Abstract
Purpose
Both intravenous dexamethasone and dexmedetomidine prolong the analgesic duration of interscalene blocks (ISB) after arthroscopic shoulder surgery. This study compared their relative effectiveness and the benefit of their use in combination.
Methods
This single-centre, double-blinded, parallel three-group superiority trial randomized 198 adult patients undergoing ambulatory arthroscopic shoulder surgery. Patients received preoperative ISB with 30 mL 0.5% bupivacaine and 50 µg dexmedetomidine or 4 mg dexamethasone or both of these agents as intravenous adjuncts. The primary outcome was analgesic block duration. Secondary outcomes included the quality of recovery 15 score (range: 0–150) on day 1 and postoperative neurologic symptoms in the surgical arm.
Results
Block durations (n = 195) with dexamethasone (median [range], 24.5 [2.0–339.5] hr) and both adjuncts (24.0 [1.5-157.0] hr) were prolonged compared with dexmedetomidine (16.0 [1.5–154.0] hr). When analyzed by linear regression after an unplanned log transformation because of right-skewed data, the corresponding prolongations of block duration were 59% (95% confidence interval [CI], 28 to 97) and 46% (95% CI, 18 to 80), respectively (both P < 0.001). The combined adjuncts were not superior to dexamethasone alone (-8%; 95% CI, -26 to 14; P = 0.42). Median [IQR] quality of recovery 15 scores (n = 197) were significantly different only between dexamethasone (126 [79–149]) and dexmedetomidine (118.5 [41–150], P = 0.004), but by an amount less than the 8-point minimum clinically important difference.
Conclusion
Dexamethasone is superior to dexmedetomidine as an intravenous adjunct for prolongation of bupivacaine-based ISB analgesic duration. There was no additional benefit to using both adjuncts in combination.
Trial registration
www.clinicaltrials.gov(NCT03270033); registered 1 September 2017.
Résumé
Objectif
La dexaméthasone et la dexmédétomidine intraveineuses prolongent toutes deux la durée analgésique des blocs interscaléniques (BIS) après une chirurgie arthroscopique de l’épaule. Cette étude a comparé leur efficacité relative et les avantages d’une utilisation des deux agents en combinaison.
Méthode
Cette étude de supériorité monocentrique en trois groupes parallèles à double insu a randomisé 198 patients adultes subissant une chirurgie arthroscopique de l’épaule en ambulatoire. Les patients ont reçu un BIS préopératoire composé de 30 mL de bupivacaïne 0,5 % avec 50 μg de dexmédétomidine, 4 mg de dexaméthasone, ou la combinaison de ces deux agents comme adjuvants intraveineux. Le critère d’évaluation principal était la durée analgésique du bloc. Les critères d’évaluation secondaires comprenaient le score de qualité de récupération (QoR) 15 (plage : 0-150) au jour 1 et les symptômes neurologiques postopératoires dans le bras opéré.
Résultats
Les durées des blocs (n = 195) avec la dexaméthasone (médiane [plage], 24,5 [2,0-339,5] heures) et la combinaison des deux adjuvants (24,0 [1,5-157,0] heures) ont été prolongées par rapport à la dexmédétomidine (16,0 [1,5-154,0] heures). Lorsqu’elles ont été analysées par régression linéaire après une transformation logarithmique non planifiée en raison de données biaisées vers la droite, les prolongations correspondantes de la durée du bloc étaient de 59 % (intervalle de confiance [IC] 95 %, 28 à 97) et de 46 % (IC 95 %, 18 à 80), respectivement (les deux P < 0,001). La combinaison des adjuvants n’était pas supérieure à la dexaméthasone seule (-8 %; IC 95 %, -26 à 14; P = 0,42). Les scores médians [ÉIQ] de qualité de récupération 15 (n = 197) n’étaient significativement différents qu’entre la dexaméthasone (126 [79-149]) et la dexmédétomidine (118,5 [41-150], P = 0,004), mais la différence observée était inférieure à la différence minimale de 8 points nécessaire pour être considérée cliniquement importante.
