Abstract
Purpose
Sepsis has high incidence and mortality rates, particularly in the intensive care unit (ICU). Corticosteroids may improve outcomes, and vitamin C may add benefit. We aimed to assess whether vitamin C and corticosteroids improved outcomes compared with corticosteroids alone.
Methods
This historical cohort study (11 December 2016 to 21 February 2018) was conducted in the ICU of a quaternary referral hospital. Patients with an ICU admission diagnosis of sepsis or septic shock who received vitamin C and hydrocortisone within 72 hr were compared with those who received only hydrocortisone. All patients received standard sepsis care including source control, antibiotics, and fluid resuscitation. Most patients received thiamine as standard ICU care. The primary outcome was hospital mortality. Secondary outcomes included ICU mortality, ventilator-free days, vasopressor-free days, dialysis use, and duration of ICU admission.
Results
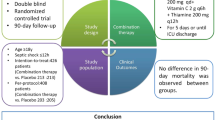
One hundred and forty-four patients were included in the study. The mean (standard deviation [SD]) age was 64 (15) yr; 39% were female; and the mean (SD) Acute Physiology And Chronic Health Evaluation IV score was 89 (30). Eighty-eight patients did not receive vitamin C and 52 received vitamin C. There was no observed difference in hospital mortality between the non-vitamin C (36%) and vitamin C (39%) groups (adjusted odds ratio for hospital death, 0.52; 95% confidence interval, 0.20 to 1.34; P = 0.18). There were no statistically significant differences in any secondary outcomes.
Conclusion
In this small observational study of ICU patients with septic shock, the addition of vitamin C to hydrocortisone therapy did significantly affect hospital mortality or other measures of mortality or organ dysfunction.
Résumé
Objectif
Le sepsis comporte une incidence et des taux de mortalité élevés, particulièrement à l’unité de soins intensifs (USI). Les corticostéroïdes pourraient améliorer les pronostics, et la vitamine C pourrait être bénéfique. Notre objectif était d’évaluer si la vitamine C et les corticostéroïdes amélioraient les devenirs par rapport à un traitement de corticostéroïdes seulement.
Méthode
Cette étude de cohorte historique (réalisée entre le 11 décembre 2016 et le 21 février 2018) a été réalisée à l’USI d’un hôpital quaternaire. Les patients ayant un diagnostic de sepsis ou de choc septique lors de leur admission à l’USI et ayant reçu de la vitamine C et de l’hydrocortisone dans les premières 72 heures ont été comparés à ceux n’ayant reçu que de l’hydrocortisone. Tous les patients ont reçu des soins standard pour le sepsis, soit un contrôle de la source de l’infection, un traitement antibiotique et une réanimation liquidienne. La plupart des patients ont reçu de la thiamine, un traitement standard à l’USI. Le critère d’évaluation principal était la mortalité hospitalière. Les critères d’évaluation secondaires comprenaient la mortalité à l’USI, les jours sans respirateur, les jours sans vasopresseurs, le recours à la dialyse et la durée de séjour à l’USI.
Résultats
Cent quarante-quatre patients ont été inclus dans notre étude. L’âge moyen (écart type [ÉT]) était de 64 (15) ans; 39 % étaient de sexe féminin; et le score APACHE IV moyen (ÉT) de 89 (30). Quatre-vingt-huit patients n’ont pas reçu de vitamine C et 52 en ont reçu. Aucune différence n’a été observée en matière de mortalité hospitalière entre les groupes sans vitamine C (36 %) ou avec vitamine C (39 %) (rapport de cotes ajusté pour la mortalité hospitalière, 0,52; intervalle de confiance 95 %, 0,20 à 1,34; P = 0,18). Il n’y a eu aucune différence statistiquement significative en ce qui touchait aux critères d’évaluation secondaires.
