Abstract
Background
International practice guidelines make different recommendations for postoperative troponin testing to detect perioperative myocardial infarction and myocardial injury after noncardiac surgery. To gain insights into current testing patterns, we evaluated predictors of routine troponin testing after three commonly performed major noncardiac surgeries.
Methods
We conducted a population-based historical cohort study of adults having major orthopedic, colorectal, or vascular surgery in Ontario, Canada from 1 January 2010 to 31 December 2017. We used hierarchical logistic regression modelling to assess the association of patient, surgery, and hospital factors with postoperative troponin testing, while accounting for clustering at the hospital level. We characterized hospital-level variation by the intraclass correlation coefficient (ICC), which was adjusted for various characteristics.
Results
The cohort included 176,454 eligible patients. Hospital-specific adjusted testing rates ranged from 0–20.1% for orthopedic surgery, 0–43.8% for colorectal surgery, and 19.6–88.0% for vascular surgery. Older age, urgent surgery status, and surgery duration were consistently associated with higher rates of testing for all three surgeries. Higher Revised Cardiac Risk Index scores were associated with higher odds of testing for orthopedic and colorectal surgery, but not for vascular surgery. Even after adjustment, the ICCs were 9.2%, 7.4%, and 24.1% for orthopedic, general, and vascular surgery, respectively.
Conclusions
Troponin testing varied substantially across hospitals for selected major noncardiac surgery procedures even after accounting for differences in patient-level cardiac risk factors. Our observations lend support to a more standardized approach for troponin testing after noncardiac surgery.
Résumé
Contexte
Les directives de pratique internationales émettent différentes recommandations en ce qui concerne les dosages postopératoires de troponines afin de détecter l’infarctus du myocarde et les lésions myocardiques périopératoires après une chirurgie non cardiaque. Pour mieux comprendre les habitudes de test actuelles, nous avons évalué les prédicteurs de dosage de troponines de routine après trois chirurgies non cardiaques majeures couramment réalisées.
Méthode
Nous avons réalisé une étude de cohorte historique basée sur la population d’adultes bénéficiant d’une chirurgie orthopédique, colorectale ou vasculaire majeure en Ontario, au Canada, entre le 1er janvier 2010 et le 31 décembre 2017. Nous avons utilisé un modèle de régression logistique hiérarchique afin d’évaluer l’association des facteurs liés au patient, à la chirurgie et à l’hôpital avec les dosages de troponines postopératoires, tout en tenant compte des groupements au niveau hospitalier. Nous avons caractérisé la variation hospitalière par le coefficient de corrélation intraclasse (CCI), qui a été ajusté pour tenir compte de diverses caractéristiques.
Résultats
La cohorte comprenait 176 454 patients éligibles. Les taux de tests ajustés propres à l’hôpital variaient de 0 à 20,1 % pour les chirurgies orthopédiques, de 0 à 43,8 % pour les chirurgies colorectales et de 19,6 à 88,0 % pour les chirurgies vasculaires. Un âge plus avancé, un statut de chirurgie urgente et la durée de la chirurgie étaient systématiquement associés à des taux plus élevés de dosages pour les trois chirurgies. Des scores plus élevés sur l’Indice de risque cardiaque révisé étaient associés à des probabilités plus élevées de dosages pour les chirurgies orthopédiques et colorectales, mais pas pour les chirurgies vasculaires. Même après ajustement, les CCI étaient de 9,2 %, 7,4 % et 24,1 % pour les chirurgies orthopédiques, générales et vasculaires, respectivement.