Conclusion
La dexaméthasone est supérieure à la dexmédétomidine en tant qu’adjuvant intraveineux pour prolonger la durée analgésique d’un BIS à base de bupivacaïne. Aucun avantage supplémentaire n’a été observé lors de l’utilisation combinée des deux adjuvants.
Enregistrement de l’étude
www.clinicaltrials.gov (NCT03270033); enregistrée le 1er septembre 2017.
Similar content being viewed by others
Significant pain after ambulatory arthroscopic shoulder surgery is common and among the most frequent reasons for unplanned postoperative admission.1,2,3 Interscalene blocks (ISB) are recommended for use in this population because they reduce acute postoperative pain and opioid use.1 Interscalene blocks consisting of a single injection of local anesthetic with co-administered adjunctive medications can provide a mean analgesic duration of 24 hr,4 avoiding the associated practical challenges and complications of maintaining a plexus catheter infusion.5,6
Both dexamethasone, a potent glucocorticoid, and dexmedetomidine, a highly selective α2 adrenergic agonist with sedative and analgesic properties, prolong the analgesic duration of ISB when administered as intravenous adjuncts.7,8,9,10 Despite this increased research interest, we are aware of only one other randomized trial that used both intravenous adjuncts.11 This relatively small study (n = 22 per group) of shoulder arthroscopy patients found a 3.5-fold increase in ISB analgesic duration with the combination of dexamethasone and dexmedetomidine compared with dexamethasone alone. Nevertheless, without a dexmedetomidine only control group it is unclear if the results were due to a synergistic effect of the combination of adjuncts or an isolated effect of dexmedetomidine.
In this study, we addressed this limitation and attempted to confirm the remarkable treatment effect observed by Kang et al.11 in a larger sample from a separate population. The objectives were to better establish the relative analgesic effectiveness of intravenous dexamethasone and dexmedetomidine, and any added benefit of using them in combination for single injection ISB with bupivacaine. We hypothesized that the combination would offer superior analgesia to either adjunct used on its own in patients undergoing arthroscopic shoulder surgery.
Methods
Ethics approval
The study protocol for this investigation included obtaining written informed consent from all patients. It was approved by the University of Manitoba Biomedical Research Ethics Board (B2017:053, 8 June 2017) and the Winnipeg Regional Health Authority Research Access and Approval Committee (2017-027, 25 August 2017). Health Canada (HC2017:001, 3 May 2017) provided a no objection letter granting permission for the analgesic use of dexamethasone and dexmedetomidine. The study was registered on 1 September 2017 at clinicaltrials.gov (NCT03270033).
Study design, setting, and population
This single-centre, double-blinded, randomized-controlled superiority trial involved three parallel groups. Patients were randomized to receive ISB using 30 mL of 0.5% bupivacaine and one of the following intravenous adjunctive regimens: (i) 50 µg dexmedetomidine, (ii) 4 mg dexamethasone, or (iii) both of these agents (combination group). The doses were chosen to maximize sensory block duration while minimizing side effects.4,9,12
The study was conducted at the Pan Am Clinic in Winnipeg, Manitoba, Canada. This is an established stand-alone ambulatory surgical centre with a high volume of arthroscopic shoulder surgery functioning within a universal public health insurance system. Patients at least 18 yr of age undergoing arthroscopic shoulder surgery were included. Exclusion criteria were patient refusal, diabetes, pregnancy, contraindication to ISB or the study drugs in the view of the attending anesthesiologist, systemic glucocorticoid use in the last two weeks, epidural or intraarticular corticosteroid injection in the past three months, daily opioid use for the last two weeks, active peptic ulcer disease, end-stage renal disease, cirrhotic liver disease, and previous participation in the study.
Study procedures
Consent and randomization
Eligibility and interest in participating were assessed by preoperative telephone call. Prospective participants were e-mailed the informed consent document to review. On the day of surgery, written consent was obtained after review of the document with study staff. Participants were randomized in a 1:1:1 allocation ratio. GraphPad Prism version 6.04 for Windows (GraphPad Software, La Jolla, CA, USA) was used to generate the randomization table in blocks of 20, which was stored on a password-protected spreadsheet by a study staff member not involved in the conduct of the study (L.G.). A separate research associate (F.F.) not involved in the study sequentially obtained blocks of 20 patient assignments, stored them in a locked drawer, and prepared the study interventions.
