Abstract
Purpose
Carbetocin, an oxytocin analog, given as a postpartum hemorrhage prophylaxis in elective Cesarean deliveries, frequently causes tachycardia and hypotension. Phenylephrine infusion has been shown to prevent spinal anesthesia-induced hypotension. The goal of this study was to evaluate if a slow infusion of carbetocin would reduce maternal heart rate variation and hemodynamic disturbances compared with a rapid bolus in parturients receiving a prophylactic phenylephrine infusion during elective Cesarean delivery.
Methods
In this double-blinded randomized controlled trial, 70 healthy parturients were allocated to either a bolus group or an infusion group. At cord clamping, participants in the bolus group received carbetocin 100 µg as a rapid intravenous bolus, while participants in the infusion group received carbetocin 100 µg over 10 min. The primary outcome was the variation in maternal heart rate from baseline during the 20 min following cord clamping. Secondary outcomes included blood pressure, cardiac output, and stroke volume variations during the study period, measured with the ClearSight™ hemodynamic monitor.
Results
Maximum heart rate variation was not different between the groups: bolus group, mean (standard deviation) 29.8 (25.2)% vs infusion group, 27.2 (23.3)%; P = 0.67. The increase in heart rate occurred significantly earlier in the bolus group than in the infusion group (median [interquartile range] time, 105 [69–570] sec vs 485 [255–762] sec; P = 0.02; group × time interaction: two-way repeated measures ANOVA, P = 0.04). There was no significant difference in maximum variations for the other hemodynamic parameters between the groups.
Conclusion
Carbetocin infused over ten minutes did not reduce the magnitude of maternal heart rate variation but delayed its occurrence. This finding could be relevant to the anesthesiologist caring for parturients in whom a slight increase in maternal heart rate is clinically undesirable.
Study registration
www.clinicaltrials.gov (NCT03404544); registered 19 January 2018.
Résumé
Objectif
Lorsque la carbétocine, un analogue de l’ocytocine, est administrée à titre de prophylaxie pour les hémorragies du post-partum dans les accouchements par césarienne programmée, cet agent provoque fréquemment une tachycardie et une hypotension. Il a été démontré qu’une perfusion de phényléphrine prévenait l’hypotension induite par la rachianesthésie. L’objectif de cette étude était d’évaluer si une perfusion lente de carbétocine réduirait la variation de fréquence cardiaque maternelle et les perturbations hémodynamiques par rapport à un bolus rapide chez les parturientes recevant une perfusion prophylactique de phényléphrine pendant un accouchement par césarienne programmée.
Méthode
Dans cette étude randomisée contrôlée à double insu, 70 parturientes en bonne santé ont été allouées à un groupe bolus ou à un groupe perfusion. Lors du clampage du cordon, les participantes du groupe bolus ont reçu 100 μg de carbétocine sous forme de bolus intraveineux rapide, tandis que les participantes du groupe perfusion ont reçu 100 μg de carbétocine sur dix minutes. Le critère d’évaluation principal était la variation de la fréquence cardiaque maternelle par rapport aux valeurs de base au cours des 20 minutes suivant le clampage du cordon. Les critères secondaires comprenaient la tension artérielle, le débit cardiaque et les variations du volume d’éjection au cours de la période d’étude, tels que mesurés avec le moniteur hémodynamique ClearSight™.
Résultats
La variation maximale de fréquence cardiaque n’était pas différente entre les groupes : groupe bolus, moyenne (écart type) 29,8 (25,2) % vs groupe perfusion, 27,2 (23,3) %; P = 0,67. L’augmentation de la fréquence cardiaque s’est produite significativement plus tôt dans le groupe bolus que dans le groupe perfusion (temps médian [écart interquartile], 105 [69-570] sec vs 485 [255-762] sec; P = 0,02;× interaction groupe x temps : ANOVA bidirectionnelle à mesures répétées, P = 0,04). Il n’y avait pas de différence significative dans les variations maximales pour les autres paramètres hémodynamiques entre les groupes.
Conclusion
La carbétocine perfusée pendant dix minutes n’a pas réduit l’ampleur de la variation de la fréquence cardiaque maternelle, mais a retardé son apparition. Cette découverte pourrait être pertinente pour l’anesthésiologiste qui prend soin de parturientes chez qui une légère augmentation de la fréquence cardiaque maternelle serait cliniquement indésirable.
