequently refined accordingly. Data were extracted independently by two authors (L. B. and M. G.).17 For each study, we extracted the perioperative complication rate of patients with a specific NMD or NMDs in general, and the use of cholinesterase inhibitors (in patients with MG), NMBAs, volatile anesthetics, and sugammadex in the study population.
For each case study, we extracted the age, sex, publication date, neuromuscular diagnosis (including information if the diagnosis was known before surgery), type of surgery (including emergency or elective), anesthesia technique, anesthetic agents used, administration of NMBAs and their antagonists, whether neuromuscular monitoring was used, and the perioperative disease course (complicated or uncomplicated). In the patients with MG, we registered the perioperative use of cholinesterase inhibitors. In the patients with a complicated disease course, we registered the specific complications. All perioperative complications were registered, without distinction between complications, that were very likely caused by the anesthesia or by a more uncertain etiology. A case was classified as complicated when an adverse event or unexpected condition of a patient occurred during or following a medical action resulting in irreversible damage and/or need for medical treatment.
For each review article, we extracted which NMDs were associated with MH or AIR when exposed to volatile anesthetics and/or succinylcholine, and which NMBAs and antagonists appear to be safe in patients with a specific NMD, or NMDs in general.
Stage 5: Synthesis of results
Case reports and case series were assessed for perioperative complications. In case of more than one anesthetic in the same patient, available data from all anesthetics were used for the statistical analysis. The anesthetic agents/NMBAs used, the type of surgery, anesthetic technique, and the use of neuromuscular monitoring were presented as numbers and percentages for the whole study population, and separately for the patients with a complicated and uncomplicated perioperative disease course, respectively. Additionally, the difference between those characteristics in the patients with a complicated and uncomplicated disease course were analyzed as categorical data using logistic regression with these characteristics as the independent variable, publication year as covariate, and a complicated/uncomplicated disease course as the dependent variable. We used the publication year as covariate since anesthetic practice varied during the study period (e.g., sugammadex was introduced during the study period, and neuromuscular monitoring became stricter and commonly used). We considered P values of < 0.05 significant. Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 25.0 (IBM Corp., Armonk, NY, USA).
Since there are no high-quality methodologies for an objective quality assessment of narrative reviews, selection based on quality of the review articles would lead to a significant selection bias. Therefore, we did not perform a quality assessment, and information regarding the authors’ opinion on the topics described in stage 3 is presented as categorical data without triage.
Results
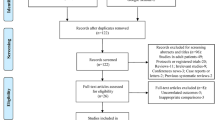
We identified 4,525 articles matching our search strategy. After removal of duplicates and screening titles and abstracts, 490 articles were considered relevant (Cohen’s κ coefficient, 0.78). Subsequently, we reviewed the full-texts and included 219 articles for data extraction (Cohen’s κ coefficient, 0.89). The study selection process and reasons for exclusion are summarized in the Figure. Details on the included articles can be found in ESM eAppendix 2.
Clinical studies
We did not identify any prospective clinical studies on the effect of anesthetic interventions on perioperative complications in patients with NMDs. The only three prospective clinical studies identified investigated the pharmacodynamics of nondepolarizing NMBAs in NMD patients. These studies showed that the train-of-four (TOF) ratio before administration of an NMBA is a good predictor for atracurium and rocuronium requirement in patients with MG.18,19 Another pharmacodynamic study showed that, in patients with oculopharyngeal muscular dystrophy (OPMD), the onset time of cisatracurium was prolonged while the duration of action was normal compared with healthy controls.20
Retrospective studies
We identified 21 retrospective studies matching our inclusion criteria of which eight studied described perioperative complications in MG patients undergoing thymectomy.21,22,23,24,25,26,27,28 Patients with MG had more preoperative complications and a higher frequency of reintubation following thymectomy than non-MG patients did.21 Postoperative myasthenic crisis (POMC) occurred in 0–18.2% of patients22,25,26,27 and was shown to be associated with a history of a previous myasthenic crisis and unstable MG.22 Prolonged mechanical ventilation varied between 4.5–13.1% (> 24 hr)23,25 and 3.7% (> 48 hr).21 Apart from the study of Mouri et al.,26 which reported less POMC when sugammadex was used, perioperative complications could not be associated with specific anesthetic agents/management.
Other studies were on the anesthetic management of patients with myotonic dystrophy type I (DMI)29,30 and II,31,32 amyotrophic lateral sclerosis,33,34,35 Duchenne muscular dystrophy,36 glycogen storage disease,37 inclusion body myositis,38 Lambert–Eaton myasthenic syndrome,39 mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes mitochondrial encephalomyopathy40 and OPMD.41 Opioid use was possibly associated with postoperative complications in a small retrospective study on the anesthetic management of DMI.30 In this group of “other studies” the complication rates varied from 0% to 54%. Respiratory complications were frequently reported. Because sample sizes were small, lack of any complications or a control cohort perioperative complication could not be associated with specific anesthetic agents/management. Results of the retrospective studies included are summarized in Table 1.