Preparation of interventions
All patients received the standardized ISB local anesthetic solution of 30 mL 0.5% bupivacaine (Hospira Healthcare Corporation; Kirkland, QC, Canada). The intervention drugs were diluted in separate 100-mL bags of normal saline. Bag A contained dexmedetomidine (dexmedetomidine hydrochloride, 4 µg·mL−1, Hospira Healthcare Corporation; Kirkland, QC, Canada) or placebo (using sterile technique, a needle puncture mark was made in the bag’s injection port to render it indistinguishable from the intervention arm). Bag B was prepared similarly, with dexamethasone (Dexamethasone Omega Unidose, 10 mg·mL−1, Omega Laboratories Limited; Montreal, QC, Canada) or placebo. With these measures we hoped to render the bags indistinguishable to caregivers. The bags were administered in any order starting at the time of ISB performance. Bag A was administered over ten minutes, consistent with the manufacturer’s recommendation for dexmedetomidine while bag B was administered over a period of five to ten minutes to avoid perineal discomfort associated with rapid administration of dexamethasone. While anesthesia team members could perceive differences in sedation when dexmedetomidine was used, outcome assessors were not involved in perioperative care, and recovery room nurses were rarely involved in block performance. Thus, we expected to reliably blind at least outcome assessors and patients but did not directly test blinding of the latter.
Perioperative care
In the absence of a contraindication, patients received oral naproxen 500 mg in the preoperative holding area. Interscalene block was performed preoperatively after the application of electrocardiogram, pulse oximeter, and non-invasive blood-pressure cuff and the administration of midazolam and/or fentanyl for sedation, at the discretion of the attending anesthesiologist and/or their resident. Following skin preparation (4% chlorhexidine) and subcutaneous local infiltration, the block was performed under direct ultrasound-guidance with a prepped and draped ultrasound probe using an in-plane approach with a 22G 50-mm ultrasound needle (Pajunk UniPlex NanoLine; Geisingen, Germany). The bupivacaine solution was injected incrementally with frequent aspiration to surround the C5 to C7 nerve roots. No agents other than the prepared ISB solution were administered for the ISB. After completion of the ISB, the attending anesthesiologist provided anesthesia according to the usual pattern of care for that surgeon (i.e., either concomitant general anesthesia or block alone). The only intraoperative restrictions were that the patient should not receive dexmedetomidine, dexamethasone, or any other steroid or alpha 2 adrenergic agonist as part of the anesthetic management. Recovery room nurses provided routine care in the post anesthesia care unit and assessed block success or failure postoperatively. The block was considered “failed” if the patient required opioid analgesia for surgical site pain. Prior to discharge from the surgical centre, the patient was given instructions for analgesic management and a prescription for oral analgesics of the surgeon’s choice.
Outcomes
The primary outcome was duration of analgesia after ISB as measured by time from block administration to the first-time shoulder pain was experienced after the surgery. This outcome was assessed by patient recall during a telephone call on postoperative day 1, with serial daily telephone calls made if the patient had not yet reported shoulder pain. Patients were not prospectively requested to specifically make note of the time of first shoulder pain but as part of their consent they were informed that pain control would be assessed during the postoperative day 1 telephone call. Secondary outcomes were chosen to corroborate analgesic effectiveness and measure adverse effects. They were assessed by paper chart review unless otherwise noted. Related to analgesia, we measured block success (as defined above), cumulative analgesic consumption at discharge from the recovery room and at the time the primary outcome occurred (during a telephone call). The time frame for the latter outcome was used in the absence of a protocolized postoperative analgesic regimen to determine if differences in co-analgesic use for the duration of the block had potentially affected perceived block durations. Related to adverse effects we measured the use of intraoperative vasopressors or antimuscarinics, the use of intraoperative antihypertensives, the occurrence of serious adverse events related to block performance including seizure, systemic local anesthetic toxicity, pneumothorax/hemothorax, epidural spread of local anesthetic, unplanned postoperative admission to hospital from the recovery room or during the first postoperative night, and recovery room length of stay. We also administered questionnaires to calculate the quality of recovery 15 (QoR-15) scores13 during the postoperative day 1 telephone interview to assess global quality of recovery (QoR). This validated and standardized 15-item QoR questionnaire assesses dimensions of emotional wellbeing, psychological support, physical independence, pain, and physical comfort including nausea and vomiting. Finally, at a standardized telephone interview conducted by research assistants two weeks after surgery, we assessed for persistent hoarse voice, shortness of breath, and neurologic symptoms in the surgical limb arm that were new in the postoperative period. Postoperative neurologic symptoms (PONS) were defined slightly differently from in our previous work4 in that we also included distal limb pain. Patients with any one or more of transient or persistent numbness or paresthesia anywhere in the operative limb, pain in the forearm or hand, or weakness in the hand or fingers were re-assessed at six months. Those with persistent symptoms at six months had a detailed telephone assessment by a fourth-year anesthesia resident (D.R.).
