Abstract
Objective
Postoperative neurologic symptoms (PONS) in the operative arm are important complications of shoulder surgery and interscalene blockade (ISB). This systematic review aimed to compare the risk of PONS between ISB and other techniques, and the relative safety of different agents used in ISB.
Methods
Our systematic review followed Cochrane review methodology and was registered in PROSPERO. A search of MEDLINE (Ovid), EMBASE (Ovid), and CENTRAL (Wiley) from inception to June 2020 was completed. We included randomized or quasi-randomized trials of patients (> five years old) undergoing shoulder surgery with any ISB technique as an intervention, compared with any other nonregional or regional technique, or ISB of alternate composition or technique. The primary outcome was PONS (study author defined) assessed a minimum of one week after surgery.
Results
Fifty-five studies totalling 6,236 participants (median, 69; range, 30–910) were included. Another 422 otherwise eligible trials were excluded because PONS was not reported. Heterogeneity in when PONS was assessed (from one week to one year) and the diagnostic criteria used precluded quantitative meta-analysis. The most common PONS definition, consisting of one or more of paresthesia, sensory deficit, or motor deficit, was only used in 16/55 (29%) trials. Risk of bias was low in 5/55 (9%) trials and high in 36/55 (65%) trials, further limiting any inferences.
Conclusion
These findings highlight the need for a standardized PONS outcome definition and follow-up time, along with routine, rigorous measurement of PONS in trials of ISB.
Study registration
PROSPERO (CRD42020148496); registered 10 February 2020.
Résumé
Objectif
Les symptômes neurologiques postopératoires (SNPO) dans le bras opéré sont une complication importante des chirurgies de l’épaule sous bloc interscalénique (BIS). Cette revue systématique visait à comparer le risque de SNPO entre le BIS et d’autres techniques, ainsi que l’innocuité relative de divers agents utilisés pour réaliser un BIS.
Méthode
Notre revue systématique a suivi la méthodologie de révision Cochrane et a été enregistrée dans le registre PROSPERO. Une recherche a été menée dans les bases de données MEDLINE (Ovid), EMBASE (Ovid) et CENTRAL (Wiley) de leur création à juin 2020. Nous avons inclus les études randomisées ou quasi-randomisées de patients (> cinq ans) bénéficiant d’une chirurgie de l’épaule avec n’importe quelle technique de BIS en tant qu’intervention, comparée à toute autre technique régionale ou non régionale, ou à un BIS de composition ou de technique alternative. Le critère d’évaluation principal était les SNPO (définis par l’auteur de l’étude) évalué au moins une semaine après la chirurgie.
Résultats
Cinquante-cinq études totalisant 6236 participants (médiane, 69; intervalle, 30-910) ont été incluses. Quatre cent vingt-deux autres études autrement admissibles ont été exclues parce que les SNPO n’y étaient pas rapportés. L’hétérogénéité du moment auquel les SNPO ont été évalués (d’une semaine à un an) et les critères diagnostiques utilisés ont empêché la réalisation d’une méta-analyse quantitative. La définition la plus courante des SNPO, consistant en la présence de paresthésie, de déficit sensoriel et/ou de déficit moteur, n’a été utilisée que dans 16/55 (29 %) des études. Le risque de biais était faible dans 5/55 (9 %) des études et élevé dans 36/55 (65 %) des études, limitant davantage toute autre inférence.
Conclusion
Ces résultats soulignent la nécessité d’une définition normalisée du critère de SNPO et du temps de suivi, ainsi que la nécessité d’une mesure systématique et rigoureuse des SNPO dans les études portant sur les blocs interscaléniques.
Enregistrement de l’étude
PROSPERO (CRD42020148496); enregistrée le 10 février 2020.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Shoulder surgery is common and often performed in an ambulatory setting.1 Interscalene brachial plexus blockade (ISB) has long been used as a sole anesthetic or co-anesthetic in a wide array of shoulder procedures.2,3 The benefits of ISB and other regional techniques include reduced postoperative nausea and vomiting, avoidance of airway manipulation, improved postoperative analgesia, and avoidance of systemic opioids.3
Postoperative neurologic symptoms (PONS) are recognized complications of both regional anesthesia and orthopedic surgery.2,4 In shoulder surgery specifically, PONS in the operative arm can be precipitated by direct trauma from surgical dissection, ISB needle manipulation, or intraneural injection.5,6,7,8 They can also be due to ischemia from nerve traction during surgical positioning, or nerve compression from high volumes of ISB injectate or intra-articular surgical irrigation.2 Many cases behave as neuropraxias and resolve over weeks to months, but a minority persist as enduring nerve injuries.5,9,10,11 Symptoms may include persistent pain, paresthesia, sensory or motor loss in a peripheral nerve, or radicular distribution, but no formal unified definition exists.9,10
Comparative observational studies4,5,11,12 and reviews13 have reported the raw incidence of PONS in ISB vs other techniques with some suggestion of an increased risk in ISB. Nevertheless, these analyses did not account for surgical and other factors confounding block selection and technique. Randomized controlled trials (RCTs) should balance these confounding factors across study groups but previous systematic reviews of RCTs in this area3,14,15,16 have focused on analgesic endpoints, with PONS considered as a secondary outcome or not at all. By restricting this review to RCTs and quasi-randomized trials, and focusing on PONS as the primary outcome, we hoped to improve our current understanding of the role of ISB in the development of PONS. The objectives were to: 1) compare the risk of PONS from ISB (with any technique or agent) to any other regional or nonregional analgesic modality and; 2) compare the risk of PONS between different techniques or agents used for ISB.
Methods
This systematic review followed Cochrane review methodology17 and is reported according PRISMA guidelines.18 The protocol was prospectively registered on 10 February 2020 in PROSPERO (CRD42020148496).
Database and search strategy
We searched the following databases from inception to June 2020: MEDLINE (Ovid), EMBASE (Ovid), and Cochrane Central Register of Controlled trials (CENTRAL-Wiley). Abstracts from five conferences held between January 2016 and June 2020 were also hand searched (see Appendix).
Population, interventions, comparators, outcomes and study designs
We included randomized or quasi-randomized trials, reported in English, with no restriction on year of publication, and excluding editorials, letters, and notes. Nonrandomized and observational trials were excluded. We included only trials of human participants, in which at least 80% or more of the included population were undergoing shoulder surgery and were at least five years old. For the purposes of this review, we defined shoulder surgery as involving incision into and/or insertion of instruments into the shoulder (e.g., excluding closed shoulder reduction), with or without additional surgery. Trials of children less than five years old were excluded because of the difficulty in diagnosis of PONS.