Case reports
We included 168 case reports or case series reporting a total of 212 anesthetics in 197 different patients. There was a slight female predominance in the reported cases (117/212; 55%). Median [interquartile range] age was 41 [27–57] yr. Myasthenia gravis was a frequent neuromuscular diagnosis (67/212; 32%), followed by DMI (38/212; 18%) and McArdle’s disease (22/212; 10%). The neuromuscular diagnosis was known before surgery in 176/194 (91%) cases. The neuromuscular diagnosis may have been more frequently known before surgery in the uncomplicated group than in the complicated group (adjusted odds ratio [OR], 18.6; 95% confidence interval [CI], 4.06 to 85.0; P < 0.001). Nevertheless, this analysis lacks validity because of the small proportion of patients in which the neuromuscular diagnosis was unknown before surgery in the group of patients with an uncomplicated disease course (unadjusted relative risk, 6.4; 95% CI, 1.7 to 23.9). Gastrointestinal surgery (46/212; 22%), gynecological surgery (35/212; 17%), and thymectomy (29/212; 14%) were frequent indications for surgery. In 155/187 (83%) cases, surgery was elective. Surgery may have been more frequently elective in the uncomplicated group than in the complicated group (adjusted OR, 2.34; 95% CI, 1.07 to 5.1; P = 0.03). Demographics and details regarding the neuromuscular diagnosis and type of surgery are summarized in Table 2.
In 145/212 (68%) of the reported cases, surgery, anesthesia, and perioperative course of disease were uncomplicated. At least one complication was reported in 67/212 (32%) cases. Death was reported in 5/212 (2%) patients. Unexpected postoperative intensive care unit (ICU) admission (19/212; 9%), unplanned postoperative reintubations (18/212; 9%), and unplanned postoperative need for mechanical ventilation (17/212; 8%) were frequently reported complications. All reported complications and their frequencies are summarized in Table 3.
Succinylcholine may have been more frequently used in patients with a complicated disease course (adjusted OR, 0.05; 95% CI, 0.01 to 0.39; P = 0.004). Nevertheless, this analysis lacks validity because of the small proportion of patients in which the neuromuscular diagnosis was unknown before surgery in the group of patients with an uncomplicated course of disease (unadjusted relative risk, 0.13; 95% CI, 0.20 to 0.86). Volatile anesthetics may have been more frequently used in patients with a complicated disease course (adjusted OR, 0.38; 95% CI, 0.20 to 0.73; P = 0.004). Based on the available data from all included cases, there was no significant difference in the use of NMBAs (adjusted OR, 0.62; 95% CI, 0.31 to 1.24; P = 0.17) and the use of neuromuscular monitoring between the patients with a complicated (adjusted OR, 1.90, 95% CI, 0.82 to 4.43; P = 0.14) and uncomplicated course of disease. There was no significant difference in the use of NMBA antagonists between patients with an uncomplicated and complicated disease course (adjusted OR, 0.58; 95% CI, 0.25 to 1.36; P = 0.21). Sugammadex was associated with an uncomplicated perioperative disease course in univariate analysis (OR, 3.40; 95% CI, 1.25 to 9.3; P = 0.02). Based on the available data from all included cases, there was no significant difference effect after adding publication year as a covariate (adjusted OR, 2.57; 95% CI, 0.86 to 7.7; P = 0.09). Propofol infusion syndrome was not reported. Details regarding the anesthetic technique, use of NMBAs, NMBA antagonists, and neuromuscular monitoring can be found in Table 4.
Review articles
We included 27 review articles matching our inclusion criteria. Four focused solely on MH-related NMDs. The others discussed the anesthetic management of several specific NMDs or NMDs in general, and some included a section about MH-related NMDs.
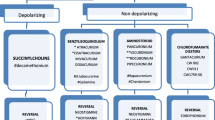
There is consensus concerning the risk of succinylcholine in patients with an NMD. The authors of all except one review article stated that the use of succinylcholine should be avoided. In addition, all authors except one stated that nondepolarizing NMBA are safe in NMD patients as long as they are used with caution (i.e., dose reduction and/or with neuromuscular monitoring). All authors except one stated that sugammadex is safe for patients with NMDs. The use of volatile anesthetics in NMD patients remains a matter of debate among the authors of the included reviews. Recommendations regarding the safe use of anesthetic agents based on the reviews included are summarized in Table 5.
Several review articles considered central core disease (CCD), King-Denborough syndrome (KDS), multi-minicore disease (MmD), and Native American myopathy as MH-related NMDs.7,11,13,42,61,62,63 Centronuclear myopathy62,63 and periodic paralysis7,62 were both considered as MH-related NMDs by two reviews articles and possibly MH associated by one review article.7,64 Association with MH susceptibility was not assumed in any of the review articles on Emery–Dreifuss muscular dystrophy,51 DMI52,53, and mitochondrial myopathies.11,60 Reviews were less unanimous about the MH risk of other NMDs. The association of MH with several NMDs according to the included reviews is summarized in Table 6.
Discussion
This scoping review provides an overview of the nature and extent of published clinical studies, retrospective studies, case reports, and review articles concerning anesthesia and NMDs, focusing on the clinical data. Literature on this topic consists of studies with low levels of evidence such as case reports/case series, review articles, and a few small retrospective studies. The main findings of this scoping review and their interpretation are discussed below.
Use of neuromuscular blocking agents and antagonists
Although the level of evidence identified in this scoping review was considerably low, we were able to identify some evidence on the use of NMBAs and its antagonists in patients with NMDs.