Conclusion
Les dosages de troponines varient considérablement d’un hôpital à l’autre pour certaines interventions chirurgicales non cardiaques majeures, même après avoir pris en compte les différences dans les facteurs de risque cardiaques liés au patient. Nos observations appuient une approche plus standardisée des dosages de troponines après une chirurgie non cardiaque.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cardiovascular complications after noncardiac surgery are among the most common postoperative complications.1,2,3 As a result, there has been an increasing interest in detecting postoperative myocardial infarction (MI) and myocardial injury after noncardiac surgery (MINS) with routine postoperative troponin testing. Nevertheless, international practice guidelines differ with respect to recommendations for postoperative troponin testing. American guidelines previously only recommended responsive testing in patients with symptoms of myocardial ischemia.4 More recently, the American Heart Association published a scientific statement recommending postoperative surveillance in high-risk patients.5 European guidelines recommend routine testing in patients with elevated cardiovascular risk based on the Revised Cardiac Risk Index (RCRI) and surgical procedure.6 The Canadian Cardiovascular Society (CCS) recommends broader-scale postoperative troponin testing based on patients’ risk of experiencing cardiovascular events, which is factored based on RCRI, age, cardiovascular comorbidities, and surgical urgency.7,8 There is a paucity of information on what factors are important in determining which patients receive troponin testing after noncardiac surgery or on the degree of variation in testing in clinical practice. Whether testing is done in relation to patient risk, surgical factors, or hospital factors is not known. Understanding the factors driving postoperative troponin testing may help guide strategies to achieving more standardized testing in Canada in accordance to the CCS guidelines. Accordingly, we conducted a population-based cohort study in Ontario, Canada to evaluate hospital variation in postoperative troponin testing rates for three cohorts of commonly performed noncardiac surgeries (orthopedic surgery, colorectal surgery, and vascular surgery), to identify patient, surgical, and hospital factors that are associated with troponin testing. The included surgeries encompassed different underlying comorbidity profiles and differences in usual testing practice within these different surgical specialties.8
Methods
Design and data sources
We conducted a historical cohort study using routinely collected administrative and laboratory data in Ontario, Canada. The data were housed, linked using unique encoded identifiers, and analyzed at ICES (formerly known as the Institute for Clinical Evaluative Sciences) in Toronto, Ontario, Canada. ICES is an independent, non-profit research institute whose legal status under Ontario’s health information privacy law allows it to collect and analyze health care and demographic data, without consent, for health system evaluation and improvement.Footnote 1 The primary databases accessed included: 1) Ontario Laboratory Information System (OLIS), which is an Ontario-wide repository of hospital and community laboratory information in Ontario; 2) Canadian Institute for Health Information Discharge Abstract Database, which is a database containing administrative and clinical data on patients admitted to hospital and was used to identify hospitalizations, surgeries, and comorbidities; 3) Ontario Health Insurance Plan, which is a database containing all provincial physician fee-for-service billing; and 4) Statistics Canada, which contains census data used to identify neighbourhood income data. The use of data in this project was authorized under section 45 of Ontario’s Personal Health Information Protection Act, which does not require review by a research ethics board and allows for collection, analysis, and reporting without the need for patient consent.
Study population
The study population was adapted from a previous cohort of noncardiac surgery patients in Ontario.8 We included patients aged 40–105 yr undergoing inpatient orthopedic, colorectal, or vascular surgeries from 1 January 2010 to 31 December 2017. Patients in this age group were included because they likely represent a higher-risk cohort that may benefit from routine testing. Total hip and knee joint replacement procedures were included for orthopedic surgery; large bowel and/or rectal surgeries were included for colorectal surgery; and carotid endarterectomies, endovascular abdominal aortic aneurysm (AAA) repair, and open AAA repairs were included for vascular surgery. These surgeries were chosen because they represent commonly performed surgeries with different rates of major adverse cardiovascular outcomes (i.e., orthopedic surgeries are relatively low risk and vascular surgeries are relatively high risk) and with different rates of postoperative troponin testing.8 Surgeries were identified using Canadian Classification of Health Interventions codes in the Canadian Institute for Health Information Discharge Abstract Database (Electronic Supplementary Material [ESM] eTable 1). Patients were excluded if their surgery was performed at a hospital that was not reporting laboratory information to OLIS at the time of the study. Patients who died intraoperatively were excluded. We also excluded non-Ontario residents and patients with missing information (postal code and surgery duration).
Outcome
The primary outcome was troponin testing performed within two days of the operation. Troponin testing was ascertained using OLIS. All troponin assays (conventional, high-sensitivity, plasma, serum, and T and I isotypes) were included except for point-of-care testing.