The primary intervention of interest was ISB by any technique, with any composition (local anesthetic and/or nonlocal anesthetic adjuvants), of any injectate volume, with or without concomitant catheter insertion and either preoperatively or immediately postoperatively in the postanesthesia care unit. Included comparative interventions were any type of regional or nonregional analgesic technique, or ISB by a different technique, composition, or volume, including comparisons of single injection and catheter techniques.
The primary outcome was the incidence of PONS in the operative arm at longest follow up. Postoperative neurologic symptoms were included if, as defined by study authors, they satisfied each of the following conditions: 1) include one or more of paresthesia, numbness, motor loss, sensory loss, or distal arm pain in the operative arm; 2) not a composite outcome with other adverse effects not specifically related to the operative arm such as hoarse voice or dissatisfaction with care; and 3) present at least 1 week into the postoperative period. Prior to data synthesis, criterion 1 was expanded to include nonspecific neurologic symptoms and dysfunction. Secondary outcomes included 1) hoarse voice (including documented ipsilateral superior laryngeal nerve injury) and 2) dyspnea (including documented phrenic nerve palsy), where they were not part of a composite outcome and were present at least one week into the postoperative period.
Study selection, data extraction, and risk of bias assessments
Two reviewers (S. N., S. R.) independently screened abstracts in duplicate, and only those trials that did not meet the study type, population, intervention, or comparison exclusion criteria were excluded. Both reviewers then independently reviewed the full text of remaining trials in duplicate for possible inclusion. All conflicts were resolved through consensus or by a third reviewer (T. M.) as required. For included trials, a data extraction form was developed, and data independently abstracted in duplicate. The following elements were abstracted: author, year of publication, country of origin, funding sources, main inclusion criteria, main exclusion criteria, number randomized, types of surgical procedure, surgical position (lateral or sitting), elective or emergency surgery status, duration of surgery, age, sex, weight, height, body mass index, concomitant use of general anesthesia, American Society of Anesthesiologists Physical Status class, details of technique, injectate components (local anesthetic and adjuvant name and dose, adjuvant route (perineural or intravenous), total ISB injectate volume, timing (i.e., pre- or postoperatively), type of PONS (i.e., paresthesia, motor loss), duration of follow up, method of follow up (i.e., patient report by telephone assessment vs in-person physical examination), and use of a baseline neurologic examination. All conflicts were resolved through consensus, or by a third reviewer as required. All included trials were also assessed for risk of bias using the Cochrane Risk of Bias 2.0 tool.19 Assessments on each study were independently performed by two reviewers (G. L., M. M.) and compared. Disagreements were resolved using consensus or a third party (T. M.) when necessary. Additional information on risk of bias assessment is found in the Electronic Supplementary Material (ESM) eAppendix: Supplementary methods and results.
Data synthesis
Registered plans for meta-analysis were abandoned on account of high risk of bias in many trials and heterogeneity in clinical comparator groups, PONS diagnostic criteria, and the timing of PONS assessment. Data were instead synthesized and summarized descriptively.
Results
Description of included trials and study populations
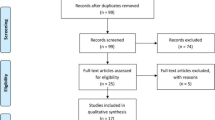
From 893 unique records identified by our search strategy, 55 trials (total of 6,236 participants; median sample size, 69; range, 30–910 participants) met our inclusion criteria (Figure). It should be noted that 422 additional trials were excluded for lack of PONS report.
The 55 trials were published between 1999 and 2021, with 16 trials published in 2010 or earlier. No trials reported hoarseness or dyspnea without also reporting PONS, but only eight and five trials measured these secondary outcomes, respectively. Trials and population characteristics are summarized in Table 1, ESM eTable 1, and eAppendix: Supplementary methods and results. No patients under the age of 18 were included in any of the included trials and all blocks were done preoperatively.
Interventions of included trials
Thirty-nine trials (71%) consisted exclusively of single injection techniques. Of these, 27/39 compared different techniques or compositions of single injection ISB and 12/39 compared ISB with other blocks.20,21,22,23,24,25,26,27,28,29,30,31 Of the remaining 16 trials, 13 consisted solely of catheter techniques, including ten that compared ISB catheters of different technique or composition32,33,34,35,36,37,38,39,40,41 and three42,43,44 that compared ISB catheter with other catheter techniques. The remaining three trials compared ISB catheter to ISB single injection techniques45,46 and wound infiltration.47
A nerve stimulator was used as the sole technique for needle (and catheter) guidance for at least one study group in 16 trials.9,26,33,34,35,37,38,39,40,48,49,50,51,52,53,54 Three additional trials compared ISB with nerve stimulator to ultrasound guidance44,55 or paresthesia.56 Ultrasound approach was in plane, out of plane, or unclear in 29, five,24,31,42,43,57 and two45,58 of the remaining 36 trials, respectively.
Median and modal loading volume of block solution for catheter trials was 30 mL (range, 10–40 mL). Median and modal volume of single injection ISB was 20 mL (range 5–55 mL). Only one study compared different volumes of local anesthetic across ISB groups.59 Perineural epinephrine (dose range, 2.5–5 µg·mL-1) was used in 16 trials. Other perineural adjuvants administered per protocol included dexamethasone (eight trials; dose range, 1–8 mg),53,58,60,61,62,63,64,65 dexmedetomidine (two trials; dose range, 41.5–150 µg),57,66 sodium bicarbonate (two trials), magnesium sulphate (two trials),55,56,67,68 and triamcinolone acetonide (one trial).54 Additional detail on interventions is in ESM eAppendix.
Risk of bias
Only five trials were assessed as low risk of bias,28,29,63,66,69 14 trials were assessed as some concerns of bias,40,41,42,50,58,60,61,62,65,67,70,71,72,73 and 36 were judged as high risk of bias (Table 2).20,21,22,23,24,25,26,27,30,31,32,33,34,35,36,37,38,39,43,44,45,46,47,48,49,51,52,53,54,55,56,57,59,68,74,75 Only seven trials (13%) were assessed as low risk of bias for selective outcome reporting (domain 5). For the remaining 48 trials, there were some concerns of bias, typically due to an inability to confirm the reported PONS analysis was planned a priori. Some concerns of bias or high risk of bias due to deviations from the intended intervention were also common (domain 2, 44% of trials) and risk of bias due to measurement of the outcome (domain 4, 35% of trials). Both domains are influenced by insufficient reporting on blinding or lack of blinding of patients, caregivers, and outcome assessors.