Statistical analysis
The power analysis for this study was based on published results from previous work.4,9 With a two-tailed alpha error of 0.05 and a standard deviation of 5.0 hr in each group, 189 total patients (63 per group) would provide greater than 90% power to detect a difference of 3.0 hr in block duration and account for 5% attrition.
The primary outcome, block duration, was analyzed rounded to the nearest 0.1 hr, but is reported rounded to the nearest 0.5 hr. Patients typically only reported time of first pain to the nearest 0.5 hr even though anesthesiologists recorded time of block administration to the nearest 0.1 hr. Block duration was first analyzed according to the intention to treat principle, while a preplanned sensitivity analysis of the primary outcome excluded patients who had “failed” ISB identified in the recovery room or protocol violations. These analyses were performed with univariate regression and post hoc pairwise contrasts adjusted using Holm’s method. A multivariable analysis of the primary outcome was also preplanned as an opportunity to explore what clinical factors beyond the adjuncts might be relevant to the outcome. Given the large number of candidate predictors, only predictors with a P value ≤ 0.1 on screening univariate tests were included. These were entered into the multivariable model and removed with backwards stepwise elimination, starting in order of least significance to obtain a final model where all variables were significant at P < 0.05 and no excluded variable regained significance when re-entered into the model. Before these analyses were carried out, an unplanned log transformation of the block duration was deemed necessary because of the skewed distribution of block duration and the presence of several extreme outliers. Both the univariate and multivariable log transformed models of block duration had their residuals inspected via histograms, while estimated response vs residual scatter plots and quantile-quantile normal plots were constructed to assess homoscedasticity and conditional normality, respectively. Cook’s distance was calculated for each observation to measure its influence on the model fit. In the final models, resultant coefficients for the pairwise comparisons of the three groups have been exponentiated and presented as percent increase or decrease in block duration, relative to either a reference group or a unit change in the predictor.
Continuous secondary outcomes (i.e., not block duration) were analyzed with the Kruskal–Wallis test and binary variables were analyzed with likelihood ratio tests from univariate logistic regression models. The Dunn test was used for pairwise comparisons following a significant Kruskal–Wallis test. The P values presented for omnibus tests of secondary outcomes are unadjusted for multiple testing. All tests were two-sided and all outcomes pre-specified. Analyses were performed with R version 3.6.2 (https://www.R-project.org, Vienna, Austria). The authors approved these analysis plans before the study results were known.
Results
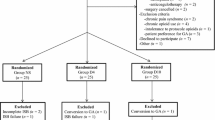
All 198 patients enrolled between 19 September 2017 and 13 April 2018 (Figure 1) were allocated to study interventions and received an ISB. All patients provided outcome data except one who did not answer repeated follow-up telephone calls. An additional two patients never experienced shoulder pain despite follow-up extension to one week. They contributed to all outcome analyses except for those involving block duration. One patient in each of the dexamethasone and dexmedetomidine groups was noted to have diet-controlled diabetes after randomization. Their outcomes, along with those of the four patients in the combination group who received extra dexamethasone or neither allocated adjunct, are included in all analyses except where protocol violations are excluded. Because of these issues, we recruited nine more patients than the 189 originally planned.