Conclusion
Dans cette petite étude observationnelle portant sur des patients de l’USI en choc septique, l’ajout de vitamine C à un traitement d’hydrocortisone n’a pas eu d’impact significatif sur la mortalité hospitalière ou les autres mesures de mortalité ou d’atteintes organiques.
Similar content being viewed by others
Sepsis and septic shock have high incidence and mortality rates.1 Advances in our understanding of sepsis pathophysiology have led to the concept of metabolic resuscitation,2 which proposes that in sepsis, key vitamins acting as cofactors and coenzymes are depleted and need replacing for effective cellular metabolism. Nevertheless, evidence for the benefits of vitamin supplementation is mixed.
Sepsis may also impair endogenous corticosteroid production and effectiveness.3 Replacement can potentially reduce a patient’s inflammatory response,4 and large randomized-controlled trials have shown that treatment with supra-physiologic doses of corticosteroids may improve clinical outcomes including shock duration, mechanical ventilation, and intensive care unit (ICU) length of stay.5,6
Patients with sepsis also have depleted vitamin C, potentially increasing susceptibility to tissue damage by reactive oxygen species resulting in organ failure and death.7,8 Animal models suggest that replacing or elevating plasma vitamin C levels may improve vascular function and response to vasoconstrictors.9,10 Clinical data are less convincing. Initial studies suggested that intravenous repletion may improve organ failure scores11 and prevent multiorgan failure in critically ill postsurgical patients.12 Nevertheless, more recent work did not see improvement in markers of organ failure, inflammation, or vascular injury in ICU patients with sepsis and acute respiratory distress syndrome.13
Vitamin C and corticosteroids may interact cooperatively in sepsis, in addition to their individual benefits. Vitamin C improves the functionality of the corticosteroid receptor,14 making corticosteroids more effective. Conversely, corticosteroids have been shown to increase cellular vitamin C uptake.15 The combination of vitamin C and hydrocortisone, along with thiamine, has been shown in a recent before-after study to be associated with reduced mortality for ICU patients with sepsis and septic shock.2 Secondary outcomes further suggested prevention of progressive organ failure, decreased duration of vasopressor use, and improved procalcitonin clearance in the treatment group.2 Nevertheless, this study had several limitations. Most importantly, controls did not routinely receive corticosteroids, which may have negatively impacted their outcomes. Further, it was a single-centre, non-blinded, before-after study and the arms were conducted during different times of the year. Recently, a randomized-controlled trial was conducted looking at the benefits of vitamin C and thiamine in addition to hydrocortisone in septic shock. They found no mortality benefit and no benefit in any secondary outcomes except a significantly lower sequential organ failure assessment (SOFA) score in the intervention group compared with the control group at three-days.16
Further high-quality research is imperative prior to widespread implementation of combined vitamin C and hydrocortisone in critically ill septic patients, as clinical equipoise remains. Several randomized-controlled trials are already underway, including the Canadian LOVIT (Lessening Organ dysfunction with VITamin C) trial,17 which is directly comparing vitamin C with placebo in septic ICU patients. The use of vitamin C in our ICU was an experimental therapy based on preliminary data and was not the standard of care.
While large randomized trials were ongoing, we performed a historical cohort study to assess whether vitamin C and hydrocortisone compared with hydrocortisone alone was associated with a decreased hospital mortality in ICU patients with sepsis or septic shock. We additionally sought to assess the association of vitamin C with the following secondary outcomes: ICU mortality, ICU length of stay, vasopressor-free days, ventilator-free days, and use of dialysis.
Methods
This study was approved by the University of British Columbia Clinical Research Ethics Board (H18-01037) who waived the requirement for written informed consent.
Study design and setting
We conducted a historical cohort study of patients who were admitted between 12 December 2016 and 21 February 2018 with a diagnosis of septicemia, sepsis, or septic shock. Patients were identified using our local clinical Provincial Critical Care Database.18,19 The start date represents the first use of high-dose intravenous vitamin C to treat sepsis at our institution. We only included patients who received at least one dose of hydrocortisone. Patients were excluded if hydrocortisone or vitamin C was administered beginning more than 72 hr after ICU admission, or if it was a readmission. Patients who initially received corticosteroids other than hydrocortisone were excluded as they were likely to have a different primary indication for corticosteroid treatment.