Enregistrement de l’étude
www.clinicaltrials.gov (NCT03404544); enregistrée le 19 janvier 2018.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In 2018, the Society of Obstetricians and Gynecologists of Canada reaffirmed their recommendation to use carbetocin as the first line uterotonic agent to prevent uterine atony during elective Cesarean delivery.1 Carbetocin is a structural analog of oxytocin with an approximate half-life of 40 min, so can be administered as a single-dose injection rather than a continuous infusion.2 Like oxytocin, carbetocin reduces the risk of postpartum hemorrhage by inducing rhythmic uterine contractions, by increasing the frequency of already existing contractions, and by strengthening uterine tone.3
Carbetocin and oxytocin have similar mechanisms of action and hemodynamic profiles. Hypotension and tachycardia are frequent clinical side effects.4 Several studies have shown that a slow infusion of oxytocin can mitigate the maternal hemodynamic side effects, and some experts have suggested that a similar approach may also improve the hemodynamic profile of carbetocin.5,6,7,8 This question is clinically relevant if peripartum hemodynamic stability is particularly important in certain parturients. In daily clinical practice, carbetocin is often administered as a rapid “push“ intravenous bolus (personal communication from several colleagues). This practice contradicts current recommendations in the drug monograph, which stipulate that carbetocin should be administered as an intravenous bolus over one minute.2
The medical literature has convincingly shown the efficacy and safety of prophylactic phenylephrine infusion to maintain maternal arterial pressure within a clinically acceptable range in parturients undergoing Cesarean delivery under spinal anesthesia. This practice is now recommended in the international consensus statement on the management of hypotension with vasopressors during Cesarean delivery under spinal anesthesia and is considered the standard of care at our centre.9
We focused on heart rate for the following reasons: 1) it is part of standard monitoring and is readily available in all instances; 2) it is a surrogate indicator of maternal cardiac output and as such, may be of significant clinical use to all anesthesiologists caring for obstetric patients even when a cardiac output monitor is not available;10 and 3) early detection of heart rate variations may be of clinical importance in a variety of medical conditions. In accordance with published literature, we considered that a variation of 10% in heart rate would be clinically relevant, especially when heart rate stability is clinically required.7
Phenylephrine infusion indirectly influences maternal heart rate through baromodulatory mechanisms. It is unclear whether slow infusion of carbetocin would have any clinical impact on maternal heart rate and hemodynamics when pregnant women receive clinically relevant doses of phenylephrine. Therefore, the objective of our study was to compare the effect of an intravenous infusion of carbetocin over ten minutes with that of a rapid bolus on maternal heart rate changes in parturients undergoing elective Cesarean deliveries under spinal anesthesia with a prophylactic intravenous phenylephrine infusion. We hypothesized that, with a phenylephrine infusion, the mode of administration of carbetocin would not significantly alter maternal heart rate and hemodynamics.
Methods
This prospective, randomized, double-blinded, controlled trial was conducted at the CEMTL – Installation Maisonneuve-Rosemont, affiliated to the University of Montreal, between June 2018 and January 2020 (ClinicalTrials.gov registration number, NCT03404544; registered 19 January 2018). Following institutional ethical review board approval (project number: 2018-1213) and after receiving a non-objection letter from Health Canada authorizing the rapid injection of carbetocin for this trial (file number: HC6-24-c211131), participants were approached at the obstetric investigation clinic (OIC) at the CEMTL the week preceding their scheduled Cesarean delivery. Patients’ written informed consent was obtained and preoperative data were collected at the OIC. Inclusion criteria were an American Society of Anesthesiologists status of II, elective Cesarean delivery under spinal anesthesia, term gestation (≥ 37 weeks gestation), and adult age (18 yr old and above). Exclusion criteria were multiple gestation, morbid obesity (body mass index > 40 kg· m-2 at first prenatal medical visit), coagulopathy before or during pregnancy, active labour, polyhydramnios, uterine leiomyoma, hypotensive or hypertensive illness (including pre-eclampsia and eclampsia), hemodynamic-altering medications (e.g., antihypertensive medications), placental implantation abnormalities, maternal cardiac pathology, urgent Cesarean delivery, contraindications to spinal anesthesia, planned general anesthesia for Cesarean delivery, planned uterine exteriorization for hysterotomy repair, known allergy to carbetocin, and refusal to participate. Withdrawal criteria were administration of intravenous ephedrine or glycopyrrolate during the study period; unplanned administration of an additional uterotonic; onset of postpartum hemorrhage defined as > 1,000 mL blood loss or uncontrolled bleeding after delivery of the baby; and unexpected uterine exteriorization for hysterotomy repair.