Surgeries were performed by eight surgeons (range 7–51 procedures) with 39 different attending anesthesiologists either performing blocks directly or through the supervision of a third year or higher anesthesiology resident (n = 61). Five anesthesiologists performed or supervised more than ten study ISBs, and 25 less than five ISBs. Patients, procedures, and perioperative care were similar between groups (Table 1).
The intention to treat, log transformed, omnibus analysis of block duration revealed significant differences between groups (P < 0.001) (Table 2). In pairwise contrasts, compared with dexmedetomidine alone, both dexamethasone and the combination of adjuncts prolonged block duration, but there was no added benefit to the combination over dexamethasone alone. Exclusion of failed blocks and protocol violations, and the multivariable analysis did not change this interpretation. In a post hoc sensitivity analysis, exclusion from the models of the four most influential observations (three block durations of 2.0 hr or less and 1 of 339.5 hr) as measured by Cook’s distance, also did not change the interpretation. A table of block duration recorded from each group (eTable), a box plot of these values (eFig 1), and quantile-quantile normal plot of log-transformed block duration (eFig 2) can be found in the electronic supplement.
Among secondary outcomes (Table 3), postoperative day 1 QoR-15 scores differed between groups (Kruskal–Wallis test P = 0.02, unadjusted for multiple secondary outcome testing). This result was due to the difference between dexamethasone and dexmedetomidine (P = 0.004, Dunn test) as opposed to the combination vs dexmedetomidine or dexamethasone (P = 0.05, 0.15, respectively). The 7.5-point difference in medians between the dexamethasone and dexmedetomidine groups is less than the 8-point minimum clinically important difference established for this instrument.14 Post hoc analyses of the five separate QoR-15 dimension scores showed that only between group differences in pain and emotional state scores were statistically significant.
All 14-day follow-up cases of hoarse voice and dyspnea were resolved by six months. Nevertheless, PONS persisted at six months in eight of 53 patients with PONS symptoms at 14 days (Table 4). Some had pre-existing symptoms in the surgical arm, while others had symptoms that may or may not have been directly related to the ISB.
Transient paresthesias during block performance were reported in four patients, one of whom had hoarseness and persistent distal surgical arm pain at 14 days but not six months postoperatively. An additional two patients in the dexmedetomidine group experienced bradycardia during block performance. Four patients visited an emergency room less than 24 hr after recovery room discharge. In the dexmedetomidine group, two patients sought additional analgesia and another attended for dyspnea and was diagnosed with pneumonia. The fourth patient received dexamethasone and required a dressing change for incisional bleeding. No patient was admitted to hospital.
Discussion
We found that a single 4-mg intravenous injection of dexamethasone prolonged block duration in patients undergoing ambulatory arthroscopic shoulder surgery under ISB with 30 mL of 0.5% bupivacaine compared with a single intravenous injection of dexmedetomidine 50 µg. This prolongation is substantial, with median block duration increasing from 16.0 to 24.6 hr, or by 59% (95% confidence interval, 28 to 97) when interpreting exponentiated β coefficients of block duration from the log transformed, intention to treat analysis as percent change in geometric mean). To our knowledge, this comparison has not previously been reported. In addition, combining both adjuncts did not result in a statistically significant advantage in analgesic block duration over dexamethasone alone. Taken together, this study provides strong evidence that intravenous dexamethasone is superior to intravenous dexmedetomidine for prolongation of ISB analgesia, and that combining the two adjuncts is unlikely to substantially increase block duration further.
We are aware of only one smaller randomized trial that has compared the combination of intravenous dexamethasone and dexmedetomidine with intravenous dexamethasone alone.11 Our findings are in stark contrast with that study where a more than 3.5-fold increase in time to a visual analogue scale pain score of more than 4 on 10 was reported with dexamethasone (0.11 mg·kg−1) plus dexmedetomidine (1 µg·kg−1) compared with dexamethasone alone (n = 22 per group). Our larger study size increases the likelihood that our results reliably reflect the true intervention effect, at least under the conditions in which the adjuncts were used in the study. Kang et al.’s disparate results may represent a spurious finding from the small sample size, or be a consequence of a different definition of analgesic block duration, differences in ISB solution (15 mL 0.5% ropivacaine with 1:200,000 epinephrine), or larger adjunct doses.