This study was carried out in the Vancouver General Hospital (VGH) ICU, which was affiliated with the University of British Columbia. This was a quaternary, closed, 34-bed mixed medical and surgical unit staffed by board-certified intensivists; 95% of admitted patients require mechanical ventilation. The nurse-to-patient ratio was approximately 1:1.2.
Data collection
Patient demographics and clinical characteristics were collected from the Provincial Critical Care Database.18,19 These included the Acute Physiology And Chronic Health Evaluation (APACHE) IV scores,20 source of sepsis, hospital and ICU admission and discharge times, mortality, and SOFA scores21 for the first seven days of ICU admission. Durations of interventions were also documented; these included mechanical ventilation, vasoactive agents, and renal replacement therapy. Ventilator-free days and vasoactive-free days were subsequently calculated. Data on hydrocortisone, thiamine, and vitamin C prescription were collected from the pharmacy database. Chart correlation was used to verify medication prescriptions and/or source of sepsis as required.
Exposure
Exposure was defined as patients who were prescribed vitamin C at a dose of at least 1,500 mg every six hours. Patients who received vitamin C for 96 hr were considered to have completed a full course,2 and any vitamin C administered after this was not recorded.
Patient management
Patient management decisions were at the discretion of treating physicians. There was no pre-defined sepsis protocol in the VGH ICU. Patients were treated following the Surviving Sepsis Campaign guidelines,22 including rapid administration of antibiotics, source control, fluid resuscitation as required, vasopressor support as required, and hydrocortisone in patients with refractory septic shock. Hydrocortisone was typically mixed as 50 mg in 50 mL of 5% dextrose solution and infused over 15–30 min. Thiamine was regularly supplemented on admission to ICU at our institution, following recommendations for prophylactic treatment for thiamine deficiency in critically ill patients.23 While not a universal standard of care, the vast majority of patients in our ICU receive thiamine, which was administered as 200 mg mixed in 50 mL of 5% dextrose solution, infused over 30 min. When vitamin C was prescribed, 1,500 mg was typically mixed in 50 mL of 5% dextrose solution and infused over 15 min. Vitamin C was considered an experimental treatment, not a standard of care.
Statistical analysis
No corrections were made for multiple comparisons as this analysis was exploratory. The primary outcome of hospital death and the secondary outcome of ICU death were analyzed with a multivariable logistic regression model. Three covariates were included in each model to maintain a 15:1 ratio of events to predictors and to prevent overfitting. The covariates used in the final analysis of hospital death were APACHE IV score, year of admission, and whether or not the patient was admitted directly from the emergency department. Covariates for ICU death were the APACHE IV score and source of sepsis. The APACHE IV score was chosen a priori for clinical significance. Other covariates were added using forward selection based on statistical significance to the model. Linearity in log-odds was tested for continuous variables. Effect sizes (adjusted odds ratios [aOR]) for the above endpoints were reported as point estimates with 95% confidence intervals (CI). The secondary endpoints of ventilator-free days, vasopressor-free days, and ICU length of stay were analyzed using a t-test; need for dialysis was analyzed using a Chi square test. P values were reported for primary and secondary endpoint analysis and P < 0.05 was considered statistically significant. All statistical analysis was performed using R version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria).24
Results
Baseline demographics and clinical characteristics
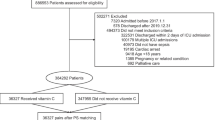
There were 2,078 admissions to the VGH ICU between 12 December 2016 and 21 February 2018, and 535 of these patients were admitted with sepsis, septic shock, or septicemia (Figure). Overall, 395 patients were excluded for the reasons listed in the Figure. The remaining 140 patients were included in the final analysis; 52 were treated with vitamin C and 88 were not. There were no observed differences between the two groups with regards to age, sex, source of sepsis, or location of admission (Table 1). There were differences in admission year, APACHE IV score, and SOFA score; admission year and APACHE IV score were used as covariates in the logistic regression model for hospital mortality.