Revised Cardiac Risk Index
The RCRI is an externally validated clinical prediction tool for major postoperative cardiovascular complications after elective noncardiac surgery.9 It is composed of the following six components (with one point given for each component met): 1) high-risk surgery, 2) ischemic heart disease, 3) heart failure, 4) cerebrovascular disease, 5) diabetes requiring preoperative insulin use, and 6) chronic kidney disease (creatinine > 176.8 µmol·L-1). High-risk surgery was defined as any surgery that was intraperitoneal, intrathoracic, or suprainguinal vascular surgery. Colorectal surgeries and AAA repair procedures met the high-risk component of the RCRI. Consistent with previous studies, chronic kidney disease (International Statistical Classification of Diseases and Related Health Problems 10th Revision [ICD-10]: N032-N037, N052-N057, N18, N19, N250, Z490-Z492, Z940, Z992) and diabetes10 were used as a surrogate for elevated creatinine and diabetes requiring preoperative insulin, respectively.11 History of ischemic heart disease was defined via ICD-10 I20-25 and history of cerebrovascular disease was defined via ICD-10 I60-64, G45. Heart failure was defined using a previously validated algorithm.12
Statistical analysis
Baseline characteristics are reported across strata based on surgery type and were compared using the Kruskal–Wallis test and Chi square test for continuous and categorical variables, respectively. Determination of which patients met the recommendations for testing was described previously8 and was based on the CCS guidelines.7 Briefly, the guidelines recommended testing in elective surgery if patients met any of these three criteria: 1) age of 65 yr or older, 2) significant cardiovascular disease, 3) RCRI ≥ 1. For urgent surgery, testing was recommended if patients met any of the following two criteria: 1) age 65 yr or older and 2) significant cardiovascular disease. For elective surgery patients, the guidelines also recommend preoperative brain natriuretic peptide (BNP) or N-terminal prohormone of BNP (NT-proBNP) testing to help risk-stratify patients. We did not have access to these laboratory values and thus interpreted the guidelines without BNP or NT-proBNP as described previously.8 We calculated hospital-specific proportions of patients who met CCS criteria for postoperative troponin testing, as well as the overall hospital-specific proportion of patients who underwent testing among those who met CCS criteria. To account for differences in patient case-mix across hospitals, we also calculated indirectly standardized hospital-specific testing rates using a logistic regression risk-adjustment model.13,14 The indirectly standardized rate was defined as
This regression model adjusted for patient age, sex, neighbourhood median income quintile, rural residency, and comorbidities (congestive heart failure, ischemic heart disease, cerebrovascular disease, anemia/blood disease, atrial fibrillation, cancer, chronic obstructive pulmonary disease, dyslipidemia, hypertension, and peripheral vascular disease).
Hierarchical logistic regression modelling was then used to evaluate the association between patient, surgery, and hospital characteristics with troponin testing for each surgery separately. Variables were selected a priori based on the clinical literature and expert opinion. Model parameters were estimated using maximum likelihood estimation, which was approximated via the adaptive Gaussian quadrature. Model-based standard errors were used to construct 95% confidence intervals (CIs). These models incorporated hospital-specific random effects to account for the clustering of patients within hospitals. We sequentially adjusted for 1) patient factors, 2) patient and surgical factors, and 3) patient, surgical, and hospital factors. Patient factors included demographics, median neighbourhood income quintile, rurality, RCRI, and comorbidities (i.e., anemia/blood disease, atrial fibrillation, cancer, chronic obstructive pulmonary disease, dyslipidemia, hypertension, and peripheral vascular disease). Surgical factors included urgency of admission, duration of surgery, and subtype of surgery. Hospital factors included hospital size (defined as number of beds) and teaching status. Odds ratios (OR) with 95% CIs represented the adjusted association of each covariate in the model with troponin testing. The intraclass correlation coefficient (ICC) and the median odds ratio (mOR) were also calculated. The ICC represents the proportion of the variation in troponin testing that is due to systematic between-hospital differences.15 It is computed as \(ICC= \frac{{V}_{A}}{{V}_{A} + \frac{{\pi }^{2}}{3}}\) where VA represents the hospital-level random effects variance. The mOR can be used to compare the magnitude of the effect of clustering on troponin testing. It is reported on the same scale as OR for other model covariates. We assessed changes in troponin testing over time using a sensitivity analysis whereby year of procedure was included in the model. The included vascular procedures (carotid endarterectomy, endovascular AAA repair, and open AAA repair) likely have different inherent perioperative cardiovascular risks, which in turn may influence the likelihood of troponin testing. As a sensitivity analysis, we assessed interhospital variation in testing rates and predictors of testing among the three vascular surgeries independently. All analyses were performed using SAS statistical software, version 9.4 (SAS Institute, Cary, NC, USA).