We chose our adjunct doses from the literature available at the time the study was designed.4,9,12 Our aim was to maximize sensory block duration while minimizing side effects. A ceiling effect for intravenous dexamethasone at or below the 4-mg dose used in this study is well established.4,7,8 Our dexmedetomidine dose was based on a systematic review recommendation for a perineural dose12 and a well-designed study showing intravenous dexmedetomidine to be noninferior to perineural dexmedetomidine for prolongation of ISB analgesic duration.9 More recent work has suggested that intravenous doses of dexmedetomidine higher than 1 µg·kg−1 are necessary to prolong the analgesic duration of brachial plexus block.10,15 Yet higher dexmedetomidine doses may cause side effects that are undesirable, especially in older patients undergoing ambulatory surgery. Intraoperative bradycardia, hypotension, and excessive postoperative sedation have been reported,15,16 while smaller studies may have been underpowered to detect important differences between groups.10,11 Further work is required to further clarify the ideal local anesthetic drug dose and volume,4,17,18 and the ideal adjunct dose and routes for dexmedetomidine.9,10
We also found statistically significant differences between the groups with regards to the QoR-15 score on the day after surgery. Scores were higher with dexamethasone compared with dexmedetomidine (Dunn non-parametric test P = 0.004), with the difference between the two group medians equal to 7.5. The statistical significance of these results should be interpreted cautiously given the multiple testing of secondary outcomes. Further, with the established minimum clinically important difference for this instrument being 8 points,14 dexamethasone did not improve QoR by a clinically important amount.
The overall rates of PONS in this study were considerably higher than several prospective studies of PONS after ultrasound guided ISB,19,20 but without significant differences between study groups. We continue to believe that these higher rates reflect our definition of PONS as any report of symptoms, including transient symptoms and those deemed to be due to the surgery or pre-existing neuropathy.4 Indeed, the reported incidence of nerve injuries after shoulder surgery has been reported to be as high as 10%.21 Compared with our previous study,4 we additionally recorded forearm or hand pain as PONS, which accounted for 19 additional outcomes at 14-day follow-up. Consistent with this inclusive definition, almost all 14-day PONS symptoms had resolved by six-month follow-up in agreement with other studies with similar high rates of early PONS.4,22,23 In several six-month patients it remains unclear whether there is nerve injury due to ISB or other cause, persistent pain due to other pathology, or even if the symptom is new in the postoperative period. Continued study of PONS using consistent and inclusive outcome definitions in randomized, controlled comparisons of regional analgesia techniques is important for establishing their safety.
The strengths of this study are its relatively large size, blinding, reporting of QoR, and the pragmatic approach to both perioperative care and measurement of block duration. The latter increases the likelihood that the observed treatment effects reflect those expected under usual care conditions. Relying on recall of first shoulder pain to measure block duration without explicit instructions or pain diaries may have limited the precision of the primary outcome measurement, but with blinding and randomization of a sufficiently large sample size, this additional variability would be expected to be evenly distributed across study groups. Another potential limitation is that relying on block duration as a primary outcome may not have captured the patient’s analgesic experience in the hours after the block wore off. Nevertheless, the post hoc analyses of the pain domain of the QoR-15 scores indicate the dexmedetomidine group patients were more likely to experience moderate or severe pain in the first 24 hr after surgery, in addition to shorter block duration. The study was also not designed to compare the adjuncts to a placebo or to detect small between-group differences in block duration or secondary outcome rates. We relied on prior literature for the former point,7,8,9,10 which is less established for dexmedetomidine. For the latter, although we cannot exclude a small difference in block duration between the combination and dexamethasone groups, our confidence intervals suggest a clinically important difference is quite unlikely. Finally, the findings of this study may not be applicable to centres using smaller volumes of bupivacaine than the 30 mL used in this study. This choice of block volume was a confluence of the typical practice at our centre and a desire to maximize the analgesic duration of the blocks.