Postoperative neurologic symptoms, hoarseness, and dyspnea outcomes
Only two out of 55 (4%) included trials were powered to determine the incidence of PONS as the primary outcome.55,56 Postoperative neurologic symptoms were assessed either by telephone interview (26/55, 47%), physical exam (21/55, 38%), or unclear methods (8/55, 15%). Repeated PONS assessments occurred in 13 trials including four that assessed for PONS three or four times.
Postoperative neurologic symptoms were most frequently assessed at one or two weeks, with the latest assessment at one year. Reported incidences of PONS tended to be highest at earlier assessment points, decreasing markedly between two weeks and one month (Table 3). No cases of PONS were reported in 38 trials with sample sizes ranging from 30 to 336 participants, including 19 trials with assessments at one week. At least one case of PONS (incidence range, 0.8–32%) was reported in 17 trials with sample sizes ranging from 41 to 910 participants, including ten with repeated PONS assessments (ESM eTable 2). Of 27 trials comparing single injection ISB with ISB of different technique or composition, 24 trials measured PONS between one and three weeks. Incidence ranged from 0% (19 trials, 30–256 participants per trial) to 32% (13/41 participants). Of 12 trials comparing single injection ISB with other regional techniques, ten reported PONS between one and four weeks. The incidence ranged from 0% (nine trials, 44–336 participants per trial) to 6% (4/60 participants). Of 16 trials comparing ISB catheter with other techniques or alternative ISB catheter technique or composition, ten reported PONS between one and three weeks. The incidence ranged from 0% (ten trials, 36–303 participants per trial) to 3% (2/62 participants).
When summarized by type of surgery, in 23 trials of arthroscopy patients, PONS was measured between one and three weeks after surgery in all but three trials. No cases were reported in 15 trials (trial size range, 44–336 participants) and the incidence ranged from 1/58 (2%) to 13/41 (32%) in the remaining eight trials. In two trials of shoulder arthroplasty, PONS incidence ranged from 0/129 at three months to 2/70 (3%) at six weeks. No cases of PONS were reported among 302 patients undergoing open (nonarthroplasty) shoulder procedures across five trials assessing PONS between one week and one month after surgery. In the remaining 25 trials, the surgical populations were mixed or unclear. No PONS cases were reported in 17 (trial size range, 30–218 participants), of which 16 measured PONS between one and four weeks. In the remaining eight trials, the incidence ranged from 0.8% (2/240) at three months to 15% (15/99) at one week.
There were inconsistencies in how PONS diagnostic criteria were defined in the included trials (Table 4 and ESM eTable 2), ranging from ambiguous, nonspecific definitions (e.g., neurologic complications) to specific definitions (e.g., numbness, pain, tingling, or motor weakness in the operative arm). The most common definition criteria for PONS were the presence of one or more of paresthesia, sensory deficit, or motor deficit, used in 16/55 (29%) trials. Paresthesia (reported in 55% of trials), motor deficits (49% of trials), and numbness (40% of trials) were the most common criteria. Pain was a part of the diagnostic criteria in 18% of trials, and dysesthesia in 7% of trials. Of the included trials, 25% had nonspecific definitions of PONS and 9% were not classified (e.g., “other”).
Of eight trials measuring dyspnea, four with a sample size range of 60–910 had zero outcomes.43,45,54,58 Two trials of ISB adjuncts by the same group reported dyspnea at two weeks in 7/280 (2.5%) patients63 and 2/198 (1.0%) patients,69 with neither study reporting persistent symptoms at six months. Another two studies reported dyspnea at three months in 1/69 (1.4%) patients47 and seven days in 2/120 (1.7%) patients.42
Of five trials measuring hoarseness, two trials54,58 of 218 and 910 patients each reported zero outcomes. The remaining three trials reported an incidence of hoarseness between one and four weeks of 1/120 (0.8%),42 7/280 (2.5%),63 and 17/198 (8.6%) patients.69 In the latter two trials, no cases of hoarseness persisted at six months.
Discussion
Statement of findings
We found that PONS were infrequently reported in RCTs of ISB use. Of 477 otherwise eligible trials, 422 (88%) were excluded solely for not measuring PONS. Further, across the 55 included studies that reported PONS, outcome definitions were inconsistent and results were usually at high risk of bias. Only 16/55 (29%) trials used the most common PONS definition. Consistent with concerns of bias and heterogeneity, incidence varied greatly across trials, even when comparing trials with similar follow-up periods. Because of these limitations in the existing literature, the predefined objectives related to quantifying the risk of PONS with ISB, including comparing ISB techniques and compositions, could not be achieved.
Contextualized with previous research
Randomized controlled trials are the only study design capable of balancing surgical, patient, and block related predictors of PONS across study groups, thereby providing the most unbiased estimates of PONS incidence in ISB vs in comparator blocks. Nevertheless, large observational studies4,5,11,12 comparing the incidence of PONS in ISB with other commonly used peripheral nerve blocks remain prominent in the literature despite their limitations. Specific details of block technique and composition are typically lacking, and comparisons of the incidence of PONS with ISB vs with other techniques are inadequately adjusted for confounding associations between type of block and other potential predictors of PONS. Additional smaller series of a few hundred patients receiving ISB76,77 provide estimates of PONS incidence but no comparison group. Reported incidence ranges in these studies vary but fall within the wide ranges of incidence reported in the randomized trials included in this review. This variation may be caused by variable definitions of PONS across studies, as was also found in the trials in this review. Where measured longitudinally, the incidence of PONS was typically highest soon after surgery and decreased over time, as was seen empirically across the trials in this review. The type of surgery and the surgical position may also influence the rate of PONS, as specific neurologic injuries are associated with different types of shoulder surgery.2
A recent systematic review of randomized trials comparing the analgesic effects of perineural dexamethasone vs placebo for any brachial plexus blockade also included “neurologic complications” as a secondary outcome. Just 8/33 trials reported neurologic complications, and events occurred in only one of these,15 echoing the challenges encountered in this review.
It could be argued that it is unreasonable to expect that an adverse outcome like PONS would be routinely measured with the same rigor as typical primary outcomes like analgesia. Nevertheless, we believe that, since ISB is a commonly used regional technique and neurologic complications are an important patient safety outcome,78 PONS outcome reporting should be improved and standardized. The development of a standardized PONS outcome would require input from and consensus among content experts, patients, and other stakeholders. The ideal standardized PONS outcome would be easily and reliably measured to facilitate consistent use in clinical research. The most commonly used and specific PONS definitions identified in this review would deserve scrutiny in this regard.