On the day of surgery, all participants received an antacid prophylaxis of sodium citrate 30 ml per mouth. In the operation room, an 18G or 20G intravenous catheter was sited in an upper limb vein and standard monitoring and the ClearSight™ hemodynamic monitor (Edwards Lifesciences, Irvine, CA, USA) was applied. The ClearSight monitor provides noninvasive hemodynamic measurements through digital photoplethysmography pulse wave analysis. This technology measures arterial blood pressure and has been validated in the obstetric population.11 It also traces a pulse contour wave, which is the base of an algorithm used to calculate stroke volume and cardiac output. This pulse contour wave analysis-based cardiac output measurement technique has been validated in several populations but not in the obstetric population.12,13 Standard vital signs were measured and collected with the Dräger Infinity® C700 (Draeger Medical Canada Inc, Mississauga, ON, Canada) automated information management system.
Participants were preloaded with a 1,000-mL lactated Ringer bolus over five to ten minutes upon obtaining the intravenous line, followed by an infusion at 300 mL·hr-1. Patients were helped into the seated position for the spinal anesthesia procedure during bolus infusion. Standardized spinal anesthesia was performed by the attending anesthesiologist between the L2 and L5 interspaces with a 25G or 27G Whitacre spinal needle. The anesthetic solution consisted of hyperbaric bupivacaine (0.75%) 10.5 mg with fentanyl 15 µg and morphine 0.15 mg. Immediately after spinal anesthesia administration, the patient was placed in the supine position with the right hip wedged with a Oasis Flat Bottom Chest Roll, model OA212 (Trulife, Trenton, ON, Canada) to reduce potential aortocaval compression. Cephazolin 2 g iv as well as metoclopramide 10 mg iv was given to all patients after spinal anesthesia was administered. Nausea and vomiting episodes not responding to hypotensive treatment (vide infra) were treated with ondansetron 4 mg iv.
A phenylephrine infusion set at 0.5 µg·kg-1·min-1 was initiated at the time of spinal injection. Hypotensive episodes were defined as two consecutive systolic blood pressure (SBP) measurements < 20% of presurgical baseline value or as an episode of nausea and were treated with boluses of phenylephrine 1.5 µg·kg-1. Presurgical baseline SBP was determined by averaging three consecutive measurements taken in the waiting room of the operating room. Bradycardia with and without hypotension were treated with ephedrine iv and glycopyrrolate iv, respectively, (protocol implemented but not utilized as bradycardia did not occur). In cases of hypertensive episodes, defined as a SBP > 120% of baseline value for two consecutive measurements, the phenylephrine infusion rate was decreased by 0.2 µg·kg-1·min-1. At any moment, the attending anesthesiologist could deviate from this protocol for patient safety.
Participants were randomized into two groups: a bolus group and an infusion group. In the bolus group, participants received carbetocin 100 µg iv as a bolus as fast as possible (“push”) while in the infusion group, participants received carbetocin 100 µg iv as an infusion delivered by a pump over ten minutes. The patient, the attending anesthesiologist, and the research personnel collecting intraoperative data were blinded to patient group allocation. The study drug was prepared by the research nurse who was not involved in collecting intraoperative data. To preserve blinding, the research nurse prepared two syringes for each participant, in a double-dummy design (bolus group: one 3 ml syringe containing 1 mL of carbetocin 100 µg or 1 mL of normal saline; infusion group: one 10 mL syringe containing 1 mL of carbetocin 100 µg diluted with 9 mL of normal saline for a total volume of 10 mL, or 10 mL of normal saline). The volume in the 3 mL syringe was injected as an intravenous push bolus at cord clamping, as per routine institutional practice, and the volume in the 10 mL syringe was infused over ten minutes, initiated at cord clamping.
