Abstract
Purpose
Programmed intermittent epidural bolus (PIEB) provides better analgesia for labour pain than continuous epidural infusion does. Nevertheless, commonly used PIEB regimens are associated with high sensory block. We hypothesized that a PIEB technique with slower bolus delivery speed would produce lower sensory levels.
Methods
We recruited term nulliparous women with singleton pregnancies during the first stage of labour. All participants had an American Society of Anesthesiologists Physical Status score of II-III, had epidural catheters placed at L3/4, and had epidural analgesia maintained with PIEB 10 mL every 40 min using 0.0625% bupivacaine with fentanyl 2 µg·mL-1. Women were randomized to receive PIEB delivered at 250 mL·hr-1 (G250) or 125 mL·hr-1 (G125). The study was completed six hours after the loading dose or at full cervical dilatation, whichever occurred first. The primary outcome was the presence of sensory block to ice ≥ T6 in at least one assessment during the study period (maximum six hours).
Results
We analyzed data from 90 women. The proportion of women presenting sensory block ≥ T6 at any time was not different between G125 and G250 groups (60.0% vs 64.4%; difference, -4.4%; 95% confidence interval [CI], -24.5 to 15.6; P = 0.66). The median [interquartile range] highest sensory block level was also not different between G125 and G250 groups (T6 [T7-T5] vs T5 [T7-T5], P = 0.39). Women in the G125 group had a lower incidence of hypotension than women in the G250 group did (11.1% vs 33.3%; difference, -22.2%; 95% CI, -38.8 to -5.67; P = 0.01). Quality of analgesia and patient satisfaction were not different between groups.
Conclusion
The maintenance of epidural analgesia with a PIEB delivery speed of 125 mL·hr-1 did not produce lower sensory block levels when compared with 250 mL·hr-1. The slower injection speed regimen was associated with lower incidence of hypotension, but this secondary finding warrants confirmation in a future trial.
Trial registration
www.ClinicalTrials.gov (NCT03236298); registered 1 August 2017.
Résumé
Objectif
L’administration programmée intermittente de bolus périduraux (PIEB, pour programmed intermittent epidural bolus) fournit une meilleure analgésie pour la douleur du travail que l’analgésie péridurale par perfusion continue. Néanmoins, les régimes de PIEB couramment utilisés sont associés à un bloc sensoriel élevé. Nous avons émis l’hypothèse qu’une technique de PIEB avec une vitesse d’administration plus lente du bolus produirait des niveaux sensoriels inférieurs.
Méthode
Nous avons recruté des femmes nullipares à terme ayant des grossesses uniques au cours de la première étape du travail obstétrical. Toutes les participantes avaient un score de statut physique II-III de l’American Society of Anesthesiologists, des cathéters périduraux placés au niveau L3/4 et une analgésie péridurale maintenue avec des PIEB de 10 mL de bupivacaïne 0,0625 % et de 2 μg·mL-1 de fentanyl, administrés toutes les 40 minutes. Les femmes ont été randomisées à recevoir des PIEB administrés à une vitesse de 250 mL·h-1 (G250) ou 125 mL·h-1 (G125). L’étude se terminait six heures après la dose de charge ou lors de la dilatation cervicale complète, selon la première éventualité. Le critère d’évaluation principal était la présence d’un bloc sensoriel à la glace ≥ T6 lors d’au moins une évaluation au cours de la période à l’étude (pour un maximum de six heures).
Résultats
Nous avons analysé les données de 90 femmes. La proportion de femmes présentant un bloc sensoriel ≥ T6 à tout moment n’était pas différente entre les groupes G125 et G250 (60,0 % vs 64,4 %; différence, -4,4 %; intervalle de confiance [IC] à 95 %, -24,5 à 15,6; P = 0,66). Le niveau médian [écart interquartile] le plus élevé de bloc sensoriel n’était pas non plus différent entre les groupes G125 et G250 (T6 [T7-T5] vs T5 [T7-T5], P = 0,39). Les femmes du groupe G125 avaient une incidence d’hypotension plus faible que les femmes du groupe G250 (11,1 % vs 33,3 %; différence, -22,2 %; IC 95 %, -38,8 à -5,67; P = 0,01). La qualité de l’analgésie et la satisfaction des patientes n’étaient pas différentes d’un groupe à l’autre.