Medication administration
Of the 52 patients in the vitamin C group, 49 patients (94%) were prescribed 1,500 mg of vitamin C every six hours and three (6%) were prescribed 1,500 mg every 12 hr. One patient in the vitamin C group had a delayed hydrocortisone start, beginning 25 hr after vitamin C. Twenty-one patients (40%) completed 96 hr of treatment with vitamin C. Forty-nine patients (94%) were initially prescribed a minimum of 200 mg of hydrocortisone or equivalent daily.
Among those patients who did not receive vitamin C, 68 of 88 patients (77%) were initially prescribed a minimum of 200 mg of hydrocortisone or equivalent daily.
Sixty-nine patients (78%) in the non-vitamin C group and 50 patients (96%) in the vitamin C group were prescribed at least 200 mg of thiamine daily.
Clinical outcomes
There was no observed difference in hospital mortality between the non-vitamin C (36%) and vitamin C (39%) groups (Table 2). The aOR for hospital death was 0.52 (95% CI, 0.20 to 1.34; P = 0.95) for the vitamin C group compared with the non-vitamin C group. There was also no observed statistical difference in any secondary outcomes, including ICU mortality (31% vs 23%; aOR, 0.83; 95% CI, 0.30 to 2.31; P = 0.39), mean (standard deviation [SD]) duration of ICU admission [11.1 (13.3) vs 9.3 (11.7); P = 0.41], mean (SD) ventilator-free days [16.0 (11.8) vs 17.8 (11.8), P = 0.40], mean (SD) vasopressor-free days [16.9 (11.8) vs 19.7 (11.5), P = 0.17], or use of renal replacement therapy (44% vs 44%, P = 1.00) for the vitamin C group compared with the non-vitamin C group.
Discussion
Despite important similarities in demographic and clinical characteristics observed between the two patient cohorts, combining vitamin C and hydrocortisone did not significantly reduce hospital mortality compared with hydrocortisone alone in this sample of ICU patients with sepsis, septic shock, or septicemia. Furthermore, we did not observe a significant reduction in ICU mortality, ICU length of stay, vasopressor-free days, ventilator-free days, or requirement for renal replacement therapy.
Our results are in contrast to the apparent benefit shown by Marik et al.2 in another non-randomized study, where the combination of vitamin C, hydrocortisone, and thiamine appeared to drastically reduce hospital mortality, prevent worsening of organ failure, and decrease requirements for vasopressors. Two early meta-analyses of Marik et al.’s work and two very small randomized-controlled trials agreed that vitamin C appeared to decrease mortality and vasopressor duration, although the decrease in ICU length of stay was not consistently significant.25,26 The randomized CITRIS-ALI trial also suggested that vitamin C may decrease 28-day mortality, ventilator requirements, and ICU length of stay for patients with sepsis and acute respiratory distress syndrome, but did not show any differences in the primary outcomes of changes in SOFA score, C-reactive protein, and thrombomodulin.13 Our results are more consistent with the recent meta-analysis by Wei et al. and the randomized trial by Fujii et al., which both concluded that there was no difference in mortality when patients were treated with vitamin C.16,27
The lack of mortality benefit in the current analysis may reflect the lack of randomization in our study, in that vitamin C may have been given to sicker patients. Nevertheless, the baseline characteristics of the two groups appeared similar, with no significant differences in age, sex, or source of sepsis, and the difference in APACHE IV score, a predictor of hospital mortality based on pathophysiologic characteristics prior to ICU admission,20 was accounted for by inclusion as a modelling covariate. Additionally, previously reported results indicating an improvement in mortality are limited by study design. The results of Marik et al.2 may have been primarily driven by administration of corticosteroids, which the control group did not routinely receive.2 Importantly, independent corticosteroid administration has shown improved outcomes, including mortality.5 Taken together, it is possible that the combination treatment of vitamin C and hydrocortisone is truly not superior to hydrocortisone alone.