Strengths and limitations
The methods of this review were registered a priori and guided by the procedures of the Cochrane and PRISMA statements. The methodological and clinical heterogeneity of the existing literature was underestimated and prevented a meta-analysis that would quantify the risk of PONS with ISB vs other techniques, or among different ISB techniques. Variation in PONS definitions and follow-up time were the main sources of methodological heterogeneity while variation in the type of surgery, comparator groups, ISB technique, and composition contributed to clinical heterogeneity. Additionally, high risk of bias among included trials, possibly due to PONS being reported as a secondary outcome, would further limit the quality of evidence derived from a quantitative analysis. Inclusion of trials published in languages other than English may have increased the number of included trials but would likely not provide a sufficiently homogeneous pool of trials upon which to perform a meta-analysis.
Implications
This systematic review provides insight into the methodological deficiencies of the literature with regards to the measurement of PONS as an outcome, despite heterogeneity and high risk of bias preventing the originally planned quantitative assessment of the risk of PONS after ISB vs other techniques, or among ISB techniques. The development and implementation of a standardized PONS definition would help to improve the value of the individual trials and facilitate comparisons across trials, as was attempted in the current work and supported elsewhere.78 Additionally, routinely including PONS as an outcome in regional anesthesia trials, defining it in registries and blinding PONS outcome assessors to group assignment, would minimize bias in PONS outcome reporting. Together, these methodologic improvements could improve patient safety by identifying how surgical approach, ISB technique, and composition influence PONS outcomes.
Conclusion
Limitations in the existing literature prevented a quantitative comparison of the risk of PONS associated with ISB. This finding highlights the need for a standardized PONS outcome definition and follow-up time and improved consistency and methodologic rigor in how PONS outcomes are assessed.
References
Warrender WJ, Syed UA, Hammoud S, et al. Pain management after outpatient shoulder arthroscopy: a systematic review of randomized controlled trials. Am J Sports Med 2017; 45: 1676-86.
Dwyer T, Henry PD, Cholvisudhi P, Chan VW, Theodoropoulos JS, Brull R. Neurological complications related to elective orthopedic surgery: part 1: common shoulder and elbow procedures. Reg Anesth Pain Med 2015; 40: 431-42.
Abdallah FW, Halpern SH, Aoyama K, Brull R. Will the real benefits of single-shot interscalene block please stand up? A systematic review and meta-analysis. Anesth Analg 2015; 120: 1114-29.
Sites BD, Taenzer AH, Herrick MD, et al. Incidence of local anesthetic systemic toxicity and postoperative neurologic symptoms associated with 12,668 ultrasound-guided nerve blocks: an analysis from a prospective clinical registry. Reg Anesth Pain Med 2012; 37: 478-82.
Lam KK, Soneji N, Katzberg H, et al. Incidence and etiology of postoperative neurological symptoms after peripheral nerve block: a retrospective cohort study. Reg Anesth Pain Med 2020; 45: 495-504.
Siddiqui U, Abdallah FW, Ahmed MM, Albaum JM, Brull R. Patient‐reported rates of postoperative neurological symptoms following regional anaesthesia in clinical research trials. Anaesthesia 2021; 76: 1572-6.
Neal JM, Barrington MJ, Brull R, et al. The second ASRA practice advisory on neurologic complications associated with regional anesthesia and pain medicine: executive summary 2015. Reg Anesth Pain Med 2015; 40: 401-30.
O’Flaherty D, McCartney CJ, Ng SC. Nerve injury after peripheral nerve blockade—current understanding and guidelines. BJA Educ 2018; 18: 384-90.
Borgeat A, Ekatodramis G, Kalberer F, Benz C. Acute and nonacute complications associated with interscalene block and shoulder surgery: a prospective study. Anesthesiology 2001; 95: 875-80.
Candido KD, Sukhani R, Doty R Jr, et al. Neurologic sequelae after interscalene brachial plexus block for shoulder/upper arm surgery: The association of patient, anesthetic, and surgical factors to the incidence and clinical course. Anesth Analg 2005; 100: 1489-95.
Fredrickson MJ, Kilfoyle DH. Neurological complication analysis of 1000 ultrasound guided peripheral nerve blocks for elective orthopaedic surgery: a prospective study. Anaesthesia 2009; 64: 836-44.
Liu SS, Gordon MA, Shaw PM, Wilfred S, Shetty T, Yadeau JT. A prospective clinical registry of ultrasound-guided regional anesthesia for ambulatory shoulder surgery. Anesth Analg 2010; 111: 617-23.
Brull R, McCartney CJ, Chan VW, El-Beheiry H. Neurological complications after regional anesthesia: contemporary estimates of risk. Anesth Analg 2007; 104: 965-74.
Hughes MS, Matava MJ, Wright RW, Brophy RH, Smith MV. Interscalene brachial plexus block for arthroscopic shoulder surgery: a systematic review. J Bone Joint Surg Am 2013; 95: 1318-24.
Kirkham KR, Jacot-Guillarmod A, Albrecht E. Optimal dose of perineural dexamethasone to prolong analgesia after brachial plexus blockade: a systematic review and meta-analysis. Anesth Analg 2018; 126: 270-9.
Kay J, Memon M, Hu T, et al. Suprascapular nerve blockade for postoperative pain control after arthroscopic shoulder surgery: a systematic review and meta-analysis. Orthop J Sports Med 2018; DOI: https://doi.org/10.1177/2325967118815859.
Higgins JP, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions, 2nd Edition. Chichester, UK: John Wiley & Sons; 2019: 726.
Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151: 264-9.
Sterne JA, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; DOI: https://doi.org/10.1136/bmj.l4898.
Kim BG, Han JU, Song JH, Yang C, Lee BW, Baek JS. A comparison of ultrasound-guided interscalene and supraclavicular blocks for post-operative analgesia after shoulder surgery. Acta Anaesthesiol Scand 2017; 61: 427-35.
Aliste J, Bravo D, Fernández D, Layera S, Finlayson RJ, Tran DQ. A randomized comparison between interscalene and small-volume supraclavicular blocks for arthroscopic shoulder surgery. Reg Anesth Pain Med 2018; 43: 590-5.