Conclusion
Le maintien de l’analgésie péridurale avec une vitesse d’administration des PIEB de 125 mL·h-1 n’a pas entraîné de taux de blocs sensoriels inférieurs par rapport à une vitesse de 250 mL·h-1. Le régime de vitesse d’injection plus lente a été associé à une incidence plus faible d’hypotension, mais cette constatation secondaire mérite d’être confirmée dans une étude future.
Enregistrement de l’étude
www.ClinicalTrials.gov (NCT03236298); enregistrée le 1er août 2017.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Programmed intermittent epidural bolus (PIEB) is a technique of epidural analgesia based on the automatic delivery of boluses of local anesthetic into the epidural space at fixed time intervals.1 Previous studies have shown that PIEB can decrease breakthrough pain2,3,4,5,6,7,8 and motor block,4 increase maternal satisfaction,1,5,7 and reduce local anesthetic consumption,1,2,4,5,6 compared with continuous epidural infusion.
Little attention has been paid to the actual pattern of epidural spread associated with this technique. From our previous studies, the PIEB technique seems to be associated with high sensory block levels.9,10,11,12,13 Although the high sensory block levels associated with PIEB have not been associated with clinically significant adverse events, a sensory block above T10 is unnecessary for labour analgesia. On a more practical note, at our institution, the current monitoring guidelines during labour epidurals require that the anesthesiologist should be informed when a sensory block ≥ T6 is encountered by the nursing team. Thus, optimizing the PIEB regimen with a balance between risks and benefits is important.
Local anesthetic distribution/spread in the epidural space is influenced by multiple factors, such as individual characteristics, patient positioning, volume and local anesthetic concentration, injection level, and epidural injection pressure and epidural space compliance among others.14 Our group has conducted three clinical studies attempting to optimize our PIEB regimen for labour analgesia by modifying bolus intervals, bolus volumes, and local anesthetic concentration. None of these changes decreased the incidence of high sensory block levels without compromising the analgesic effect.9,10,11,12 In vivo and in vitro studies support the idea that faster delivery rates and higher administration pressures may lead to more extensive spread of local anesthetic into the epidural space.13,15,16,17 Lange et al. have compared delivery speeds of 100 and 300 mL·hr-1 and were unable to find significant clinical differences but did not specifically address the pattern of sensory block.13 As all of our previous studies were conducted with an injection speed of 250 mL·hr-1, we thought of adjusting the PIEB delivery speed in an attempt to confine the epidural spread within appropriate levels.
The objective of this study was to investigate if slower delivery rates of the PIEB bolus could decrease the epidural spread of a 10-mL bolus of bupivacaine 0.0625% plus fentanyl 2 µg·mL-1, when administered at 40-min intervals in nulliparous women undergoing labour epidural analgesia during the first stage of labour. We hypothesized that reducing the PIEB delivery speed would reduce the incidence of women presenting an upper sensory block level ≥ T6.
Methods
The study was a triple-blind randomized controlled trial, approved by the Research Ethics Board at Mount Sinai Hospital (REB#17-0157-A, Toronto, ON, Canada) and registered at ClinicalTrials.gov on 1 August 2017 (NCT03236298). Written informed consent for study participation was obtained from each patient.
We recruited nulliparous women with a singleton fetus in vertex presentation, gestational age ≥ 37 weeks, with an American Society of Anesthesiologists Physical Status score of II and III, who were labouring (regular uterine contractions at least every five minutes with progressive cervical dilation), had a cervical dilation between 2 and 5 cm, and describing verbal numerical rating scores (VNRS) of ≥ 5 when requesting epidural analgesia (on a scale where 0 = no pain and 10 = worst imaginable pain). Exclusion criteria were any contraindication to epidural analgesia; unintentional dural puncture; allergy to lidocaine, bupivacaine or fentanyl; use of pharmacological analgesics within four hours of epidural request; and refusal to participate in the trial.