First, succinylcholine may have been associated with a complicated perioperative course of disease in the reported cases (unadjusted relative risk, 0.13; 95% CI, 0.20 to 0.86), and there was consensus among all but one of the authors of the review articles. We did not identify any clinical studies or retrospective studies on this topic. Therefore, the true severity and prevalence of life-threatening side effects such as hyperkalemia, rhabdomyolysis, and myotonia remain uncertain. Due to the association of severe side effects of succinylcholine in patients with NMDs and the availability of alternative NMBAs, prospective clinical studies on this topic are probably not feasible and future research on this topic will be limited to retrospective studies. Until more evidence on the risks of succinylcholine becomes available, the use of succinylcholine should probably be avoided when feasible.
Second, the use of NMBAs was not associated with perioperative complications in the case reports included in this scoping review (adjusted OR, 0.62; 95% CI, 0.31 to 1.24, P = 0.17). Apart from one, all authors of the review articles considered the use of nondepolarizing NMBAs as safe, or safe as long as they are used with caution (e.g., after dose reduction and/or when using concomitant neuromuscular monitoring). The presence of a complicated disease course was not associated with the use of neuromuscular monitoring in the case reports included in this scoping review. Nevertheless, the sample size was probably insufficient to answer this research question as reflected in the remarkably wide confidence interval (OR, 1.90; 95% CI, 0.82 to 4.43).
Except for pharmacokinetic and pharmacodynamic studies, we did not identify any clinical studies or retrospective studies on the effect of nondepolarizing NMBAs or neuromuscular monitoring on perioperative disease course in patients with NMDs.20 Future research should focus on pharmacokinetic and pharmacodynamic studies on different nondepolarizing NMBAs and NMDs to give more insights into whether and how pharmacodynamics and pharmacokinetics are altered for each specific NMBA and/or NMD. This might help guide personalized dose adjustments in patients with specific NMDs. Furthermore, the effect of nondepolarizing NMBAs and neuromuscular monitoring on perioperative disease course in patients with NMDs might be interesting areas for future studies. Especially considering the results from studies in the healthy population.65 Until these studies have been conducted, nondepolarizing NMBAs can probably be used with caution (use of neuromuscular monitoring and dose adjustments in individual cases). Although reliable evidence in favor of using of neuromuscular monitoring in patients with NMDs is missing, neuromuscular monitoring is harmless and has been shown to be associated with less postoperative residual paralysis in the general population.65 If available, use of neuromuscular monitoring is therefore recommended whenever muscle relaxation is required by NMBAs in NMD patients.
Third, the use of sugammadex was associated with less POMC after thymectomy in a large cohort of MG patients.26 Other studies did not show beneficial effect of sugammadex in patients with NMDs. But due to the small sample size and lack of a control cohort or sometimes even lack of any complication, the methodology and power of these studies was insufficient to answer this research question. Furthermore, sugammadex was only introduced a few years ago in the USA and Canada. Therefore, large and well-performed clinical studies in this specific subgroup of patients are not yet available. In the case reports included in this scoping review, there was no statistically significant difference in the use of sugammadex between NMD patients with a complicated versus uncomplicated disease course. Nevertheless, its use has only been reported in 30 case reports, which is an inadequate sample size to draw conclusions as reflected in the wide confidence interval (adjusted OR, 2.57; 95% CI, 0.86 to 7.7; P = 0.09).
We identified two cases of incomplete reversal of rocuronium-induced neuromuscular blockade with sugammadex in MG patients.66,67 In both cases, the TOF ratio was restored sufficiently after administration of neostigmine. Exacerbation of MG symptoms, sometimes leading to a myasthenic crisis, occurs frequently after transsternal thymectomy.24 Worsening of myasthenia status with increase in muscle weakness might be caused by the interruption of immunosuppressive agents, perioperative sepsis, surgical stress, pregnancy, or interference from anesthetic drugs (volatile anesthetics or opioids). Acetylcholinesterase inhibitors are an effective treatment for MG, and complete recovery of the TOF ratio by neostigmine administration after prior use of sugammadex may therefore be explained by its anticholinergic effect rather than by its reversal of the neuromuscular blockade by rocuronium.
Apart from one large retrospective study in MG patients,26 there is currently insufficient evidence on the risks and benefits of sugammadex in NMD patients. Future pharmacokinetic and pharmacodynamic studies could offer clarification of cases of incomplete reversal of muscle relaxations as discussed above. Given the beneficial effect of sugammadex on pulmonary complications in the general population,68 this might be an interesting area for future prospective clinical studies and/or retrospective studies. Until these studies have been conducted, the effect of sugammadex on reversal of neuromuscular blockade should be appropriately monitored with TOF ratio recovery and specific attention to the 100% twitch recovery before extubation.
Volatile anesthetics
The use of volatile anesthetics in patients with NMDs remains a matter of debate. There are no prospective or retrospective studies on this topic. Analysis of the case reports included in this scoping review indicated that use of volatile anesthetics may be associated with a complicated perioperative disease course (adjusted OR, 0.38; 95% CI, 0.20 to 0.73; P = 0.004).