A member of the research team collected intraoperative data such as gravimetrically estimated blood loss volume; total volume of infused crystalloids; total dose and number of boluses of phenylephrine, ephedrine and glycopyrrolate administered; and side effects. Blood pressure, heart rate, and oxygen saturation were collected every minute from the standard anesthesia monitor (GE Healthcare, Chicago, IL, USA). Stroke volume, cardiac output, and blood pressure were measured every five seconds from the ClearSight monitor. These hemodynamic parameters were collected from the time of intrathecal injection until the end of the study period, i.e., 20 min after the beginning of drug administration.
The obstetricians were asked to proceed with in situ hysterotomy repair. Uterine tone was assessed by the attending obstetrician as adequate or inadequate at the end of the ten-minute infusion. Nevertheless, obstetricians were invited to communicate any concerns about uterine tone to the anesthesiologist at any time. Patients with inadequate uterine tone were cared for by the attending anesthesiologist and obstetrician according to best clinical practice standards and were withdrawn from further analyses.
Group allocation randomization tables were generated with a free online randomization tool (http://www.randomization.com/) using randomly permutated blocks. Six blocks of ten participants each and two blocks of five participants each were generated. This allowed for a balanced sample size across treatment groups and an increase in the odds of similarity between the groups, as well as the conduct of an interim analysis after 40 participants were recruited, followed by a futility analysis. Only the research nurse who was not involved in intraoperative data collection had access to the group allocation unless unblinding became necessary as per good clinical practice regulations.
The primary outcome of this study was the maximum variation in maternal heart rate within the 20 min following T0 (T0 was the beginning of drug administration). The maximum heart rate variation for each patient was calculated by subtracting the baseline heart rate value from the highest heart rate and dividing it by the baseline heart rate value recorded at any timepoint during these 20 min. The baseline heart rate was the heart rate measured at T0. The alternative hypothesis was that the maximum heart rate variation would be lower by a magnitude of at least 10% in the infusion group compared with the bolus group. The secondary outcomes included the time to reach the maximum recorded heart rate; changes in SBP, cardiac output and stroke volume; phenylephrine requirements; estimated intraoperative blood loss; uterine tone assessment, and side effects of carbetocin (flushing, headache, thoracic pain, abdominal, tremor, dyspnea, nausea, and vomiting). Variations in cardiac output and stroke volume were calculated similarly to the maximum heart rate variation while the SBP variation was obtained by subtracting the baseline SBP from the lowest SBP and dividing by the baseline SBP value recorded at any timepoint during the 20 min following T0.
Statistical analysis
Unless otherwise specified, continuous data are presented as mean (standard deviation [SD]) or median [interquartile range (IQR)] according to the distribution of the variables, and categorical data are presented as n/total N (%). The distribution of the continuous variables was assessed using plots, normality tests (Shapiro–Wilk, Kolmogorov–Smirnov), and skewness and kurtosis indices. Groups were compared using Student’s t test or Mann–Whitney–Wilcoxon test for continuous parameters (according to their distribution), and the Chi square or Fisher’s exact test for categorical parameters. Maternal hemodynamic parameters between the two groups in terms of percent change from baseline (T0) were compared using a two-way repeated measures ANOVA including a term for the group, a term for the time, and a term for the group × time interaction. When the interaction term was statistically significant, contrasts were produced to compare groups at specific time points or to compare time points between groups. For statistical purposes, every blood pressure measurement taken at each minute was included in the ANOVA analysis, while only timepoints at each 20 sec were considered for heart rate, cardiac output and stroke volume.
A noncontrolled preliminary assessment in our centre on 20 parturients undergoing Cesarean delivery under similar anesthetic conditions as those described in our Methods section showed a mean (SD) decrease of heart rate from 93 to 79 (12) beats·min-1 in the infusion group vs the bolus group. We determined with an online sample size calculator (https://clincalc.com/stats/samplesize.aspx) that 56 patients (28 patients per group) would be necessary to detect a 10% decrease in heart rate in the infusion group compared with the bolus group, with an α value of 0.05 and a power of 80%. To allow for intraoperative withdrawal of patients, we included 70 participants. An interim analysis was performed when 40 out of 70 participants were recruited. The efficacy threshold that would have justified stopping recruitment was determined with the O’Brien Flemming method to be a P value < 0.0061 for the primary outcome analysis. The interim analysis of the primary outcome was performed by the research nurse who informed the principal investigator of the P value of the primary outcome comparison. This allowed the principal investigator to decide how the study should proceed. To take into account the interim analysis, the statistically significant threshold for the final analysis of the primary outcome was corrected to 0.0481. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) or higher and significance was determined according to a two-sided 0.05 significance level (except for the primary analysis). No adjustment for multiple testing and no imputation of missing data were done.