An intravenous infusion of 250 mL of lactated Ringer’s solution was administered during epidural catheterization. The epidural catheter was placed with patients in the sitting position, and the epidural puncture site was determined by pre-procedural spinal ultrasound assessment. After skin preparation with chlorhexidine 2% in alcohol 70%, skin was infiltrated with lidocaine 2%. A 17G Tuohy needle was inserted in the L3/4 interspace using loss of resistance to air or saline at the discretion of the anesthesiologist. A closed-end, multi-orifice, wire-reinforced epidural catheter (Arrow FlexTip plus; Arrow International Inc., Reading, PA, USA) was advanced 5 cm into the epidural space with the needle bevel oriented cephalad and secured. Patients were then positioned supine with left uterine displacement and 30° head-of-bed elevation. Three minutes after a 3-mL test dose of bupivacaine 0.125% with fentanyl 3.3 µg·mL-1 was administered, a 12-mL loading dose of the same solution was injected in 6-mL increments, three minutes apart.
The eligibility criterion to continue in the study was to achieve VNRS ≤ 1/10 within 20 min after administration of the loading dose was completed. The CADD-Solis Ambulatory Infusion System (Smith Medical, St. Paul, MN, USA) was set with the bolus delivery speed either at 125 or 250 mL·hr-1 (10 mL given in 4.8 min or 2.4 min). Patients were randomly allocated to two groups using block randomization with a block size of 4 at the time of enrolment: group 125 (G125) with a bolus delivery speed of 125 mL·hr-1 and group 250 (G250) with a bolus delivery speed of 250 mL·hr-1. The randomization scheme was prepared by our biostatistician and was implemented by a research assistant not participating in patient assessment. The attending nurse connected the epidural catheter to the CADD system. Analgesia was maintained with 0.0625% bupivacaine with 2 µg·mL-1 fentanyl. The PIEB boluses were fixed at 10 mL, and the PIEB interval was fixed at 40 min. The first bolus was initiated 60 min after administration of the loading dose was completed. Patients had the option of using 5-mL patient controlled epidural analgesia (PCEA) boluses, with a ten-minute lockout interval and an hourly maximum of 30 mL. Patients were educated to use a PCEA button only when uncomfortable. In case of a patient complaining of ineffective pain management with the rescue of PCEA, a nurse could administer a top-up of 10 mL of 0.125% bupivacaine. If ineffective, the in-charge anesthesiologist managed the pain with a rescue bolus of 5 mL of bupivacaine 0.25% followed by another 5 mL of the same solution if necessary. If the patient did not respond to the rescue top-ups, they were considered ineligible for study inclusion and were further managed as per the staff anesthesiologist’s preference.
Sensory block to ice was assessed bilaterally at the mid-clavicular line using an ice bag from caudad to cephalad, and the upper sensory block level was recorded as the highest dermatome at which the patient still did not feel normal sensation compared with at the frontal part of the head or cheek. Sensory block to pinprick was assessed in a similar fashion using a hand-held Neurotip (Owen Mumford Ltd, Oxfordshire, UK). Upper sensory block to ice and pinprick, pain score (VNRS 0-10 where 0 = no pain and 10 = worst pain imaginable), noninvasive blood pressure, and motor block were assessed at 20 and 60 min after completing the loading dose, and every 60 min until the end of the study. Motor block was assessed by the modified Bromage score (0 = able to raise extended legs; 1 = unable to raise extended legs but able to flex knees; 2 = unable to flex knees but able to flex ankle; 3 = unable to flex ankle). At the last assessment, patients were asked about their satisfaction with the labour epidural analgesia (rating from 0 to 10, 0 = not satisfied at all, 10 = fully satisfied). The study continued for six hours after the loading dose or until the cervix was fully dilated, whichever occurred first. We chose 360 min for this study period because this PIEB regimen study focused on the first stage of labour, as in our previous studies.9,10,11,12 The data of PCEA demands and rescue boluses were extracted from the pump history by the investigator at the end of the study. Hourly local anesthesia consumption was calculated from the average of total consumption of bupivacaine during the study period without the loading dose.