Ultimately, we have determined that intravenous dexamethasone significantly prolongs ISB duration compared with intravenous dexmedetomidine. On postoperative day 1, it may also lead to an improvement in QoR-15 scores that is less than the accepted minimum clinically important difference. We did not find an advantage, analgesic or otherwise, to using both adjuncts together compared with dexamethasone alone. The superior analgesic block duration observed with intravenous dexamethasone implies that dexmedetomidine at doses of 50 µg or less should not be considered a substitute for 4 mg of dexamethasone when the latter is not contraindicated. In addition, co-administration of dexmedetomidine with dexamethasone for the sole purpose of prolonging analgesic block duration should be avoided based on the lack of any additional benefit compared with dexamethasone alone.
References
Toma O, Persoons B, Pogatzki-Zahn E, Van de Velde M, Joshi GP. PROSPECT guideline for rotator cuff repair surgery: systematic review and procedure-specific postoperative pain management recommendations. Anaesthesia 2019; 74: 1320-31.
Sultan J, Marflow KZ, Roy B. Unplanned overnight admissions in day-case arthroscopic shoulder surgery. Surgeon 2012; 10: 16-9.
Westermann RW, Pugely AJ, Ries Z, Martin CT, Gao Y, Wolf BR. Causes and predictors of 30-day readmission after shoulder and knee arthroscopy: an analysis of 15,167 cases. Arthrosc J Arthrosc Relat Surg 2015; 31(P1035–40): E1.
Holland D, Amadeo RJ, Wolfe S, et al. Effect of dexamethasone dose and route on the duration of interscalene brachial plexus block for outpatient arthroscopic shoulder surgery: a randomized controlled trial. Can J Anesth 2018; 65: 34-45.
Marhofer P, Anderl W, Heuberer P, et al. A retrospective analysis of 509 consecutive interscalene catheter insertions for ambulatory surgery. Anaesthesia 2015; 70: 41-6.
Fredrickson MJ, Leightley P, Wong A, Chaddock M, Abeysekera A, Frampton C. An analysis of 1505 consecutive patients receiving continuous interscalene analgesia at home: a multicentre prospective safety study. Anaesthesia 2016; 71: 373-9.
Desmet M, Vanneste B, Reynvoet M, et al. A randomised controlled trial of intravenous dexamethasone combined with interscalene brachial plexus blockade for shoulder surgery. Anaesthesia 2015; 70: 1180-5.
Chalifoux F, Colin F, St-Pierre P, Godin N, Brulotte V. Low dose intravenous dexamethasone (4 mg and 10 mg) significantly prolongs the analgesic duration of single-shot interscalene block after arthroscopic shoulder surgery: a prospective randomized placebo-controlled study. Can J Anesth 2017; 64: 280-9.
Abdallah FW, Dwyer T, Chan VW, et al. IV and perineural dexmedetomidine similarly prolong the duration of analgesia after interscalene brachial plexus block: a randomized, three-arm, triple-masked, placebo-controlled trial. Anesthesiology 2016; 124: 683-95.
Kang RA, Jeong JS, Yoo JC, et al. Effective dose of intravenous dexmedetomidine to prolong the analgesic duration of interscalene brachial plexus block: a single-center, prospective, double-blind, randomized controlled trial. Reg Anesth Pain Med 2018; 43: 488-95.
Kang RA, Jeong JS, Yoo JC, et al. Improvement in postoperative pain control by combined use of intravenous dexamethasone with intravenous dexmedetomidine after interscalene brachial plexus block for arthroscopic shoulder surgery: a randomised controlled trial. Eur J Anaesthesiol 2019; 36: 360-8.
Vorobeichik L, Brull R, Abdallah FW. Evidence basis for using perineural dexmedetomidine to enhance the quality of brachial plexus nerve blocks: a systematic review and meta-analysis of randomized controlled trials. Br J Anaesth 2017; 118: 167-81.