Results
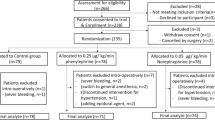
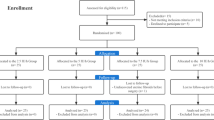
Seventy participants were included in this study (Fig. 1). Six parturients were withdrawn for clinical reasons: two in the bolus group (unplanned exteriorization of the uterus in both participants) and four in the infusion group (an unplanned exteriorization of the uterus in one participant; a second dose of carbetocin in two participants, which was requested by the obstetrician before the ten-minute interval was over because the uterine tone was unsatisfactory; and intraoperative pain in one participant, leading the attending anesthesiologist to derogate from the protocol and give the carbetocin as a bolus). The incidence of withdrawal was not significantly different between the groups. A further four patients in the bolus group were withdrawn from primary and secondary analyses because their vital signs, including heart rate, were only partially recorded or not recorded at all because of technical errors. These participants were also withdrawn from primary and secondary outcome analyses, leaving 29 patients in the bolus group and 31 patients in the infusion group being analyzed. In a further six of the 60 remaining participants (four in the bolus group and two in the infusion group), hemodynamic data other than heart rate and blood pressure were lost because of technical difficulties with the ClearSight monitor. These six patients were included in the primary analysis and secondary analyses of heart rate and blood pressure but were not included in the analyses of secondary outcomes related to cardiac output and stroke volume. The interim analysis after 40 participants were recruited showed a P value of 0.765 for the comparison of the primary outcome between groups, which allowed the trial to continue.
The groups were similar with regard to their baseline characteristics, baseline hemodynamic parameters, and intraoperative data (Table 1 and Table 2). There were no differences between the groups in heart rate variation and variation in other hemodynamic parameters (Table 3). Nevertheless, the median [IQR] time to reach the maximum heart rate was significantly shorter in the bolus group than in the infusion group (bolus: 105 [69–570] sec vs infusion: 485 [255–762] sec; P = 0.02). Analysis of hemodynamic parameters over time showed a statistically significant difference in heart rate between groups (group × time interaction: two-way repeated measures ANOVA, P = 0.04). Contrasts showed a significant difference between groups for 12 of the 61 time points (specifically at 100, 120, 340, 440, 460, 540, 640, 680, 820, 880, 1,080, and 1,140 sec) (Fig. 2).
Heart rate variation (a), systolic blood pressure variation (b), cardiac output variation (c), and stroke volume variation (d) measured over the 20 min following the intervention in parturients who received carbetocin as a bolus or as an infusion. Data are expressed as mean (standard deviation) percentage of variation from baseline values
There was no difference in side effects between the groups (Table 4). Among the participants presenting with at least one episode of hypotension, two patients in the bolus group and three patients in the infusion needed more than one bolus of phenylephrine to treat the hypotensive episode (P = 0.43). Six patients in each group received a total of 17 boluses of phenylephrine (P = 0.57) (Table 2). The maximum number of boluses of phenylephrine given to a single patient was four, in the bolus group. There were no episodes of bradycardia, including rebound bradycardia after the administration of a phenylephrine bolus, in any patients. The uterine tone at ten minutes was considered adequate by the attending obstetricians (eight obstetricians were involved in the study) in all patients that completed the study.