Baseline noninvasive blood pressure was recorded as the mean of three measurements between uterine contractions. Maternal hypotension was defined as a 20% decrease from baseline systolic blood pressure and was treated with a 250-mL lactated Ringer’s rapid infusion and/or 5 mg of intravenous ephedrine at the discretion of the anesthesiologist.
Outcomes
The primary outcome was the presence of a sensory block level to ice ≥ T6 for a patient at any time during the study period. Secondary outcomes included highest upper sensory block level to ice and pinprick; hourly upper sensory bock levels to ice and pinprick; hourly degree of motor block; pain scores; hourly consumption of local anesthetic; and presence of hypotension.
Statistics
Data from this randomized control trial were analyzed on an intention-to-treat basis. We compared the incidence of the primary outcome between the two groups using the Chi square test. The difference in the incidence between two groups was estimated using linear probability model. The secondary outcomes were compared between the two groups using the Wilcoxon rank sum test for continuous variables and the Chi square test or Fisher’s exact test (when more than 20% of cells had expected frequencies < 5) for categorical variables. In a further post hoc exploratory analysis, we calculated the ratio of sensory block assessment equal or greater than T6 (R) for each patient and compared the rate of R ≥ 25% (at least 25% of sensory block assessment ≥ T6) between the two treatment groups using the Chi square test. To estimate the difference in the incidence or median for the secondary outcomes between two groups, including the presence of R ≥ 25%, the linear probability model and quantile regression model were applied respectively. The difference in the distribution of the upper sensory block to ice and pinprick between two groups was also assessed graphically. No adjustment for multiple comparisons for secondary outcomes was conducted. Data were managed using SAS® software (SAS Institute Inc., Cary, NC, USA) and a two-sided P value of 0.05 was used to determine statistical significance.
The sample size justification was based on the primary outcome and the hypothesis. Our primary outcome was the presence of a sensory block level to ice ≥ T6 at any time during the study. From our previous study, the rate of the primary outcome was reported as 66%.9 It was our hypothesis that there would be a 50% decrease in the primary outcome in the intervention group (G125) compared with in the control group (G250). We estimated that a total of 80 patients (40 for each group) would be required to achieve 80% power to detect the 50% reduction in the rate of primary outcome (from 66% to 33%), assuming a two-sided significance level of 0.05. The Chi square test was used for the power analysis. We planned to recruit 90 patients (45 for each group) to account for possible data loss no greater than 10%.
Results
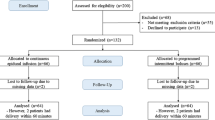
The study was conducted between 1 August 2017 and 22 January 2020. Ninety women were recruited and randomized evenly into two treatment groups (Fig. 1). Data from the 90 women were analyzed. Of the 90 women, nine (10%) violated the protocol (Fig. 1) and had missing outcomes. Since we had accounted for 10% loss to follow-up in our sample size estimation, no permutation for the missing data was conducted.
Patient demographics, as well as anesthetic and obstetric data, are presented in Table 1. The distribution of sensory block to ice during the study is shown in Table 2. The incidence of sensory block to ice ≥ T6 at any time during the study was not statistically different between the two treatment groups (G125 60.0% vs G250 64.4%; difference, -4.4%; 95% confidence interval [CI], -24.5 to 15.6; P = 0.66,), nor was the mean [interquartile range] highest sensory block level (T6 [T7-T5] vs T5 [T7-T5], P = 0.39). Nevertheless, in an exploratory analysis, the incidence of women presenting sensory block to ice ≥ T6 in more than 25% of the assessments was significantly lower in the G125 than in the G250 group (31.1% vs 53.3%; difference, -22.2%; 95% CI, -42.1 to -2.3; P = 0.03). A similar result was obtained for the sensory block to pinprick (Table 3). Besides, the analysis results for the case when only included the sensory block levels assessed between 2 and 6 hours after completion of the loading dose were consistent (Electronic Supplementary Material; eTable 1 and eTable 2). Of note, each participant had 14 (range, 10-14) sensory assessments during the study period. The overall incidence of hypotension was significantly lower in G125 (11.1% vs 33.3%; difference, -22.2%; 95% CI, -38.8 to -5.7; P = 0.01), but the need for pharmacological treatment was not statistically different.