Aliste J, Bravo D, Finlayson RJ, Tran DQ. A randomized comparison between interscalene and combined infraclavicular-suprascapular blocks for arthroscopic shoulder surgery. Can J Anesth 2018; 65: 280-7.
Desroches A, Klouche S, Schlur C, Bauer T, Waitzenegger T, Hardy P. Suprascapular nerve block versus interscalene block as analgesia after arthroscopic rotator cuff repair: a randomized controlled noninferiority trial. Arthroscopy 2016; 32: 2203-9.
Wiegel M, Moriggl B, Schwarzkopf P, Petroff D, Reske AW. Anterior suprascapular nerve block versus interscalene brachial plexus block for shoulder surgery in the outpatient setting: a randomized controlled patient- and assessor-blinded trial. Reg Anesth Pain Med 2017; 42: 310-8.
Dhir S, Sondekoppam RV, Sharma R, Ganapathy S, Athwal GS. A comparison of combined suprascapular and axillary nerve blocks to interscalene nerve block for analgesia in arthroscopic shoulder surgery: an equivalence study. Reg Anesth Pain Med 2016; 41: 564-71.
Hadzic A, Williams BA, Karaca PE, et al. For outpatient rotator cuff surgery, nerve block anesthesia provides superior same-day recovery over general anesthesia. Anesthesiology 2005; 102: 1001-7.
Aliste J, Bravo D, Layera S, et al. Randomized comparison between interscalene and costoclavicular blocks for arthroscopic shoulder surgery. Reg Anesth Pain Med 2019; DOI: https://doi.org/10.1136/rapm-2018-100055.
Abdallah FW, Wijeysundera DN, Laupacis A, et al. Subomohyoid anterior suprascapular block versus interscalene block for arthroscopic shoulder surgery: a multicenter randomized trial. Anesthesiology 2020; 132: 839-53.
Kim DH, Lin Y, Beathe JC, et al. Superior trunk block: a phrenic-sparing alternative to the interscalene block: a randomized controlled trial. Anesthesiology 2019; 131: 521-33.
Taha AM, Yurdi NA, Elahl MI, Abd-Elmaksoud AM. Diaphragm-sparing effect of the infraclavicular subomohyoid block vs low volume interscalene block. A randomized blinded study. Acta Anaesthesiol Scand 2019; 63: 653-8.
Lee JJ, Hwang JT, Kim DY, et al. Effects of arthroscopy-guided suprascapular nerve block combined with ultrasound-guided interscalene brachial plexus block for arthroscopic rotator cuff repair: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc 2017; 25: 2121-8.
Albrecht E, Bathory I, Fournier N, Jacot-Guillarmod A, Farron A, Brull R. Reduced hemidiaphragmatic paresis with extrafascial compared with conventional intrafascial tip placement for continuous interscalene brachial plexus block: a randomized, controlled, double-blind trial. Br J Anaesth 2017; 118: 586-92.
Borgeat A, Aguirre J, Marquardt M, Mrdjen J, Blumenthal S. Continuous interscalene analgesia with ropivacaine 0.2% versus ropivacaine 0.3% after open rotator cuff repair: the effects on postoperative analgesia and motor function. Anesth Analg 2010; 111: 1543-7.
Borgeat A, Ekatodramis G, Guzzella S, Ruland P, Votta-Velis G, Aguirre J. Deltoid, triceps, or both responses improve the success rate of the interscalene catheter surgical block compared with the biceps response. Br J Anaesth 2012; 109: 975-80.
Borghi B, Facchini F, Agnoletti V, et al. Pain relief and motor function during continuous interscalene analgesia after open shoulder surgery: a prospective, randomized, double-blind comparison between levobupivacaine 0.25%, and ropivacaine 0.25% or 0.4%. Eur J Anaesthesiol 2006; 23: 1005-9.
Choromanski DW, Patel PS, Frederick JM, Lemos SE, Chidiac EJ. The effect of continuous interscalene brachial plexus block with 0.125% bupivacaine vs 0.2% ropivacaine on pain relief, diaphragmatic motility, and ventilatory function. J Clin Anesth 2015; 27: 619-26.
Eroglu A, Uzunlar H, Sener M, Akinturk Y, Erciyes N. A clinical comparison of equal concentration and volume of ropivacaine and bupivacaine for interscalene brachial plexus anesthesia and analgesia in shoulder surgery. Reg Anesth Pain Med 2004; 29: 539-43.
Yang CW, Jung SM, Kwon HU, et al. A clinical comparison of continuous interscalene brachial plexus block with different basal infusion rates of 0.2% ropivacaine for shoulder surgery. Korean J Anesthesiol 2010; 59: 27-33.
Casati A, Borghi B, Fanelli G, et al. Interscalene brachial plexus anesthesia and analgesia for open shoulder surgery: a randomized, double-blinded comparison between levobupivacaine and ropivacaine. Anesth Analg 2003; 96: 253-9.
Casati A, Vinciguerra F, Scarioni M, et al. Lidocaine versus ropivacaine for continuous interscalene brachial plexus block after open shoulder surgery. Acta Anaesthesiol Scand 2003; 47: 355-60.
Borgeat A, Kalberer F, Jacob H, Ruetsch YA, Gerber C. Patient-controlled interscalene analgesia with ropivacaine 0.2% versus bupivacaine 0.15% after major open shoulder surgery: the effects on hand motor function. Anesth Analg 2001; 92: 218-23.
Wiesmann T, Feldmann C, Muller HH, et al. Phrenic palsy and analgesic quality of continuous supraclavicular vs. interscalene plexus blocks after shoulder surgery. Acta Anaesthesiol Scand 2016; 60: 1142-51.
Beaudet V, Williams SR, Tétreault P, Perrault MA. Perioperative interscalene block versus intra-articular injection of local anesthetics for postoperative analgesia in shoulder surgery. Reg Anesth Pain Med 2008; 33: 134-8.
Oh JH, Rhee KY, Kim SH, Lee PB, Lee JW, Lee SJ. Comparison of analgesic efficacy between single interscalene block combined with a continuous intra-bursal infusion of ropivacaine and continuous interscalene block after arthroscopic rotator cuff repair. Clin Orthop Surg 2009; 1: 48-53.
Sabesan VJ, Shahriar R, Petersen-Fitts GR, et al. A prospective randomized controlled trial to identify the optimal postoperative pain management in shoulder arthroplasty: liposomal bupivacaine versus continuous interscalene catheter. J Shoulder Elbow Surg 2017; 26: 1810-7.