Discussion
To our knowledge, our study is the first to compare the effects of a controlled infusion of carbetocin 100 µg over ten minutes with the effects of a rapid bolus on heart rate and other hemodynamic parameters in parturients receiving a prophylactic intravenous infusion of phenylephrine as part of their anesthetic management. Our results show that the maximum heart rate variation from baseline registered during the 20 min following cord clamping was similar between groups. Nevertheless, we found that maternal heart rate increased earlier in the bolus group than in the infusion group, that is, around the second minute following the intervention. Similarly, Moertl et al. and Rosseland et al. observed transient tachycardia within the first two minutes after healthy parturients received a bolus of carbetocin during their Cesarean delivery.4,7 In both studies, however, this tachycardia was mirrored by maternal hypotension. In contrast, our results show that the maternal heart rate increased despite successful control of arterial pressure in both groups with a phenylephrine infusion. Phenylephrine lowers maternal heart rate through increased systemic vascular resistance and reflex baromodulation rather than via a direct pharmacologic action on myocardial conduction.10,14 While clinicians tightly control intraoperative blood pressure with a phenylephrine infusion, they do not do so as aggressively for heart rate unless it reaches clinically concerning values. In patients who do not receive beta-receptor agonists or anticholinergic agents, heart rate could therefore be more sensitive in detecting mild intraoperative hemodynamic fluctuations, including those induced by carbetocin.
Our results differ from those of Dell-Kuster et al. who compared the administration of carbetocin 100 µg as a bolus over one minute with a rapid free-flow infusion over a median duration of 3 min 52 sec.15 The authors did not detect any difference between the groups in secondary outcomes such as maternal heart rate, blood pressure, and vasopressor requirements. This difference of less than three minutes between the two groups may not be pharmacokinetically relevant, considering the 40-min half-life of carbetocin; the plasma concentration of the molecule might not have been different between the groups. Our study aimed at resolving this issue by comparing a practice that is common in our setting—pushing carbetocin—with giving carbetocin as a controlled infusion over ten minutes. This time difference between the groups to reach peak plasma concentration of carbetocin may explain the earlier occurrence of tachycardia in the bolus group despite an overall similar maximum heart rate variation over the study period. This delayed increase in the plasma concentration of carbetocin in the infusion group may also support the apparent trend towards a sustained higher heart rate in the infusion group from the fifth minute on after cord clamping (Fig. 2). Concerns with regards to postpartum hemorrhage related to uterine atony in the infusion group may arise. The work by Khan et al. showed that the 95% effective dose (ED95) of carbetocin is 15 µg. Therefore, patients in the infusion group would have received 15 µg in 1 min 30 sec and 30 µg (two times the ED95) in three minutes. Postpartum hemorrhage should therefore not be a concern related to the mode of administration of carbetocin as long as the full recommended 100 µg dose is administered.
Boluses of oxytocin and carbetocin increase maternal cardiac output and, to a lesser extent, stroke volume through uterine contraction-induced autotransfusion and tachycardia.4,7,10 The phenylephrine-induced increase in systemic vascular resistance as well as decrease in stroke volume and cardiac output are well described, are dose-dependent, and have been shown to partially blunt the hemodynamic effects of oxytocin and possibly of carbetocin.10,16,17 Rosseland et al. observed a decrease in maternal blood pressure as well as an increase in cardiac output and stroke volume after a bolus of carbetocin 100 µg while using a phenylephrine infusion set at a rate of 0.25 µg·kg-1·min-1.4 In our trial, the phenylephrine infusion rate was set at 0.5 µg·kg-1·min-1, double that of the Rosseland et al. study. This higher rate may explain the greater blood pressure, and therefore cardiac output and stroke volume stability, observed in our participants compared with those of Rosseland et al. Interestingly, cardiac output and heart rate variation curves followed a parallel course (Figs 2a and 2c). Although we did not quantify this association, this observation may agree with the findings of Dyer et al. that heart rate is a surrogate marker of maternal cardiac output.10