There was no difference in quality of analgesia, hourly local anesthetic consumption, and patient satisfaction (Table 4).
Discussion
Our results show that PIEB with a bolus delivery speed of 125 mL·hr-1 did not reduce the incidence of sensory block levels ≥ T6 compared with 250 mL·hr-1. Furthermore, the quality of labour analgesia and overall patient satisfaction were not different. The slower delivery speed determined a lower overall incidence of hypotension; however, the incidence of hypotension requiring treatment was similar in both groups.
The injection of local anesthetic solutions into the epidural space generates a circumferential spread that bathes the epidural space all around and is thought to be the most effective in producing analgesia. A non-circumferential, irregular spread creates asymmetric, less effective analgesia. Hogan showed this in human cadavers and speculated that the epidural solution would spread from the high injection pressure area (injection point) to the more distant areas with low pressure.15 Mowat et al. observed in pigs that manual boluses were associated with increased longitudinal spread of local anesthetic along the epidural space compared with continuous epidural infusion.16 The authors highlighted in the discussion of their results that the injection pressure generated by manual bolus was far greater than that generated by continuous infusion and concluded that the wide epidural spread correlated positively with high injection pressure. Oliver et al. investigated dye distribution into cadaveric porcine epidural space using multiple infusion pumps.17 Although this was a small study, an infusion rate of 125 mL·hr-1 produced less extensive epidural spread compared with 500 mL·hr-1. Of note, all these studies were conducted in vitro or in animal models, and the results cannot be directly applied to the clinical setting.
In clinical studies, the effects of the injection of the local anesthetic solution into the epidural space on the epidural pressure and resulting epidural spread are not fully understood. Usubiaga et al. reported that the high residual epidural pressure rather than the peak pressure determined the upper level of epidural analgesia.18 Bromage proposed the concept of epidural compliance, which decreases with advancing age, and argued that the small epidural compliance in the elderly might lead to a wider epidural spread.14 Contrary to their points of view, Hirabayashi et al. suggested that higher epidural compliance is related to wider epidural spread,19 and Paul and Wildsmith concluded that there is no correlation between either peak or residual epidural pressure and sensory block.20 Cardoso et al. also investigated the relationship between epidural pressure and epidural spread, and observed that although faster epidural injection generated higher peak epidural pressure than slower epidural injection, the pressure dramatically decreased in 30 seconds after the cessation of the injection in both groups.21 To summarize these studies, we can only say that epidural pressure is, in part, related to the spread of sensory block. Nevertheless, based on the available literature, we cannot determine the exact relationship between epidural pressure and epidural spread.
As PIEB gains popularity in obstetric anesthesia, some researchers have investigated the correlation between bolus delivery speed and pressure generated at the proximal end of the epidural catheter in in vitro studies. Klumpner et al. found that increasing bolus delivery speed resulted in higher peak pressure, more so in closed-end multi-orifice catheters than in open-ended singe-orifice catheters.22 Similarly, Krawczyk et al. reported that smaller closed-end multi-orifice catheters and faster bolus delivery speed generated higher peak pressure.23 Both studies monitored the pressure connecting a three-way adapter to an epidural catheter, a digital pressure transducer and a PIEB system. As the distal end of the catheter was placed in atmospheric pressure, the results cannot be directly translated into clinical practice.