Results
Cohort assembly
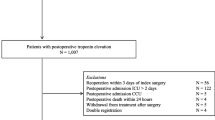
We identified 691,379 patients undergoing orthopedic, colorectal, and vascular procedures in Ontario (Fig. 1). We excluded patients based on age (< 40 or > 105; 31,108 patients), non-Ontario residency (N = 175), intraoperative death (N = 166), and missing information (location, surgical duration, hospital size; N = 6,869). After excluding hospitals that were not reporting laboratory information and selecting the subset of eligible orthopedic, colorectal, and vascular procedures, the final cohort consisted of 176,454 patients. Orthopedic procedures accounted for 79.1% (139,570/176,454) of the cohort, colorectal surgery accounted for 15.6% (27,530/176,454), and vascular surgery accounted for the remaining 5.3% (9,354/176,454). There were 58, 74, and 21 hospitals represented in the orthopedic, colorectal, and vascular cohorts, respectively.
Baseline characteristics
Mean (standard deviation [SD]) ages were 69.0 (10.6) yr for orthopedic, 67.2 (12.5) yr for colorectal, and 71.8 (9.1) yr for vascular procedures (Table 1). Male patients accounted for 39.4% of orthopedic procedures, 51.1% of colorectal procedures, and 75.2% of vascular procedures. Comorbidity burden was generally lowest for orthopedic surgery and highest for vascular surgery. If the CCS guidelines were applied to our cohort, 73.5%, 90.8%, and 95.6% of patients would have met recommendations for postoperative troponin testing for orthopedic, colorectal, and vascular surgeries, respectively. Nevertheless, only 6.7%, 16.6%, and 50.2% received troponin testing.
Hospital testing rates
Hospital-specific troponin testing rates ranged from 0% to 33.0% for orthopedic surgery (95% CI, 0 to 37.9), 0% to 38.1% for colorectal surgery (95% CI, 0 to 60.8), and 18.0% to 84.2% for vascular surgery (95% CI, 15.6 to 87.1) (Fig. 2). Indirectly standardized hospital-specific troponin testing rates varied from 0% to 20.1% for orthopedic surgery (95% CI, 0 to 22.6), 0% to 43.8% for colorectal surgery (95% CI, 0 to 64.4), and 19.5% to 88.0% for vascular surgery (95% CI, 16.1 to 92.0; ESM eFig. 1).
Hospital testing rates. Hospital-based troponin testing rates. Blue, green, and orange bars represent orthopedic, colorectal, and vascular surgeries, respectively. Each bar represents one hospital. “X” indicates hospitals with testing rates of 0%. Errors bars are derived via the binomial confidence interval.
Factors associated with troponin testing
Univariate analyses comparing patients who were tested to those who were not tested are represented in ESM eTable 2. In general, patients undergoing orthopedic and colorectal surgery that were tested were older, had higher RCRI scores, and had higher comorbidity burden for all comorbidities assessed than patients who were not tested. In contrast, for vascular surgeries there was no difference in age between tested and non-tested groups.
Factors associated with troponin testing in hierarchical regression models are shown in Fig. 3 and ESM eTable 3. For orthopedic surgery, compared with the reference age of 40–64, ages 65–79 and ≥ 80 were significantly associated with increased adjusted odds of troponin testing with OR of 1.57 (95% CI, 1.48 to 1.67) and 2.33 (95% CI, 2.17 to 2.50), respectively. Compared with RCRI scores of 1, the adjusted odds of undergoing troponin testing were lower for scores of 0 (OR, 0.72; 95% CI, 0.69 to 0.76), and higher for increasing RCRI scores of 2 (OR, 1.62; 95% CI, 1.49 to 1.75), 3 (OR, 2.15; 95% CI, 1.89 to 2.45), and ≥ 4 (OR, 3.30; 95% CI, 2.60 to 4.19).
Forest plot. The dotted vertical line represents an odds ratio of 1. Squares represent the adjusted odds ratio of the covariate. Odds ratios of greater than 1 indicate increased odds of receiving troponin postoperatively and odds ratios of less than 1 indicate decreased odds of receiving troponin postoperatively. Error bars represent the 95% confidence interval.