Several authors have expressed concerns regarding the AIR risk in patients with NMDs in general and in muscular dystrophy patients in particular.11,12,13,44,51,54,55 Nevertheless, although AIR is a well-known perioperative complication in patients with muscular dystrophies, a causal relationship of AIR with volatile anesthetics has never been unequivocally proven. We identified only one report of an 18-year-old obese patient with Becker muscular dystrophy who suffered a hyperkalemic cardiac arrest and rhabdomyolysis after the use of isoflurane for a six-hour procedure.69 To test for susceptibility to MH, an in vitro caffeine halothane contracture test (IVCT) was performed three months later that turned out to be positive. In the absence of clear symptoms of hypermetabolism and RYR1/CACNA1S/STAC3 variants, AIR might be a better diagnostic description indicating potential harmfulness of long-term exposure of volatile anesthetics. The IVCT has high false-positive rates in dystrophic patients.70
Since we identified a very limited number of AIR cases, the risk of AIR in adult patients with NMDs seems low (Table 3). This rare occurrence within our cohort is probably due to the pediatric age group being excluded, where muscular dystrophy and AIR are frequently described. In spite of the low number of AIR cases, the use of volatile anesthetics was possibly associated with a complicated disease course rather than by AIR. There are several explanations for this finding. In animal studies, sevoflurane has been shown to reduce the contraction force of the diaphragm.71,72 In addition, volatile anesthetics enhance the effect of nondepolarizing NMBAs, as illustrated by a prolonged duration of rocuronium action if coadministered with sevoflurane.73,74,75 This may lead to residual relaxation and reduced muscle force in NMD patients. Future studies or setting up a registry of all NMD patients exposed to volatile anesthetics might help to create an unbiased cohort and examine the prevalence and severity of AIR, residual paralysis, and pulmonary complications in NMD patients exposed to volatile anesthetics.
Although associated with serious complications and risks, volatile anesthetics do have desirable pharmacokinetic properties. Since evidence on this topic is limited, the risks and benefits of volatile anesthetics in patients with NMDs should be weighed on a case-by-case basis, taking into account the extent and duration of the surgery, the degree of muscle weakness (which may be enhanced by NMBAs), and/or disease-specific considerations such as the association with AIR and MH susceptibility of some NMDs. Neuromuscular monitoring should probably be used whenever NMBAs are used in combination with volatile anesthetics.
Propofol and propofol infusion syndrome
Although patients with mitochondrial myopathies are at increased risk of propofol infusion syndrome, we did not identify any studies or case reports in the body of literature we examined. Propofol infusion syndrome occurs mainly when patients are exposed to propofol for > 48 hr in the ICU.76,77 Propofol seems safe when used for procedural sedation and/or general anesthesia lasting for shorter time periods, even if those last up to several hours. In addition to our observation, there is international consensus concerning the safety of propofol in patients with mitochondrial disorders.78 The choice between intravenous and volatile anesthesia must be made on a case-by-case basis. Based on current available evidence, we cannot recommend completely avoiding the use of either one in patients with NMDs.
Susceptibility to malignant hyperthermia
We did not identify any clinical/retrospective studies on this topic, possibly because of the low prevalence of NMDs and MH, excluding laboratory and pediatric studies and ethical limitations of prospective studies on this topic. There is consensus regarding the association of MH with RYR1-related myopathies (CCD, KDS, and MmD). Nevertheless, there are concerns about a potential relation with MH in a large number of NMDs without consensus among authors in the field of NMDs and anesthesiology (Table 6). The association of MH and some NMDs has been proven through direct genetic evidence in patients with NMDs due to mutations in RYR1,79,80 and, less frequently, in CACNA1S81,82,83 and STAC3.84,85 Patients with an NMD associated with variants in these genes might therefore be at risk for MH when exposed to triggering agents and should be referred to an MH unit to counsel and test for MH susceptibility.
There is currently no evidence for an association of other NMDs with MH. The association of some muscular dystrophies with MH made by some authors of the included review articles is probably based on misdiagnosed cases of AIR.86 Of course, patients with any Mendelian NMD may have an additional RYR1/CACNA1S/STAC3 variant resulting in MH susceptibility. Nevertheless, this risk is similar to the low MH risk in the general population.
Limitations
Our scoping review has a number of limitations. First, the level of evidence we identified was very low. There is a lack of prospective studies on the relationship between anesthetic management and perioperative complications in patients with NMDs. Such studies are difficult to conduct because of the low prevalence of most NMDs. Many retrospective studies had a small sample size, high bias risk, and insufficient methodology to study specific effects of perioperative anesthetic management on disease course. Furthermore, due to the explorative nature of our study design and the small number of case reports, we were not able to draw reliable conclusions as reflected in the remarkable ORs and wide confidence intervals. For the same reasons, we were not able to perform multivariate analysis and/or meaningful subgroup analysis for specific NMDs. While patients with different NMDs have different complications, anesthetic management should be adapted accordingly. We were not able to compare complication type, complication rate, or anesthetic management with data from a control cohort. Additionally, the cohort included in the case report/case series section is a very selective patient population since more highly complex cases with significant and clinically relevant perioperative risks are published. Finally, it was not possible to perform an objective quality assessment of the review articles, and although all reviews had been peer-reviewed before publication, quality and evidence for the statements made were variable.