To the best of our knowledge, this is only the second randomized controlled trial investigating PIEB delivery speed and clinical outcomes. Lange et al. used a combined spinal-epidural technique using a single-orifice catheter.13 Labour neuraxial analgesia was maintained with PIEB of 10 mL every 60 min and PCEA of 5 mL with a lockout of ten minutes up to three times per hour, using 0.0625% bupivacaine with fentanyl 1.95 µg·mL-1. Similar to our results, they observed that bolus delivery speeds of 100 mL·hr-1 and 300 mL·hr-1 provided similar analgesia in labouring women. From the results of Lange’s and our current study, we may conclude that a bolus delivery speed in the range of 100-300 mL·hr-1 does not produce clinical differences in pain management during labour.
Lange et al. reported that both groups in their study showed similar highest sensory block to ice with a median of T6.13 Our results are in keeping with their findings. In our study, the incidence of any sensory block to ice ≥ T6 was also similar between the groups; however, in an exploratory analysis, our results showed that G125 was associated with fewer hourly assessments in which the sensory block was ≥ T6. As the median of episodes of sensory block assessments ≥ T6 was different in both groups, we conducted the comparison of at least 25% of sensory block assessment ≥ T6. Since the median of episodes of sensory block ≥ T6 was three out of 14 assessments in the entire study population, we chose a cut-off point of 25% in our study. The difference was subtle, but we could see that the block was consistently lower in G125 (Fig. 2). The significance of these findings will need to be assessed in further studies. It is possible that the sensory block levels in both groups were assessed at slightly different times in the PIEB cycle.
Upper sensory block level over time. Subtitle 2a: sensory block level to ice; subtitle 2b: sensory block level to pinprick. G250 = control group (250 mL·hr-1); G125 = intervention group (125 mL·hr-1). The circle and cross inside the bars represent mean value, and those outside the bars are outliers.
One interesting finding of our study is that the overall incidence of hypotension was lower in G125, although the number of women requiring treatment was similar. As overall sensory block levels to both ice and pinprick were not significantly different between the two groups, it is likely that the levels of sympathetic blockade were also similar in both groups. Given that hypotension was a secondary outcome, this finding warrants confirmation in future studies.
Our study has some limitations and also presents some differences when compared with the study of Lange et al.13 The sensory block assessment was done hourly in both studies, irrespective of the time of the PIEB cycle and the use of PCEA or manual bolus; hence, the sensory block levels may have been assessed at different times of the PIEB cycle in the studied groups. Nevertheless, in our study, the median number of PCEA boluses was 0-1 in both groups, so less likely to have influenced the PIEB interval compared with Lange et al. where PCEA was used nine to ten times. Our study was done with ultrasound assessment of the spine, ensuring a better comparison between groups, given that the level of puncture is known to influence the upper sensory block level. Also, as the Lange’s study showed no difference in the level sensory block between 100- and 300-mL·hr-1 PIEB delivery, it would be of interest to investigate whether even lower PIEB delivery speeds could decrease the incidence of higher neuraxial block without compromising labour analgesia, although we might not find a clinical difference between extremely slow PIEB delivery speeds and continuous infusion. Our study was limited to nulliparous women during the first stage of labour. We purposely avoided extending the study into second stage of labour, given that labour pain during second stage is more complex because of multiple confounders and that analgesic requirements may increase and modify the upper sensory block levels. Also, as we did not measure lower sensory block levels, we could not conclude that sacral coverage is the same in both groups.
Lastly, a limitation of our study is our very primary outcome. Although our choice was based on our nursing protocol of informing the anesthesiologist when the upper sensory block is ≥ T6, this was an arbitrary choice. Furthermore, we defined the upper sensory block level as one level below that where patients felt the sensation as same as an unanesthetized area. Some may argue that the real sensory block level should be that where patients first perceived any sensation of cold or pinprick moving from caudad to cephalad,24 which may be more clinically meaningful. This is a knowledge gap in the literature. Although the correlation between sensory block levels, assessed by different methods and techniques, and effective surgical anesthesia have been well studied, this particular topic has not been studied in labour analgesia. A T10 sensory level is quoted in the literature as required for effective analgesia during the first stage of labour, based on the original anatomic studies showing that sensory nerve fibres from the uterus enter the spinal cord at the T10-L1 levels.25 Nevertheless, it is unknown what level and density of sensory block assessed by ice or pinprick best correlates with effective pain control during the first stage of labour.