Panchamia JK, Amundson AW, Jacob AK, et al. A 3-arm randomized clinical trial comparing interscalene blockade techniques with local infiltration analgesia for total shoulder arthroplasty. J Shoulder Elbow Surg 2019; 28: e325-38.
Bjørnholdt KT, Jensen JM, Bendtsen TF, Søballe K, Nikolajsen L. Local infiltration analgesia versus continuous interscalene brachial plexus block for shoulder replacement pain: a randomized clinical trial. Eur J Orthop Surg Traumatol 2015; 25: 1245-52.
Casati A, Fanelli G, Cedrati V, Berti M, Aldegheri G, Torri G. Pulmonary function changes after interscalene brachial plexus anesthesia with 0.5% and 0.75% ropivacaine: a double-blinded comparison with 2% mepivacaine. Anesth Analg 1999; 88: 587-92.
Casati A, Fanelli G, Cappelleri G, et al. A clinical comparison of ropivacaine 0.75%, ropivacaine 1% or bupivacaine 0.5% for interscalene brachial plexus anaesthesia. Eur J Anaesthesiol 1999; 16: 784-9.
Casati A, Fanelli G, Aldegheri G, et al. Interscalene brachial plexus anaesthesia with 0.5%, 0.75% or 1% ropivacaine: a double-blind comparison with 2% mepivacaine. Br J Anaesth 1999; 83: 872-5.
Casati A, Fanelli G, Albertin A, et al. Interscalene brachial plexus anesthesia with either 0.5% ropivacaine or 0.5% bupivacaine. Minerva Anestesiol 2000; 66: 39-44.
DeMarco JR, Componovo R, Barfield WR, Liles L, Nietert P. Efficacy of augmenting a subacromial continuous-infusion pump with a preoperative interscalene block in outpatient arthroscopic shoulder surgery: a prospective, randomized, blinded, and placebo-controlled study. Arthroscopy 2011; 27: 603-10.
Tandoc MN, Fan L, Kolesnikov S, Kruglov A, Nader ND. Adjuvant dexamethasone with bupivacaine prolongs the duration of interscalene block: a prospective randomized trial. J Anesth 2011; 25: 704-9.
Webb BG, Sallay PI, McMurray SD, Misamore GW. Comparison of interscalene brachial plexus block performed with and without steroids. Orthopedics 2016; 39: e1100-3.
Liu SS, Zayas VM, Gordon MA, et al. A prospective, randomized, controlled trial comparing ultrasound versus nerve stimulator guidance for interscalene block for ambulatory shoulder surgery for postoperative neurological symptoms. Anesth Analg 2009; 109: 265-71.
Liguori GA, Zayas VM, YaDeau JT, et al. Nerve localization techniques for interscalene brachial plexus blockade: a prospective, randomized comparison of mechanical paresthesia versus electrical stimulation. Anesth Analg 2006; 103: 761-7.
Fritsch G, Danninger T, Allerberger K, et al. Dexmedetomidine added to ropivacaine extends the duration of interscalene brachial plexus blocks for elective shoulder surgery when compared with ropivacaine alone: a single-center, prospective, triple-blind, randomized controlled trial. Reg Anesth Pain Med 2014; 39: 37-47.
Cummings KC 3rd, Napierkowski DE, Parra-Sanchez I, et al. Effect of dexamethasone on the duration of interscalene nerve blocks with ropivacaine or bupivacaine. Br J Anaesth 2011; 107: 446-53.
Zhai W, Wang X, Rong Y, Li M, Wang H. Effects of a fixed low-dose ropivacaine with different volume and concentrations on interscalene brachial plexus block: a randomized controlled trial. BMC Anesthesiol 2016; DOI: https://doi.org/10.1186/s12871-016-0248-4.
Woo JH, Kim YJ, Kim DY, Cho S. Dose-dependency of dexamethasone on the analgesic effect of interscalene block for arthroscopic shoulder surgery using ropivacaine 0.5%: a randomised controlled trial. Eur J Anaesthesiol 2015; 32: 650-5.
Chun EH, Kim YJ, Woo JH. Which is your choice for prolonging the analgesic duration of single-shot interscalene brachial blocks for arthroscopic shoulder surgery? intravenous dexamethasone 5 mg vs. perineural dexamethasone 5 mg randomized, controlled, clinical trial. Medicine (Baltimore) 2016; DOI: https://doi.org/10.1097/MD.0000000000003828.
McHardy PG, Singer O, Awad IT, et al. Comparison of the effects of perineural or intravenous dexamethasone on low volume interscalene brachial plexus block: a randomised equivalence trial. Br J Anaesth 2020; 124: 84-91.
Holland D, Amadeo RJ, Wolfe S, et al. Effect of dexamethasone dose and route on the duration of interscalene brachial plexus block for outpatient arthroscopic shoulder surgery: a randomized controlled trial. Can J Anesth 2018; 65: 34-45.
Khan U, Torrance E, Townsend R, Davies S, Mackenzie T, Funk L. Low-grade infections in nonarthroplasty shoulder surgery. J Shoulder Elbow Surg 2017; 26: 1553-61.
Albrecht E, Reynvoet M, Fournier N, Desmet M. Dose-response relationship of perineural dexamethasone for interscalene brachial plexus block: a randomised, controlled, triple-blind trial. Anaesthesia 2019; 74: 1001-8.
Abdallah FW, Dwyer T, Chan VW, et al. IV and perineural dexmedetomidine similarly prolong the duration of analgesia after interscalene brachial plexus block: a randomized, three-arm, triple-masked, placebo-controlled trial. Anesthesiology 2016; 124: 683-95.
Palhais N, Brull R, Kern C, et al. Extrafascial injection for interscalene brachial plexus block reduces respiratory complications compared with a conventional intrafascial injection: a randomized, controlled, double-blind trial. Br J Anaesth 2016; 116: 531-7.
Lee AR, Yi H, Chung IS, et al. Magnesium added to bupivacaine prolongs the duration of analgesia after interscalene nerve block. Can J Anesth 2012; 59: 21-7.
Rodrigues D, Amadeo RJ, Wolfe S, et al. Analgesic duration of interscalene block after outpatient arthroscopic shoulder surgery with intravenous dexamethasone, intravenous dexmedetomidine, or their combination: a randomized-controlled trial. Can J Anesth 2021; 68: 835-45.
Kahn RL, Cheng J, Gadulov Y, Fields KG, YaDeau JT, Gulotta LV. Perineural low-dose dexamethasone prolongs interscalene block analgesia with bupivacaine compared with systemic dexamethasone: a randomized trial. Reg Anesth Pain Med 2018; 43: 572-9.