Conclusion
Patients with NMDs are at increased perioperative risk due to potential cardiorespiratory involvement and more pronounced side-effects of anesthetics and NMBAs. Current evidence on this topic is mainly based on small retrospective studies, case reports, and expert opinion-based reviews. To summarize all available evidence and knowledge on this topic, the participants of the 259th ENMC international workshop on anesthesia and neuromuscular disorders will work on consensus statements on anesthesia and NMDs, genetic counseling regarding the risk of MH susceptibility in patients with RYR1-related myopathies, and a literature review on anesthesia and NMDs in pediatric patients.
Further clinical trials or large observational studies are required to investigate which anesthetic agents can be used safely and which should be avoided. Main areas of interest are the potential benefits of using neuromuscular monitoring and sugammadex and the risks possibly associated with volatile anesthetics and succinylcholine in patients with NMDs. Until these studies have been conducted, the use of succinylcholine should probably be avoided when feasible. Nondepolarizing NMBAs and sugammadex can probably be used safely with proper monitoring and dose adjustment. The use of volatile anesthetics should be guided by the extent and duration of the surgery and anesthesia, the degree of muscle weakness, and disease-specific considerations.
References
Bhatt JM. The epidemiology of neuromuscular diseases. Neurol Clin 2016; 34: 999-1021.
Benarroch L, Bonne G, Rivier F, Hamroun D. The 2020 version of the gene table of neuromuscular disorders (nuclear genome). Neuromuscul Disord 2019; 29: 980-1018.
Bhakta D, Lowe MR, Groh WJ. Prevalence of structural cardiac abnormalities in patients with myotonic dystrophy type I. Am Heart J 2004; 147: 224-7.
Posner AD, Soslow JH, Burnette WB, et al. The correlation of skeletal and cardiac muscle dysfunction in duchenne muscular dystrophy. J Neuromuscul Dis 2016; 3: 91-9.
DePalo VA, McCool FD. Respiratory muscle evaluation of the patient with neuromuscular disease. Semin Respir Crit Care Med 2002; 23: 201-9.
Silvestri NJ, Ismail H, Zimetbaum P, Raynor EM. Cardiac involvement in the muscular dystrophies. Muscle Nerve 2018; 57: 707-15.
Litman RS, Griggs SM, Dowling JJ, Riazi S. Malignant hyperthermia susceptibility and related diseases. Anesthesiology 2018; 128: 159-67.
Martyn JA, Richtsfeld M. Succinylcholine-induced hyperkalemia in acquired pathologic states: etiologic factors and molecular mechanisms. Anesthesiology 2006; 104: 158-69.
Fischer U, Reinhardt S, Albuquerque EX, Maelicke A. Expression of functional alpha7 nicotinic acetylcholine receptor during mammalian muscle development and denervation. Eur J Neurosci 1999; 11: 2856-64.
Klingler W, Lehmann-Horn F, Jurkat-Rott K. Complications of anaesthesia in neuromuscular disorders. Neuromuscul Disord 2005; 15: 195-206.
Schieren M, Defosse J, Böhmer A, Wappler F, Gerbershagen MU. Anaesthetic management of patients with myopathies. Eur J Anaesthesiol 2017; 34: 641-9.
Katz JA, Murphy GS. Anesthetic consideration for neuromuscular diseases. Curr Opin Anaesthesiol 2017; 30: 435-40.
van den Bersselaar LR, Snoeck MM, Gubbels M, et al. Anaesthesia and neuromuscular disorders: what a neurologist needs to know. Pract Neurol 2020. https://doi.org/10.1136/practneurol-2020-002633.
van den Bersselaar LR, Riazi S, Snoeck M, Jungbluth H, Voermans NC; Anaesthesia and Neuromuscular Disorders Working Group. 259th ENMC international workshop: anaesthesia and neuromuscular disorders 11 December, 2020 and 28-29 May, 2021. Neuromuscul Disord 2021. https://doi.org/10.1016/j.nmd.2021.11.005.
Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implement Sci 2010. https://doi.org/10.1186/1748-5908-5-69.
Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018; 169: 467-73.
Lockwood C, Dos Santos KB, Pap R. Practical guidance for knowledge synthesis: scoping review methods. Asian Nurs Res (Korean Soc Nurs Sci) 2019; 13: 287-94.
Mann R, Blobner M, Jelen-Esselborn S, Busley R, Werner C. Preanesthetic train-of-four fade predicts the atracurium requirement of myasthenia gravis patients. Anesthesiology 2000; 93: 346-50.
Fujimoto M, Terasaki S, Nishi M, Yamamoto T. Response to rocuronium and its determinants in patients with myasthenia gravis: a case-control study. Eur J Anaesthesiol 2015; 32: 672-80.
Caron MJ, Girard F, Girard DC, et al. Cisatracurium pharmacodynamics in patients with oculopharyngeal muscular dystrophy. Anesth Analg 2005; 100: 393-7.
Alshaikh JT, Amdur R, Sidawy A, Trachiotis G, Kaminski HJ. Thymectomy is safe for myasthenia gravis patients: analysis of the NSQIP database. Muscle Nerve 2016; 53: 370-4.
Ando T, Omasa M, Kondo T, et al. Predictive factors of myasthenic crisis after extended thymectomy for patients with myasthenia gravis. Eur J Cardiothorac Surg 2015; 48: 705-9.