In summary, the use of PIEB with injection speeds of 125 mL·hr-1 and 250 mL·hr-1 were associated with similar sensory block levels and similar incidence of women presenting with sensory block level to ice ≥ T6. Quality of labour and patient satisfaction were not different. Our findings do not support any change in our current practice, but some of our exploratory findings, such as the smaller incidence of hypotension in the 125 mL·hr-1 group, warrant further investigation.
References
Xu J, Zhou J, Xiao H, et al. A systematic review and meta-analysis comparing programmed intermittent bolus and continuous infusion as the background infusion for parturient-controlled epidural analgesia. Sci Rep 2019; DOI: https://doi.org/10.1038/s41598-019-39248-5.
Lin Y, Li Q, Liu J, Yang R, Liu J. Comparison of continuous epidural infusion and programmed intermittent epidural bolus in labor analgesia. Ther Clin Risk Manag 2016; 12: 1107-12.
McKenzie CP, Cobb B, Riley ET, Carvalho B. Programmed intermittent epidural boluses for maintenance of labor analgesia: an impact study. Int J Obstet Anesth 2016; 26: 32-8.
Capogna G, Camorcia M, Stirparo S, Farcomeni A. Programmed intermittent epidural bolus versus continuous epidural infusion for labor analgesia: the effects on maternal motor function and labor outcome. A randomized double-blind study in nulliparous women. Anesth Analg 2011; 113: 826-31.
Wong CA, McCarthy RJ, Hewlett B. The effect of manipulation of the programmed intermittent bolus time interval and injection volume on total drug use for labor epidural analgesia: a randomized controlled trial. Anesth Analg 2011; 112: 904-11.
Fettes PD, Moore CS, Whiteside JB, McLeod GA, Wildsmith JA. Intermittent vs continuous administration of epidural ropivacaine with fentanyl for analgesia during labour. Br J Anaesth 2006; 97: 359-64.
Lim Y, Sia AT, Ocampo C. Automated regular boluses for epidural analgesia: a comparison with continuous infusion. Int J Obstet Anesth 2005; 14: 305-9.
Fidkowski CW, Shah S, Alsaden MR. Programmed intermittent eidpural bolus as compared to continuous epidural infusion for the maintenance of labor analgesia: a prospective randomized single-blinded controlled trial. Korean J Anesthesiol 2019; 72: 472-8.
Epsztein Kanczuk M, Barrett NM, Arzola C, Downey K, Ye XY, Carvalho JC. Programmed intermittent epidural bolus for labor analgesia during first stage of labor: a biased-coin up-and-down sequential allocation trial to determine the optimum interval time between boluses of a fixed volume of 10 mL of bupivacaine 0.0625% with fentanyl 2 µg/mL. Anesth Analg 2017; 124: 537-41.
Zakus P, Arzola C, Bittencourt R, Downey K, Ye XY, Carvalho JC. Determination of the optimal programmed intermittent epidural bolus volume of bupivacaine 0.0625% with fentanyl 2 µg.ml-1 at a fixed interval of forty minutes: a biased coin up-and-down sequential allocation trial. Anaesthesia 2018; 73: 459-65.
Bittencourt R, Arzola C, Zakus P, Downey K, Ye XY, Carvalho JC. A biased coin up-and-down sequential allocation trial to determine the optimum programmed intermittent epidural bolus time interval between 5 mL boluses of bupivacaine 0.125% with fentanyl 2 µg·mL-1. Can J Anesth 2019; 66: 1075-81.