Our study has limitations. We designed our study with very stringent exclusion and withdrawal criteria to minimize the influence of confounding factors, such as postpartum hemorrhage, additional uterotonics, and cardiotropic vasopressors, on maternal heart rate. This allowed us to better understand the specific effects of carbetocin on the study outcomes in a controlled and ideal setting. Among these criteria, we elected to withdraw parturients needing uterine exteriorization. In situ hysterotomy repair is a standard of care in our institution, notably because of its positive effects on patient comfort, maternal heart rate, and nausea. A recent study from our research team determined that exteriorization of the uterus is associated with significantly more tachycardia than in situ uterine repair is.18 We therefore wanted to control this heart rate variation factor in our study population. Nevertheless, this strict approach overestimates the effect of the treatment on the general population and therefore affects the external validity of our results. Caution is therefore warranted and further research on a larger and more diverse obstetrical population, including patients undergoing emergent Cesarean deliveries, those receiving general anesthesia, or those at risk of uterine atony, is needed before extending the findings of our study to general clinical practice. We elected to consider a heart rate difference of 10% as clinically significant in concordance with the literature.7 Whether this threshold is indeed clinically relevant to the anesthetic management of parturients presenting with a wide range of heart diseases is debatable as many other factors may predispose these patients to peripartum cardiac decompensation. Nevertheless, it remains clinically sound for the management of selected cardiac diseases such as mitral stenosis, where slow heart rate control is highly desirable.19 Moreover, this published threshold provides a basis for consistent outcome comparisons between studies. The ClearSight monitor is known to overestimate and poorly correlate with transthoracic echocardiographic cardiac output and stroke volume measurements in women in the third trimester of pregnancy.13 Therefore, maternal cardiac output and stroke volume measurements should be interpreted cautiously in relation to their trends rather than their absolute values. Moreover, systemic vascular resistance was not recorded by the ClearSight monitor. This hemodynamic parameter provides invaluable information about cardiovascular physiology changes and would have allowed for a more comprehensive understanding of the hemodynamic changes that occurred in our participants. We did not measure the degree of right hip wedge applied to our patients with the chest roll. Aortocaval compression may therefore be a confounding factor to consider when interpreting our results. Nevertheless, we maintained blood pressure within 80% of baseline values in all of our participants, suggesting that aortocaval compression was not clinically significant in most of our patients. Finally, the patients in the bolus group received carbetocin as a rapid bolus in a few seconds to reproduce a common practice in our institution and therefore increase the external validity of our results. Nevertheless, our study was neither designed nor powered to detect any adverse effects related to such a rapid bolus. Therefore, we cannot conclude, based on the results of our study, on the safety of a rapid intravenous bolus of carbetocin.
In conclusion, in healthy parturients undergoing a Cesarean delivery under spinal anesthesia with a prophylactic infusion of phenylephrine, our results show that a carbetocin infusion over ten minutes, rather than a rapid bolus, does not reduce the magnitude of maternal heart rate increase but delays its onset by a few minutes. This finding could be relevant to anesthesiologists caring for parturients in whom a slight increase in maternal heart rate is clinically undesirable. For instance, the clinician might consider halting the infusion momentarily until the maternal heart rate can be better controlled. Further research on the pharmacokinetic and clinical effects of infusions of carbetocin in a broader obstetrical population is warranted.
References
Leduc D, Senikas V, Lalonde AB. No. 235-Active management of the third stage of labour: prevention and treatment of postpartum hemorrhage. J Obstet Gynaecol Can 2018; 40: e841-55.
Ferring Inc. Product monograph including patient medication information_Duratocin® Carbetocin Injection 1 mL vial, 100 mcg / mL intravenous and intramuscular use uterotonic agent - revision 2020. Available from URL: https://www.ferring.ca/media/1253/eng-duratocin-vial-pm-eng-21feb2020.pdf (accessed December 2021).
Hunter DJ, Schulz P, Wassenaar W. Effect of carbetocin, a long-acting oxytocin analog on the postpartum uterus. Clin Pharmacol Ther 1992; 52: 60-7.
Rosseland LA, Hauge TH, Grindheim G, Stubhaug A, Langesaeter E. Changes in blood pressure and cardiac output during cesarean delivery: the effects of oxytocin and carbetocin compared with placebo. Anesthesiology 2013; 119: 541-51.
Thomas JS, Koh SH, Cooper GM. Haemodynamic effects of oxytocin given as i.v. bolus or infusion on women undergoing caesarean section. Br J Anaesth 2007; 98: 116-9.
Bhattacharya S, Ghosh S, Ray D, Mallik S, Laha A. Oxytocin administration during cesarean delivery: randomized controlled trial to compare intravenous bolus with intravenous infusion regimen. J Anaesthesiol Clin Pharmacol 2013; 29: 32-5.
Moertl MG, Friedrich S, Kraschl J, Wadsack C, Lang U, Schlembach D. Haemodynamic effects of carbetocin and oxytocin given as intravenous bolus on women undergoing caesarean delivery: a randomised trial. BJOG 2011; 118: 1349-56.