Fujita Y, Moriyama S, Aoki S, et al. Estimation of the success rate of anesthetic management for thymectomy in patients with myasthenia gravis treated without muscle relaxants: a retrospective observational cohort study. J Anesth 2015; 29: 794-7.
Kas J, Kiss D, Simon V, Svastics E, Major L, Szobor A. Decade-long experience with surgical therapy of myasthenia gravis: early complications of 324 transsternal thymectomies. Ann Thorac Surg 2001; 72: 1691-7.
Lu W, Yu T, Longhini F, Jiang X, Qin X, Jin X. Preoperative risk factors for prolonged postoperative ventilation following thymectomy in myasthenia gravis. Int J Clin Exp Med 2015; 8: 13990-6.
Mouri H, Jo T, Matsui H, Fushimi K, Yasunaga H. Effect of sugammadex on postoperative myasthenic crisis in myasthenia gravis patients: propensity score analysis of a Japanese nationwide database. Anesth Analg 2020; 130: 367-73.
Saylan S, Akdogan A. Anesthesia and neuromuscular block management in thymectomies performed in cases of thymoma and myasthenia gravis: a retrospective study. J Clin Anal Med 2021; 12: 327-31.
Vymazal T, Krecmerova M, Bicek V, Lischke R. Feasibility of full and rapid neuromuscular blockade recovery with sugammadex in myasthenia gravis patients undergoing surgery - a series of 117 cases. Ther Clin Risk Manag 2015; 11: 1593-6.
Imison AR. Anaesthesia and myotonia--an Australian experience. Anaesth Intensive Care 2001; 29: 34-7.
Kim CS, Park JM, Park D, Kim DH, Park JS. Opioid use may be associated with postoperative complications in myotonic dystrophy type 1 with high-grade muscular impairment. Sci Rep 2021. https://doi.org/10.1038/s41598-020-76217-9.
Kirzinger L, Schmidt A, Kornblum C, Schneider-Gold C, Kress W, Schoser B. Side effects of anesthesia in DM2 as compared to DM1: a comparative retrospective study. Eur J Neurol 2010; 17: 842-5.
Weingarten TN, Hofer RE, Milone M, Sprung J. Anesthesia and myotonic dystrophy type 2: a case series. Can J Anesth 2010; 57: 248-55.
Hoeper AM, Barbara DW, Watson JC, Sprung J, Weingarten TN. Amyotrophic lateral sclerosis and anesthesia: a case series and review of the literature. J Anesth 2019; 33: 257-65.
Onders RP, Carlin AM, Elmo M, Sivashankaran S, Katirji B, Schilz R. Amyotrophic lateral sclerosis: the Midwestern surgical experience with the diaphragm pacing stimulation system shows that general anesthesia can be safely performed. Am J Surg 2009; 197: 386-90.
Liu S, Liu Z, Zhang J, et al. Anesthetic strategy for percutaneous endoscopic gastrostomy in amyotrophic lateral sclerosis patients. Int J Clin Exp Med 2017; 10: 3725-30.
Boivin A, Antonelli R, Sethna NF. Perioperative management of gastrostomy tube placement in Duchenne muscular dystrophy adolescent and young adult patients: a role for a perioperative surgical home. Paediatr Anaesth 2018; 28: 127-33.
Gurrieri C, Sprung J, Weingarten TN, Warner ME. Patients with glycogen storage diseases undergoing anesthesia: a case series. BMC Anesthesiol 2017. https://doi.org/10.1186/s12871-017-0428-x.
Mortenson AR, Sprung J, Cavalcante AN, Watson JC, Weingarten TN. Inclusion body myositis and anesthesia: a case series. J Clin Anesth 2016; 31: 282-7.
Weingarten TN, Araka CN, Mogensen ME, et al. Lambert-Eaton myasthenic syndrome during anesthesia: a report of 37 patients. J Clin Anesth 2014; 26: 648-53.
Gurrieri C, Kivela JE, Bojanić K, et al. Anesthetic considerations in mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes syndrome: a case series. Can J Anesth 2011; 58: 751-63.
Pellerin HG, Nicole PC, Trépanier CA, Lessard MR. Postoperative complications in patients with oculopharyngeal muscular dystrophy: a retrospective study. Can J Anesth 2007; 54: 361-5.
Kaur H, Katyal N, Yelam A, Kumar K, Srivastava H, Govindarajan R. Malignant hyperthermia. Mo Med 2019; 116: 154-9.
Beecroft C, Hough M. Perioperative management of patients with musculoskeletal disease and for the burns patient. Surgery 2016; 34: 405-10.
Hudson KA, Greene JG. Perioperative consultation for patients with preexisting neurologic disorders. Semin Neurol 2015; 35: 690-8.
Muckler VC, O’Brien JM, Matson SE, Rice AN. Perianesthetic implications and considerations for myasthenia gravis. J Perianesth Nurs 2019; 34: 4-15.
Sungur Z, Sentürk M. Anaesthesia for thymectomy in adult and juvenile myasthenic patients. Curr Opin Anaesthesiol 2016; 29: 14-9.
Spasojevic I, Hajdukovic D, Komarcevic M, Petrovic S, Jovanovic J, Ciric A. Specific features of anesthesia in patients with myasthenia gravis. Med Pregl 2016; 69: 305-11.
Collins S, Roberts H, Hewer I. Anesthesia and perioperative considerations for patients with myasthenia gravis. AANA J 2020; 88: 485-91.