Shatalin D, Arzola C, Downey K, Ye XY, Carvalho JC. Programmed intermittent epidural bolus for labour analgesia during first stage of labour: a sequential allocation trial to determine the effective interval time between boluses of a fixed volume of 2.5 mL of bupivacaine 0.25% plus fentanyl 8 µg·mL-1. Can J Anesth 2021; 68: 653-60.
Lange EM, Wong CA, Fitzgerald PC, et al. Effect of epidural infusion bolus delivery rate on the duration of labor analgesia: a randomized clinical trial. Anesthesiology 2018; 128: 745-53.
Bromage PR. Epidural pressures. In: Bromage PR (Ed.). Epidural Analgesia, 1st ed. Philadelphia: WB Saunders; 1978: 160-75.
Hogan Q. Distribution of solution in the epidural space: examination by cryomicrotome section. Reg Anesth Pain Med 2002; 27: 150-6.
Mowat I, Tang R, Vaghadia H, Krebs C, Henderson WR, Sawka A. Epidural distribution of dye administered via an epidural catheter in a porcine model. Br J Anaesth 2016; 116: 277-81.
Oliver M, Strowbridge S, Mistry R, Romagnoli E, Skelton V. Vertebral spread of epidural boluses with different pump flow rates in a porcine model. Int J Obstet Anesth 2016; 28: 96-7.
Usubiaga JE, Wikinski JA, Usubiaga LE. Epidural pressure and its relation to spread of anesthetic solutions in epidural space. Anesth Analg 1967; 46: 440-6.
Hirabayashi Y, Shimizu R, Matsuda I, Inoue S. Effect of extradural compliance and resistance on spread of extradural analgesia. Br J Anaesth 1990; 65: 508-13.
Paul DL, Wildsmith JA. Extradural pressure following the injection of two volumes of bupivacaine. Br J Anaesth 1989; 62: 368-72.
Cardoso MM, Carvalho JC. Epidural pressures and spread of 2% lidocaine in the epidural space: influence of volume and speed of injection of the local anesthetic solution. Reg Anesth Pain Med 1998; 23: 14-9.
Klumpner TT, Lange EM, Ahmed HS, Fitzgerald PC, Wong CA, Toledo P. An in vitro evaluation of the pressure generated during programmed intermittent epidural bolus injection at varying infusion delivery speeds. J Clin Anesth 2016; 34: 632-7.
Krawszyk P, Piwowar P, Salapa K, Lonc T, Andres J. Do epidural catheter size and flow rate affect bolus injection pressure in different programmed intermittent epidural bolus regimens? An in vitro study. Anesth Analg 2019; 129: 1587-94.
Nor NM, Russell IF. Assessing blocks after spinal anaesthesia for elective caesarean section: how different questions affect findings from the same stimulus. Int J Obstet Anesth 2013; 22: 294-7.
Bonica JJ. Pain of parturition. Clin Anaesthesiol 1986; 4: 1-31.
Acknowledgements
We would like to thank Dr. David Thomas Monks, Department of Anesthesiology, Washington University School of Medicine, for his important suggestions incorporated into this manuscript, on the occasion of his visit to our Department as a Visiting Professor. Dr. Jose Carvalho is supported by the Merit Awards Program, Department of Anesthesiology and Pain Medicine, University of Toronto.
Author contributions
Yusuke Mazda, Cristian Arzola, Kristi Downey, and Jose C.A. Carvalho contributed to all aspects of this manuscript, including study conception and design; acquisition, analysis, and interpretation of data; and drafting the manuscript. Xiang Y. Ye contributed to study design, analysis and interpretation of data, and drafting the manuscript.
Disclosures
None.
Funding statement
Departmental funds only.
Editorial responsibility
This submission was handled by Dr. Alana Flexman, Associate Editor, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mazda, Y., Arzola, C., Downey, K. et al. Programmed intermittent epidural bolus for labour analgesia: a randomized controlled trial comparing bolus delivery speeds of 125 mL·hr-1 versus 250 mL·hr-1. Can J Anesth/J Can Anesth 69, 86–96 (2022). https://doi.org/10.1007/s12630-021-02132-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-021-02132-w