Nguyen-Lu N, Carvalho JC, Farine D, Seaward G, Ye XY, Balki M. Carbetocin at cesarean delivery for labour arrest: a sequential allocation trial to determine the effective dose. Can J Anesth 2015; 62: 866-74.
Kinsella SM, Carvalho B, Dyer RA, et al. International consensus statement on the management of hypotension with vasopressors during caesarean section under spinal anaesthesia. Anaesthesia 2018; 73: 71-92.
Dyer RA, Reed AR, van Dyk D, et al. Hemodynamic effects of ephedrine, phenylephrine, and the coadministration of phenylephrine with oxytocin during spinal anesthesia for elective cesarean delivery. Anesthesiology 2009; 111: 753-65.
Akkermans J, Diepeveen M, Ganzevoort W, van Montfrans GA, Westerhof BE, Wolf H. Continuous non-invasive blood pressure monitoring, a validation study of Nexfin in a pregnant population. Hypertens Pregnancy 2009; 28: 230-42.
Truijen J, van Lieshout JJ, Wesselink WA, Westerhof BE. Noninvasive continuous hemodynamic monitoring. J Clin Monit Comput 2012; 26: 267-78.
Duclos G, Hili A, Resseguier N, et al. Clearsight™ use for haemodynamic monitoring during the third trimester of pregnancy - a validation study. Int J Obstet Anesth 2018; 36: 85-95.
Rumboll CK, Dyer RA, Lombard CJ. The use of phenylephrine to obtund oxytocin-induced hypotension and tachycardia during caesarean section. Int J Obstet Anesth 2015; 24: 297-302.
Dell-Kuster S, Hoesli I, Lapaire O, et al. Efficacy and safety of carbetocin given as an intravenous bolus compared with short infusion for caesarean section - double-blind, double-dummy, randomized controlled non-inferiority trial. Br J Anaesth 2017; 118: 772-80.
Stewart A, Fernando R, McDonald S, Hignett R, Jones T, Columb M. The dose-dependent effects of phenylephrine for elective cesarean delivery under spinal anesthesia. Anesth Analg 2010; 111: 1230-7.
Langesaeter E, Rosseland LA, Stubhaug A. Continuous invasive blood pressure and cardiac output monitoring during cesarean delivery: a randomized, double-blind comparison of low-dose versus high-dose spinal anesthesia with intravenous phenylephrine or placebo infusion. Anesthesiology 2008; 109: 856-63.
Mireault D, Loubert C, Drolet P, et al. Uterine exteriorization compared with in situ repair of hysterotomy after cesarean delivery: a randomized controlled trial. Obstet Gynecol 2020; 135: 1145-51.
Arendt KW, Lindley KJ. Obstetric anesthesia management of the patient with cardiac disease. Int J Obstet Anesth 2019; 37: 73-85.
Author contributions
Marie-Ève Boisselle, Philippe Richebé, and Christian Loubert contributed to all aspects of this manuscript, including study conception and design; acquisition, analysis, and interpretation of data; and drafting the article. Valérie Zaphiratos contributed to study conception and design, analysis and interpretation of data, and drafting the article. Annik Fortier contributed to analysis and interpretation of data and drafting the article.
Disclosures
None.
Funding statement
This study is an independent investigator-initiated trial for which Dr. Loubert received a research fund from Ferring Canada in 2019. Dr. Valérie Zaphiratos was member of the Advisory Board for, and received honorariums from, Ferring Canada in 2020. Dr. Philippe Richebé received honorariums for lectures and as medical consultant from the following companies: Abbvie, Medtronic, Biosyent, Edwards, Merck, and Avirpharma.
Editorial responsibility
This submission was handled by Dr. Sheila Riazi, Associate Editor, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Boisselle, MÈ., Zaphiratos, V.V., Fortier, A. et al. Comparison of carbetocin as a bolus or an infusion with prophylactic phenylephrine on maternal heart rate during Cesarean delivery under spinal anesthesia: a double-blinded randomized controlled trial. Can J Anesth/J Can Anesth 69, 715–725 (2022). https://doi.org/10.1007/s12630-022-02227-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-022-02227-y