For colorectal surgery, compared with ages 40–64, ages of 65–79 and ≥ 80 were associated with increased adjusted odds of troponin testing with OR of 1.46 (95% CI, 1.34 to 1.59) and 1.90 (95% CI, 1.72 to 2.10), respectively. Relative to an RCRI score of 1, the adjusted odds of testing were higher for RCRI scores of 2 (OR, 1.39; 95% CI, 1.29 to 1.51), 3 (OR, 2.54; 95% CI, 2.24 to 2.87), and ≥ 4 (OR, 3.00; 95% CI, 2.50 to 3.61).
For vascular surgery, compared with ages 40–64, ages 65–79, and ≥ 80 were associated with increased adjusted odds troponin testing with OR of 1.19 (95% CI, 1.05 to 1.35) and 1.21 (95% CI, 1.04 to 1.42), respectively. Relative to the RCRI score of 1, only an RCRI score ≥ 4 was associated with an OR of 1.44 (95% CI, 1.10 to 1.90). Type of vascular surgery was also significantly associated with testing with an OR of 1.12 (95% CI, 0.97 to 1.29) for endovascular AAA repair and 2.98 (95% CI, 2.54 to 3.49) for open AAA repair compared with carotid endarterectomies.
Hospital-level variation
Hospital-level variation in testing rates is reported in Table 2. The ICC for the null models (i.e., models that did not adjust for any patient, surgical, or hospital factors) were 12.3%, 7.6%, and 25.2% for orthopedic, colorectal, and vascular surgeries, respectively (P < 0.001). When all variables were included in the models, the ICC was 9.2%, 7.4%, and 24.1% for orthopedic, colorectal, and vascular surgeries, respectively (P < 0.001). Thus, 24.1% of the between-hospital variation in troponin testing after vascular surgery was due to systematic between-hospital differences, even after adjusting for measured patient, surgery, and hospital characteristics. The mORs for the null models were 1.91, 1.64, and 2.73 for orthopedic, colorectal, and vascular surgeries, respectively. After adjusting for patient, surgical, and hospital factors, the mORs were 1.73, 1.63, and 2.65, respectively. For orthopedic surgeries, this can be conceptualized as for all pairwise hospital comparisons; the mOR associated with troponin testing when comparing the lower testing hospital to the higher testing hospital is 1.73.
Sensitivity analyses
Restricting our sample to the years 2014–2017 did not substantially affect the results (ESM eTable 4). When stratifying vascular surgeries by surgery type (i.e., endovascular AAA repair, open AAA repair, and carotid endarterectomy), we also observed substantial hospital variation in testing rates (ESM eFig. 2) and similar levels of between-hospital variation remain unexplained after adjustment (ESM eTable 5). To test whether correlation among Toronto hospitals was biasing the results, we ran sensitivity analyses where all Toronto hospitals were grouped within one cluster. The ICC and mOR were 11.6% and 1.87 for orthopedic procedures, 9.23% and 1.73 for colorectal procedures, and 19.7% and 2.36 for vascular procedures.
Discussion
This population-based study of Ontario residents undergoing orthopedic, colorectal, or vascular surgery sought to characterize hospital-level variation in postoperative troponin testing, as well as patient-, surgery- and hospital-level factors that are predictive of testing. We found significant hospital-level variation in postoperative troponin testing for the three groups of surgical procedures included in this study. We also showed that while patient-level cardiovascular risk (characterized by the RCRI) was predictive of postoperative testing following orthopedic and colorectal surgery, it was less predictive of testing after vascular surgery. In addition, there were substantial differences in the odds of receiving testing even after accounting for all measured patient-level, surgery-level, and hospital-level characteristics, much more so that one would expect due to variation of cardiac symptoms or MINS/MI after surgery.
Of the factors we assessed in the models, older age, cardiovascular risk (via RCRI), urgency of surgery, and surgical duration were associated with higher rates of troponin testing for the three surgeries we assessed. Patients who are elderly or have higher burden of comorbidities are more likely to experience MI, which may be reflected in the increased rate of testing in these groups.16,17 Nevertheless, among vascular surgeries, cardiovascular risk (via RCRI) was not strongly associated with testing. The lack of association between RCRI and testing in vascular surgeries may be because patients were at high cardiovascular risk already and thus the impact of RCRI was less influential than other covariates compared with patients undergoing orthopedic or colorectal surgeries. Consistent with what would be expected with a higher risk cohort, much higher rates of troponin testing were also observed for vascular procedures, such that over 50% of patients received testing postoperatively, compared with 6.7% and 16.6% of orthopedic and colorectal patients, respectively.