The baseline SOFA and APACHE IV scores, as well as the ICU and hospital mortality rates, were higher than the typical values for patients with septic shock in our institution’s ICU. Moreover, a majority of patients in the cohort were supported with vasoactive agents and all patients were prescribed hydrocortisone with or without vitamin C. Together, this suggests that this cohort reflects a group of patients in refractory septic shock. The SOFA and APACHE IV scores were slightly higher in the vitamin C group, and we controlled for this by including the APACHE IV score as a covariate in the logistic regression model. The SOFA score was not included in the model as it is collinear with the APACHE IV score; we chose to include only one covariate controlling for disease severity to avoid any issues with multicollinearity. The APACHE IV score was chosen because it is a more clinically comprehensive mortality risk score and it was a more statistically significant covariate to the model when using forward selection.
Limitations
This study is non-randomized, no statistical adjustments for multiple comparisons were made, covariates were not controlled for in secondary outcomes, and external validity is limited by our single-centre design. There may be confounding by indication, given the non-randomized design; vitamin C may have been added to the management plan for sicker patients, or there may be other important unmeasured differences between groups (i.e., residual confounding). The presence of chronic diseases not included in the APACHE IV score is not routinely recorded, and thus is not included in the analysis. The high ICU mortality rate and difference between ICU and hospital mortality rates may reflect differences in the population studied, as these values are higher than for the general population of ICU patients with sepsis at our institution. No systemic changes to standards of care for septic patients in our ICU are known over the study period, but process measures such as time to antibiotics are not routinely recorded. Corticosteroid dosing was not standardized, and vitamin C treatment was delayed beyond ICU admission on rare occasions. Thiamine is routinely supplemented for critically ill patients at our institution, which may limit generalizability. Some modelling covariates were determined based on statistical significance for this particular population. Propensity score-matched analysis was not undertaken in this analysis because numbers were small, but would be valuable in future studies with high match rates. Despite these limitations, our analysis provides evidence that hospital mortality, as well as several other secondary outcomes (e.g., ICU length of stay, ventilator-free days), do not significantly differ between patients treated with vitamin C and hydrocortisone and those treated only with hydrocortisone.
Conclusions
In this small observational study of ICU patients with septic shock, the addition of vitamin C to hydrocortisone therapy did not significantly affect hospital mortality or other measures of mortality or organ dysfunction. Further studies are needed prior to widespread adoption of vitamin C as an adjunct to the current standard of care for sepsis management.
References
Walkey AJ, Lagu T, Lindenauer PK. Trends in sepsis and infection sources in the United States. A population-based study. Ann Am Thorac Soc 2015; 12: 216-20.
Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J. Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study. Chest 2017; 151: P1229-38.
Annane D, Bellissant E, Bollaert PE, Briegel J, Keh D, Kupfer Y. Corticosteroids for treating sepsis. Cochrane Database Syst Rev 2015; . https://doi.org/10.1002/14651858.CD002243.pub3.
de Kruif MD, Lemaire LC, Giebelen IA, et al. Prednisolone dose-dependently influences inflammation and coagulation during human endotoxemia. J Immunol 2007; 178: 845-51.
Venkatesh B, Finfer S, Cohen J, et al. Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med 2018; 378: 797-808.
Annane D, Renault A, Brun-Buisson C, et al. Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl J Med 2018; 378: 809-18.
Galley HF, Davies MJ, Webster NR. Ascorbyl radical formation in patients with sepsis: effect of ascorbate loading. Free Radic Biol Med 1996; 20: 139-43.