Hoppe K, Pulnien R, Lehman-Horn F, Jurkat-Rott K, Gösele M, Klingler W. Mitochondrial disorders. Anästh Intensivmed 2017; 58: S125-33.
Hsieh VC, Krane EJ, Morgan PG. Mitochondrial disease and anesthesia. J Inborn Errors Metab Screen 2017. https://doi.org/10.1177/2326409817707770.
Echeverry-Marín PC, Bustamante-Vega AM. Anesthetic implications of muscular dystrophies. Colombian Journal of Anestesiology 2018; 46: 228-39.
Ashizawa T, Gagnon C, Groh WJ, et al. Consensus-based care recommendations for adults with myotonic dystrophy type 1. Neurol Clin Pract 2018; 8: 507-20.
Hoppe K, Jurkatt-Rot K, Lehmann-Horn F, Klingler W. Anaesthesia recommendations for patients suffering from myotonic dystrophies type 1 and 2. Anästh Intensivmed 2015; 56: S667-74.
Álvarez AL, Fernandes AR. Anesthesia recommendations for patients suffering from limb-girdle muscular dystrophy. Anästh Intensivmed 2016; 57: S583-9.
Brandsema JF, Darras BT. Dystrophinopathies. Semin Neurol 2015; 35: 369-84.
Stourac P, Kosinova M. Anaesthesia recommendations for recessive myotonia congenita (Becker’s disease). Anästh Intensivmed 2019; 60: S545-53.
Jimenez N, Liston DE. Anaesthesia recommendations for patients suffering from merosin-deficient congenital muscular dystrophy. Anästh Intensivmed 2015; 56: S525-31.
Prottengeier J, Shammas K, Smith J. Anaesthesia recommendations for collagen VI-related myopathy. Anästh Intensivmed 2020; 61: S20-6.
Schaller SJ, Lewald H. Clinical pharmacology and efficacy of sugammadex in the reversal of neuromuscular blockade. Expert Opin Drug Metab Toxicol 2016; 12: 1097-108.
Parikh S, Goldstein A, Koenig MK, et al. Diagnosis and management of mitochondrial disease: a consensus statement from the Mitochondrial Medicine Society. Genet Med 2015; 17: 689-701.
Broman M, Islander G, Müller CR. Malignant hyperthermia, a Scandinavian update. Acta Anaesthesiol Scand 2015; 59: 951-61.
Riazi S, Kraeva N, Hopkins PM. Malignant hyperthermia in the post-genomics era: new perspectives on an old concept. Anesthesiology 2018; 128: 168-80.
Rosenberg H, Pollock N, Schiemann A, Bulger T, Stowell K. Malignant hyperthermia: a review. Orphanet J Rare Dis 2015. https://doi.org/10.1186/s13023-015-0310-1.
Sangkuhl K, Dirksen RT, Alvarellos ML, Altman RB, Klein TE. PharmGKB summary: very important pharmacogene information for CACNA1S. Pharmacogenet Genomics 2020; 30: 34-44.
Carvalho H, Verdonck M, Cools W, Geerts L, Forget P, Poelaert J. Forty years of neuromuscular monitoring and postoperative residual curarisation: a meta-analysis and evaluation of confidence in network meta-analysis. Br J Anaesth 2020; 125: 466-82.
dos Santos Fernandes H, Saraiva Ximenes JL, Nunes DI, Ashmawi HA, Vieira JE. Failure of reversion of neuromuscular block with sugammadex in patient with myasthenia gravis: case report and brief review of literature. BMC Anesthesiol 2019. https://doi.org/10.1186/s12871-019-0829-0.
Sugi Y, Nitahara K, Shiroshita T, Higa K. Restoration of train-of-four ratio with neostigmine after insufficient recovery with sugammadex in a patient with myasthenia gravis. A A Case Rep 2013; 1: 43-5.
Kheterpal S, Vaughn MT, Dubovoy TZ, et al. Sugammadex versus neostigmine for reversal of neuromuscular blockade and postoperative pulmonary complications (STRONGER): a multicenter matched cohort analysis. Anesthesiology 2020; 132: 1371-81.
Kleopa KA, Rosenberg H, Heiman-Patterson T. Malignant hyperthermia-like episode in Becker muscular dystrophy. Anesthesiology 2000; 93: 1535-7.
Gurnaney H, Brown A, Litman RS. Malignant hyperthermia and muscular dystrophies. Anesth Analg 2009; 109: 1043-8.
Breuer T, Maes K, Rossaint R, et al. Sevoflurane exposure prevents diaphragmatic oxidative stress during mechanical ventilation but reduces force and affects protein metabolism even during spontaneous breathing in a rat model. Anesth Analg 2015; 121: 73-80.
Ide T, Kochi T, Isono S, Mizuguchi T. Effect of sevoflurane on diaphragmatic contractility in dogs. Anesth Analg 1992; 74: 739-46.
Ye L, Zuo Y, Zhang P, Yang P. Sevoflurane enhances neuromuscular blockade by increasing the sensitivity of skeletal muscle to neuromuscular blockers. Int J Physiol Pathophysiol Pharmacol 2015; 7: 172-7.