We showed that unadjusted hospital testing rates varied significantly for the surgeries we assessed. Even after adjusting for patient factors to account for differences in hospital case mix, the variation in testing rates across hospitals remained high, suggesting that hospital-level factors—not differences in patient case mix—are strong contributors to the likelihood of troponin testing. This was characterized by relatively high ICCs. For example, we observed an ICC of 24.1% for vascular procedures. This indicates that, after adjustment for patient, surgical, and hospital characteristics, 24.1% of between-hospital variation was due to systematic between-hospital differences. Moreover, observed mORs varied between 1.6 and 2.7—indicating that hospitals testing rates were different if patients with the same characteristics are admitted to different hospitals. While we do not know why there was substantial variation in the use of troponin testing between hospitals, several reasons should be considered. First, our study was done prior to the change in CCS guidelines, so variable uptake of the guidelines it is unlikely to have affected the observed between-hospital variation. Moreover, our study period was prior to the VISION studies that showed the prognostic significance of postoperative troponin in noncardiac surgery. Second, the degree of hospital-level variation that we observed was similar to what has been shown for other preoperative testing and consults.11, 18 For example, preoperative electrocardiography and chest radiography use was associated with mORs of 2.3 and 1.6 respectively, indicating that the decision to use troponin testing might be influenced by the preference of the surgeons.11 Third, the prognostic value of postoperative troponins has been debated, especially during the study period. While the seminal VISION studies have showed a large proportion of perioperative MI and MINS, the evidence on how to manage these patients was limited. This is supported by the American Heart Association/American College of Cardiology guidelines that specifically recommend against the use of routine troponin testing postoperatively and may in fact be contributing to low testing rates in Canada. More recently, with the publication of the MANAGE trial,19 dabigatran has been identified as a potential medical therapy for patients with MINS. While this provides some needed insight into potential medical management, further research on efficacious management strategies for patients with MINS is needed.
Limitations
This study should be interpreted in the context of its limitations. First, as this study used administrative and laboratory databases, we lacked data on patient symptoms that may have led to postoperative troponin testing. Nevertheless, large observational studies have shown that ischemic symptoms after surgery are uncommon, occurring in 6% of the patients who have myocardial injury.3 Moreover, while our study shows substantial hospital-level variation, we were unable to identify which hospital-level factors are contributing to this due to limited hospital-level variables available in our data. Second, the data for this study span a seven-year period where changes in practice patterns may have occurred. We assessed if there were time varying effects by including year in a sensitivity analysis, which did not change our estimates. Third, most of the data for this study predates the 2017 CCS guidelines and as such, this study cannot make inferences on factors associated with testing in the context of the guidelines. Nevertheless, guideline adoptions rates are known to be slow and we have previously shown that in the year subsequent to the guidelines, there were no changes in troponin testing in Ontario. Fourth, we lacked physician information for this study so were not able to cluster at that level. Despite this, we clustered at the hospital level, as we hypothesized that hospital-specific practices and culture are more important factors for postoperative troponin testing. Finally, the reader is reminded that when incidences of the outcome are more common (i.e., > 10%) the OR is not a reasonable approximation of the relative risk and should not be interpreted as such.
Conclusions
In this large population-based study, we showed that troponin testing is influenced by patient, surgical, and hospital factors. Between-hospital variation is one of the strongest contributors to how routinely postoperative troponin testing after noncardiac surgery is performed. While patient-level cardiovascular risk is a major driver of testing in orthopedic and colorectal surgeries, it has less influence on testing in vascular surgeries.
Notes
About ICES. Available from URL: https://www.ices.on.ca/About-ICES (accessed January 2022).
References
Vascular Events In Noncardiac Surgery Patients Cohort Evaluation (VISION) Study Investigators; Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307: 2295-304.
Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120: 564-78.
Writing Committee for the VISION Study Investigators; Devereaux PJ, Biccard BM, Sigamani A, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2017; 317: 1642-51.
Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 130: e278-333.
Ruetzler K, Smilowitz NR, Berger JS, et al. Diagnosis and management of patients with myocardial injury after noncardiac surgery: a scientific statement from the American Heart Association. Circulation 2021; 144: e287-305.
Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: the Joint Task Force on Non-Cardiac Surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014; 35: 2383-431.
Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society Guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol 2017; 33: 17-32.
Azizi PM, Wijeysundera DN, Wijeysundera HC, Han L, Koh M, Ko DT. Troponin testing after non-cardiac surgery in Ontario: an observational study. CJC Open 2021; https://doi.org/10.1016/j.cjco.2021.03.002.
Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100: 1043-9.
Lipscombe LL, Hwee J, Webster L, Shah BR, Booth GL, Tu K. Identifying diabetes cases from administrative data: a population-based validation study. BMC Health Serv Res 2018; https://doi.org/10.1186/s12913-018-3148-0.
Wijeysundera DN, Beattie WS, Elliot RF, Austin PC, Hux JE, Laupacis A. Non-invasive cardiac stress testing before elective major non-cardiac surgery: population based cohort study. BMJ 2010; https://doi.org/10.1136/bmj.b5526.
Schultz SE, Rothwell DM, Chen Z, Tu K. Identifying cases of congestive heart failure from administrative data: a validation study using primary care patient records. Chronic Dis Inj Can 2013; 33: 160-6.
Hosmer DW, Lemeshow S. Confidence interval estimates of an index of quality performance based on logistic regression models. Stat Med 1995; 14: 2161-72.
Austin PC, Alter DA, Tu J V. The use of fixed- and random-effects models for classifying hospitals as mortality outliers: a Monte Carlo assessment. Med Decis Making 2003; 23: 526-39.
Merlo J, Chaix B, Ohlsson H, et al. A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health 2006; 60: 290-7.
Rathore V, Singh N, Mahat RK. Risk factors of acute myocardial infarction: a review. EJMI 2018; 2: 1-7.
Anand SS, Islam S, Rosengren A, et al.; INTERHEART Investigators. Risk factors for myocardial infarction in women and men: insights from the INTERHEART study. Eur Heart J 2008; 29: 932-40.
Wijeysundera DN, Austin PC, Beattie WS, Hux JE, Laupacis A. Variation in the practice of preoperative medical consultation for major elective noncardiac surgery: a population-based study. Anesthesiology 2012; 116: 25-34.
Devereaux PJ, Duceppe E, Guyatt G, et al.; MANAGE Investigators. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391: 2325-34.
Author contributions
Paymon M. Azizi, Duminda N. Wijeysundera, Harindra C. Wijeysundera, Peter C. Austin, and Dennis T. Ko contributed to all aspects of this manuscript, including study conception and design; acquisition, analysis, and interpretation of data; and drafting the article. Angela Jerath contributed to the interpretation of data. Lu Han and Maria Koh contributed to the analysis of data.
Acknowledgements
This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health (MOH) and the Ministry of Long-Term Care (MLTC). This study also received funding from CIHR. Parts of this material are based on data and information compiled and provided by CIHI and Ontario MOH. The analyses, conclusions, opinions and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred.
Disclosures
None.
Funding statement
The study is partly supported by a Canadian Institutes of Health Research grant. Additional funding was provided by Foundation Grants (FDN 154333). Dr. Dennis Ko is also supported by a Merit Award by the Department of Medicine, University of Toronto. Dr. Peter Austin is supported by a Mid-Career Award from the Heart and Stroke Foundation of Canada (HSFC). Dr. Duminda Wijeysundera is supported in part by the Endowed Chair in Translational Anesthesiology Research at St. Michael’s Hospital and University of Toronto. Drs Duminda Wijeysundera and Jerath are supported by Merit Awards from the Department of Anesthesiology and Pain Medicine at the University of Toronto. Dr. Harindra Wijeysundera is supported as a Canada Research Chair in health services research and by a phase 2 Clinician Scientist Award from the HSFC.
Editorial responsibility
This submission was handled by Dr. Stephan K. W. Schwarz, Editor-in-Chief, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is accompanied by an editorial. Please see Can J Anesth 2022; this issue.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Azizi, P.M., Wijeysundera, D.N., Wijeysundera, H.C. et al. Troponin testing after noncardiac surgery: a population-based historical cohort study on variation and factors associated with testing in Ontario. Can J Anesth/J Can Anesth 69, 572–581 (2022). https://doi.org/10.1007/s12630-022-02219-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-022-02219-y