Kang R, Jeong JS, Yoo JC, et al. Effective dose of intravenous dexmedetomidine to prolong the analgesic duration of interscalene brachial plexus block: a single-center, prospective, double-blind, randomized controlled trial. Reg Anesth Pain Med 2018; 43: 488-95.
Kang RA, Jeong JS, Yoo JC, et al. Improvement in postoperative pain control by combined use of intravenous dexamethasone with intravenous dexmedetomidine after interscalene brachial plexus block for arthroscopic shoulder surgery: a randomised controlled trial. Eur J Anaesthesiol 2019; 36: 360-8.
Vandepitte C, Kuroda M, Witvrouw R, et al. Addition of liposome bupivacaine to bupivacaine HCl versus bupivacaine HCl alone for interscalene brachial plexus block in patients having major shoulder surgery. Reg Anesth Pain Med 2017; 42: 334-41.
Desmet M, Vanneste B, Reynvoet M, et al. A randomised controlled trial of intravenous dexamethasone combined with interscalene brachial plexus blockade for shoulder surgery. Anaesthesia 2015; 70: 1180-5.
Thomas LC, Graham SK, Osteen KD, Porter HS, Nossaman BD. Comparison of ultrasound and nerve stimulation techniques for interscalene brachial plexus block for shoulder surgery in a residency training environment: a randomized, controlled, observer-blinded trial. Ochsner J 2011; 11: 246-52.
Rajpal G, Winger DG, Cortazzo M, Kentor ML, Orebaugh SL. Neurologic outcomes after low-volume, ultrasound-guided interscalene block and ambulatory shoulder surgery. Reg Anesth Pain Med 2016; 41: 477-81.
Jeong JS, Kim YJ, Woo JH, Kim CH, Chae JS. A retrospective analysis of neurological complications after ultrasound guided interscalene block for arthroscopic shoulder surgery. Anesth Pain Med 2018; 13: 184-91.
Myles PS, Grocott MP, Boney O, Moonesinghe SR; COMPAC-StEP GROUP. Standardizing end points in perioperative trials: towards a core and extended outcome set. Br J Anaesth 2016; 116: 586-9.
Author contributions
All listed authors critically reviewed the draft manuscript and approved the final version. Thomas Mutter led the project and contributed to all aspects of this study, including conception and design, analysis and interpretation of data, and drafting the manuscript. Gabrielle S. Logan and Sam Neily contributed to study design, acquisition, analysis and interpretation of data, and drafting the manuscript. Scott Richardson contributed to study design and acquisition of data. Nicole Askin contributed to study design. Marita Monterola contributed to data acquisition. Ahmed Abou-Setta contributed to study conception and design and analysis and interpretation of data.
Acknowledgements
This project was funded, in part, by the Chronic Pain Network (McMaster University Medical Centre, Hamilton, ON, Canada), through the Strategy for Patient Oriented Research. It was further supported by the Department of Anesthesiology, Perioperative and Pain Medicine Oversight and Advisory Committee, and the George and Fay Yee Centre for Health Care Innovation, both at the University of Manitoba (Winnipeg, MB, Canada).
Disclosures
None.
Funding
This project was funded, in part, by the Chronic Pain Network (McMaster University Medical Centre, Hamilton, ON, Canada), through the Strategy for Patient Oriented Research. This work was also supported by the Department of Anesthesiology, Perioperative and Pain Medicine Oversight and Advisory Committee, and the George and Fay Yee Centre for Health Care Innovation at the University of Manitoba (Winnipeg, MB, Canada).
Editorial responsibility
This submission was handled by Dr. Sheila Riazi, Associate Editor, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This work was presented as an abstract at the Canadian Anesthesiologists’ Society virtual annual meeting, June 12–13, 2021.
Supplementary Information
Below is the link to the electronic supplementary material.
Appendix: Search strategy
Appendix: Search strategy
The search strategy was designed to identify randomized and quasi-randomized controlled trials in humans in which interscalene block was part of the intervention. Below is the final Ovid MEDLINE® search strategy:
Database: Ovid MEDLINE(R) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Daily <1946 to June 02, 2020>
Search Strategy:
1 ((interscal* or Interskal* or Interescal* or mezhlestnichnaya or scalene) adj6 (bloc* or bloq* or blok* or plexusblockade or plexusbrachialis-blockade or anaesth* or anesth* or anestes* or anasth* or analg* or obezbolivanie)).ti,ab,kf.
2 isb.ti,ab,kf.
3 or/1-2
4 randomized controlled trial/ or random allocation/ or double blind method/ or single blind method/ or clinical trial/ or exp clinical trials as topic/ or placebos/ (1173727)
5 (clinical trial, phase i or clinical trial, phase ii or clinical trial, phase iii or clinical trial, phase iv or controlled clinical trial or randomized controlled trial or multicenter study or clinical trial).pt.
6 ((clinical adj trial$) or ((singl$ or doubl$ or treb$ or tripl$) adj (blind$3 or dumm$3 or mask$3)) or placebo$ or sham or randomly allocated or (allocated adj2 random$) or RCT or RCTs).tw.
7 (randomized or randomized or trial).ti.
8 or/4-7
9 3 and 8
10 retrospective studies/ or case study/ or letter/ or historical article/ or case report.tw.
11 (catalogs or comment or editorial or essays or guidebooks or handbooks or historical article or interview or journal correspondence or lectures or letter or meeting abstracts or news or newspaper article or note or short survey).pt.