Woloszczuk-Gebicka B, Wyska E, Grabowski T. Sevoflurane increases fade of neuromuscular response to TOF stimulation following rocuronium administration in children. A PK/PD analysis. Paediatr Anaesth 2007; 17: 637-46.
Baurain MJ, d’Hollander AA, Melot C, Dernovoi BS, Barvais L. Effects of residual concentrations of isoflurane on the reversal of vecuronium-induced neuromuscular blockade. Anesthesiology 1991; 74: 474-8.
Krajčová A, Waldauf P, Anděl M, Duška F. Propofol infusion syndrome: a structured review of experimental studies and 153 published case reports. Crit Care 2015. https://doi.org/10.1186/s13054-015-1112-5.
Hemphill S, McMenamin L, Bellamy MC, Hopkins PM. Propofol infusion syndrome: a structured literature review and analysis of published case reports. Br J Anaesth 2019; 122: 448-59.
De Vries MC, Brown DA, Allen ME, et al. Safety of drug use in patients with a primary mitochondrial disease: an international Delphi-based consensus. J Inherit Metab Dis 2020; 43: 800-18.
Sambuughin N, Holley H, Muldoon S, et al. Screening of the entire ryanodine receptor type 1 coding region for sequence variants associated with malignant hyperthermia susceptibility in the north american population. Anesthesiology 2005; 102: 515-21.
Riazi S, Larach MG, Hu C, Wijeysundera D, Massey C, Kraeva N. Malignant hyperthermia in Canada: characteristics of index anesthetics in 129 malignant hyperthermia susceptible probands. Anesth Analg 2014; 118: 381-7.
Monnier N, Krivosic-Horber R, Payen JF, et al. Presence of two different genetic traits in malignant hyperthermia families: implication for genetic analysis, diagnosis, and incidence of malignant hyperthermia susceptibility. Anesthesiology 2002; 97: 1067-74.
Weiss RG, O’Connell KM, Flucher BE, Allen PD, Grabner M, Dirksen RT. Functional analysis of the R1086H malignant hyperthermia mutation in the DHPR reveals an unexpected influence of the III-IV loop on skeletal muscle EC coupling. Am J Physiol Cell Physiol 2004; 287: C1094-102.
Eltit JM, Bannister RA, Moua O, et al. Malignant hyperthermia susceptibility arising from altered resting coupling between the skeletal muscle L-type Ca2+ channel and the type 1 ryanodine receptor. Proc Natl Acad Sci U S A 2012; 109: 7923-8.
Horstick EJ, Linsley JW, Dowling JJ, et al. Stac3 is a component of the excitation-contraction coupling machinery and mutated in Native American myopathy. Nat Commun 2013. https://doi.org/10.1038/ncomms2952.
Stamm DS, Aylsworth AS, Stajich JM, et al. Native American myopathy: congenital myopathy with cleft palate, skeletal anomalies, and susceptibility to malignant hyperthermia. Am J Med Genet A 2008; 146A: 1832-41.
Gray RM. Anesthesia-induced rhabdomyolysis or malignant hyperthermia: is defining the crisis important? Paediatr Anaesth 2017; 27: 490-3.
Author contributions
Luuk R. van den Bersselaar contributed to all aspects of this manuscript, including study conception and design; acquisition, analysis, and interpretation of data; and drafting the article. Madelief Gubbels contributed to the conception and design of the study; acquisition, and interpretation of data; and drafting the article. Sheila Riazi contributed to conception of the study, interpretation of data, and drafting the article. Luc Heytens contributed to the interpretation of data and drafting the article. Heinz Jungbluth contributed to conception of the study, interpretation of data, and drafting the article. Nicol C. Voermans contributed to the conception and design of the study, interpretation of data, and drafting the article. Marc M. J. Snoeck contributed to the conception and design of the study, interpretation of data, and drafting the article.
Acknowledgements
This manuscript was written with support of the European Neuromuscular Centre (ENMC) and was performed in preparation for the 259th ENMC workshop in December 2020. Several authors of this publication are members of the Netherlands Neuromuscular Center (NL-NMD) and the European Reference Network for rare neuromuscular diseases (EURO-NMD). The authors thank OnYing Chan for her help with developing the search strategy. The authors thank Karlijn Bouman and Flavien Bizot for their help with translation of the abstract. The authors thank Jeroen van Doorn for his help with the statistical analysis.
Disclosures
The authors of this scoping review, the organizers and participants of the 259th ENMC workshop, and all others involved in this scoping review do not have any conflicts of interest to declare.
Funding statement
Luuk van den Bersselaar received the 2019 Radboudumc Regional Junior Researcher Grant, in collaboration with the Canisius Wilhelmina Hospital. This scoping review was written in preparation for the 259th European Neuromuscular Centre international workshop: Anesthesia and neuromuscular disorders December 11, 2020 and May 28–29, 2021. The workshop was financially supported by the European Neuromuscular Centre.
Editorial responsibility
This submission was handled by Dr. Stephan K. W. Schwarz, Editor-in-Chief, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
van den Bersselaar, L.R., Gubbels, M., Riazi, S. et al. Mapping the current evidence on the anesthetic management of adult patients with neuromuscular disorders—a scoping review. Can J Anesth/J Can Anesth 69, 756–773 (2022). https://doi.org/10.1007/s12630-022-02230-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-022-02230-3