Stark PA, Myles PS, Burke JA. Development and psychometric evaluation of a postoperative quality of recovery score: the QoR-15. Anesthesiology 2013; 118: 1332-40.
Myles PS, Myles DB, Galagher W, Chew C, MacDonald N, Dennis A. Minimal clinically important difference for three quality of recovery scales. Anesthesiology 2016; 125: 39-45.
Li Y, Wang H, Deng Y, Yao Y, Li M. Effect of dexmedetomidine on supraclavicular brachial plexus block: a randomized double blind prospective study (Chinese). Beijing Da Xue Xue Bao Yi Xue Ban 2018; 50: 845-9.
Somsunder RG, Archana NB, Shivkumar G, Krishna K. Comparing efficacy of perineural dexmedetomidine with intravenous dexmedetomidine as adjuvant to levobupivacaine in supraclavicular brachial plexus block. Anesth Essays Res 2019; 13: 441-5.
Cummings KC 3rd, Napierkowski DE, Parra-Sanchez I, et al. Effect of dexamethasone on the duration of interscalene nerve blocks with ropivacaine or bupivacaine. Br J Anaesth 2011; 107: 446-53.
McHardy PG, Singer O, Awad IT, et al. Comparison of the effects of perineural or intravenous dexamethasone on low volume interscalene brachial plexus block: a randomised equivalence trial. Br J Anaesth 2020; 124: 84-91.
Liu SS, Gordon MA, Shaw PM, et al. A prospective clinical registry of ultrasound-guided regional anesthesia for ambulatory shoulder surgery. Anesth Analg 2010; 111: 617-23.
Sites BD, Taenzer AH, Herrick MD, et al. Incidence of local anesthetic systemic toxicity and postoperative neurologic symptoms associated with 12,668 ultrasound-guided nerve blocks: an analysis from a prospective clinical registry. Reg Anesth Pain Med 2012; 37: 478-82.
Dwyer T, Henry PD, Cholvisudhi P, et al. Neurological complications related to elective orthopedic surgery: part 1: common shoulder and elbow procedures. Reg Anesth Pain Med 2015; 40: 431-42.
Borgeat A, Ekatodramis G, Kalberer F, Benz C. Acute and nonacute complications associated with interscalene block and shoulder surgery: a prospective study. Anesthesiology 2001; 95: 875-80.
Liu SS, Zayas VM, Gordon MA, et al. A prospective, randomized, controlled trial comparing ultrasound versus nerve stimulator guidance for interscalene block for ambulatory shoulder surgery for postoperative neurological symptoms. Anesth Analg 2009; 109: 265-71.
Author contributions
Daniel Rodrigues contributed to study design, data acquisition, data interpretation, writing up the first draft of the paper, revising it, and approving the final version. Ryan Amadeo, Scott Wolfe, Jeff Leiter, Jason Old, and Peter MacDonald contributed to study design, revising the paper and approving the final version. Linda Girling, Faylene Funk, Kelsi Fidler, and Holly Brown contributed to data acquisition, revising the paper, and approving the final version. Brenden Dufault contributed to study design, data analysis, revising the paper, and approving the final version. Thomas C. Mutter contributed to study design, data interpretation, revising the paper and approving the final version.
Acknowledgements
The authors are grateful for the assistance of Alexander Schultz (BSc, MD candidate) in formatting and proofreading the manuscript.
Disclosures
None.
Funding statement
This work was supported by the University of Manitoba Department of Anesthesiology, Perioperative and Pain Medicine Oversight and Advisory Committee; and the Pan Am Clinic Foundation.
Editorial responsibility
This submission was handled by Dr. Gregory L. Bryson, former Deputy Editor-in-Chief, Canadian Journal of Anesthesia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rodrigues, D., Amadeo, R.J.J., Wolfe, S. et al. Analgesic duration of interscalene block after outpatient arthroscopic shoulder surgery with intravenous dexamethasone, intravenous dexmedetomidine, or their combination: a randomized-controlled trial. Can J Anesth/J Can Anesth 68, 835–845 (2021). https://doi.org/10.1007/s12630-021-01942-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-021-01942-2