12 (exp animal experiment/ or exp animal model/ or exp transgenic animal/ or exp male animal/ or exp female animal/ or exp juvenile animal/ or animal/ or chordata/ or vertebrate/ or tetrapod/ or exp fish/ or amniote/ or exp amphibia/ or mammal/ or exp reptile/ or exp sauropsid/ or therian/ or exp monotremate/ or placental mammals/ or exp marsupial/ or euarchontoglires/ or exp afrotheria/ or exp boreoeutheria/ or exp laurasiatheria/ or exp xenarthra/ or primate/ or exp dermoptera/ or exp glires/ or exp scandentia/ or haplorhini/ or exp prosimian/ or simian/ or exp tarsiiform/ or catarrhini/ or exp platyrrhini/ or ape/ or exp cercopithecidae/ or hominid/ or exp hylobatidae/ or exp chimpanzee/ or exp gorilla/ or exp orangutan/ or (animal or animals or pisces or fish or fishes or catfish or catfishes or sheatfish or silurus or arius or heteropneustes or clarias or gariepinus or fathead minnow or fathead minnows or pimephales or promelas or cichlidae or trout or trouts or char or chars or salvelinus or salmo or oncorhynchus or guppy or guppies or millionfish or poecilia or goldfish or goldfishes or carassius or auratus or mullet or mullets or mugil or curema or shark or sharks or cod or cods or gadus or morhua or carp or carps or cyprinus or carpio or killifish or eel or eels or anguilla or zander or sander or lucioperca or stizostedion or turbot or turbots or psetta or flatfish or flatfishes or plaice or pleuronectes or platessa or tilapia or tilapias or oreochromis or sarotherodon or common sole or dover sole or solea or zebrafish or zebrafishes or danio or rerio or seabass or dicentrarchus or labrax or morone or lamprey or lampreys or petromyzon or pumpkinseed or pumpkinseeds or lepomis or gibbosus or herring or clupea or harengus or amphibia or amphibian or amphibians or anura or salientia or frog or frogs or rana or toad or toads or bufo or xenopus or laevis or bombina or epidalea or calamita or salamander or salamanders or newt or newts or triturus or reptilia or reptile or reptiles or bearded dragon or pogona or vitticeps or iguana or iguanas or lizard or lizards or anguis fragilis or turtle or turtles or snakes or snake or aves or bird or birds or quail or quails or coturnix or bobwhite or colinus or virginianus or poultry or poultries or fowl or fowls or chicken or chickens or gallus or zebra finch or taeniopygia or guttata or canary or canaries or serinus or canaria or parakeet or parakeets or grasskeet or parrot or parrots or psittacine or psittacines or shelduck or tadorna or goose or geese or branta or leucopsis or woodlark or lullula or flycatcher or ficedula or hypoleuca or dove or doves or geopelia or cuneata or duck or ducks or greylag or graylag or anser or harrier or circus pygargus or red knot or great knot or calidris or canutus or godwit or limosa or lapponica or meleagris or gallopavo or jackdaw or corvus or monedula or ruff or philomachus or pugnax or lapwing or peewit or plover or vanellus or swan or cygnus or columbianus or bewickii or gull or chroicocephalus or ridibundus or albifrons or great tit or parus or aythya or fuligula or streptopelia or risoria or spoonbill or platalea or leucorodia or blackbird or turdus or merula or blue tit or cyanistes or pigeon or pigeons or columba or pintail or anas or starling or sturnus or owl or athene noctua or pochard or ferina or cockatiel or nymphicus or hollandicus or skylark or alauda or tern or sterna or teal or crecca or oystercatcher or haematopus or ostralegus or shrew or shrews or sorex or araneus or crocidura or russula or european mole or talpa or chiroptera or bat or bats or eptesicus or serotinus or myotis or dasycneme or daubentonii or pipistrelle or pipistrellus or cat or cats or felis or catus or feline or dog or dogs or canis or canine or canines or otter or otters or lutra or badger or badgers or meles or fitchew or fitch or foumart or foulmart or ferrets or ferret or polecat or polecats or mustela or putorius or weasel or weasels or fox or foxes or vulpes or common seal or phoca or vitulina or grey seal or halichoerus or horse or horses or equus or equine or equidae or donkey or donkeys or mule or mules or pig or pigs or swine or swines or hog or hogs or boar or boars or porcine or piglet or piglets or sus or scrofa or llama or llamas or lama or glama or deer or deers or cervus or elaphus or cow or cows or bos taurus or bos indicus or bovine or bull or bulls or cattle or bison or bisons or sheep or sheeps or ovis aries or ovine or lamb or lambs or mouflon or mouflons or goat or goats or capra or caprine or chamois or rupicapra or leporidae or lagomorpha or lagomorph or rabbit or rabbits or oryctolagus or cuniculus or laprine or hares or lepus or rodentia or rodent or rodents or murinae or mouse or mice or mus or musculus or murine or woodmouse or apodemus or rat or rats or rattus or norvegicus or guinea pig or guinea pigs or cavia or porcellus or hamster or hamsters or mesocricetus or cricetulus or cricetus or gerbil or gerbils or jird or jirds or meriones or unguiculatus or jerboa or jerboas or jaculus or chinchilla or chinchillas or beaver or beavers or castor fiber or castor canadensis or sciuridae or squirrel or squirrels or sciurus or chipmunk or chipmunks or marmot or marmots or marmota or suslik or susliks or spermophilus or cynomys or cottonrat or cottonrats or sigmodon or vole or voles or microtus or myodes or glareolus or primate or primates or prosimian or prosimians or lemur or lemurs or lemuridae or loris or bush baby or bush babies or bushbaby or bushbabies or galago or galagos or anthropoidea or anthropoids or simian or simians or monkey or monkeys or marmoset or marmosets or callithrix or cebuella or tamarin or tamarins or saguinus or leontopithecus or squirrel monkey or squirrel monkeys or saimiri or night monkey or night monkeys or owl monkey or owl monkeys or douroucoulis or aotus or spider monkey or spider monkeys or ateles or baboon or baboons or papio or rhesus monkey or macaque or macaca or mulatta or cynomolgus or fascicularis or green monkey or green monkeys or chlorocebus or vervet or vervets or pygerythrus or hominoidea or ape or apes or hylobatidae or gibbon or gibbons or siamang or siamangs or nomascus or symphalangus or hominidae or orangutan or orangutans or pongo or chimpanzee or chimpanzees or pan troglodytes or bonobo or bonobos or pan paniscus or gorilla or gorillas or troglodytes).ti,ab,kf.) not (human/ or (human$ or man or men or woman or women or child or children or patient$).ti,ab,kf.)
13 or/10-12
14 9 not 13
Conference abstracts were searched from earliest of 2016 (or oldest available) to 2020 (or most recent available at June 2020). Abstracts were searched from the following meetings: European society of Anesthesiology, American Society of Anesthesiology, International Anesthesia Research Society, Canadian Anesthesiologists’ Society, Association of Anesthetists of Great Britain and Ireland.
Rights and permissions
About this article
Cite this article
Mutter, T., Logan, G.S., Neily, S. et al. Postoperative neurologic symptoms in the operative arm after shoulder surgery with interscalene blockade: a systematic review. Can J Anesth/J Can Anesth 69, 736–749 (2022). https://doi.org/10.1007/s12630-022-02229-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-022-02229-w