Borrelli E, Roux-Lombard P, Grau GE, et al. Plasma concentrations of cytokines, their soluble receptors, and antioxidant vitamins can predict the development of multiple organ failure in patients at risk. Crit Care Med 1996; 24: 392-7.
Zhou G, Kamenos G, Pendem S, Wilson JX, Wu F. Ascorbate protects against vascular leakage in cecal ligation and puncture-induced septic peritonitis. Am J Physiol Regul Integr Comp Physiol 2012; 302: R409-16.
Armour J, Tyml K, Lidington D, Wilson JX. Ascorbate prevents microvascular dysfunction in the skeletal muscle of the septic rat. J Appl Physiol 1985; 2001(90): 795-803.
Fowler AA 3rd, Syed AA, Knowlson S, et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med 2014; . https://doi.org/10.1186/1479-5876-12-32.
Nathens AB, Neff MJ, Jurkovich GJ, et al. Randomized, prospective trial of antioxidant supplementation in critically ill surgical patients. Ann Surg 2002; 236: 814-22.
Fowler AA 3rd, Truwit JD, Hite RD, et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. JAMA 2019; 322: 1261-70.
Okamoto K, Tanaka H, Makino Y, Makino I. Restoration of the glucocorticoid receptor function by the phosphodiester compound of vitamins C and E, EPC-K1 (L-ascorbic acid 2-[3,4-dihydro-2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-2H-1-benzopyran-6-yl hydrogen phosphate] potassium salt), via a redox-dependent mechanism. Biochem Pharmacol 1998; 56: 79-86.
Fujita I, Hirano J, Itoh N, Nakanishi T, Tanaka K. Dexamethasone induces sodium-dependant vitamin C transporter in a mouse osteoblastic cell line MC3T3-E1. Br J Nutr 2001; 86: 145-9.
Fujii T, Luethi N, Young PJ, et al. Effect of vitamin C, hydrocortisone, and thiamine vs hydrocortisone alone on time alive and free of vasopressor support among patients with septic shock: the VITAMINS randomized clinical trial. JAMA 2020; 323: 423-31.
Masse MH, Ménard J, Sprague S, et al. Lessening Organ dysfunction with VITamin C (LOVIT): protocol for a randomized controlled trial. Trials 2020; . https://doi.org/10.1186/s13063-019-3834-1.
Wenner JB, Norena M, Khan N, et al. Reliability of intensive care unit admitting and comorbid diagnoses, race, elements of Acute Physiology and Chronic Health Evaluation II score, and predicted probability of mortality in an electronic intensive care unit database. J Crit Care 2009; 24: 401-7.
BC Patient Safety & Quality Council. Critical Care Database - 2019 Available from URL: https://bcpsqc.ca/improve-care/critical-care/critical-care-database-home/ (accessed July 2020),
Zimmerman JE, Kramer AA, McNair DS, Malila FM. Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today’s critically ill patients. Crit Care Med 2006; 34: 1297-310.
Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis.related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996; 22: 707-10.
Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med 2017; 45: 486-552.
Frank LL. Thiamin in clinical practice. JPEN J Parenter Enteral Nutr 2015; 39: 503-20.
The R Foundation. The R Project for Statistical Computing. Vienna, Austria; 2019. Available from URL: https://www.r-project.org/ (accessed July 2020).
Li J. Evidence is stronger than you think: a meta-analysis of vitamin C use in patients with sepsis. Crit Care 2018; . https://doi.org/10.1186/s13054-018-2191-x.
Velagapudi RK, Upadhaya S, Aburahma A, Bachuwa G. Use of vitamin c in patients with sepsis is associated with lower mortality: a meta-analysis. Chest 2018; . https://doi.org/10.1016/j.chest.2018.08.257.
Wei X, Wang Z, Liao X, et al. Efficacy of vitamin C in patients with sepsis: An updated meta-analysis. Eur J Pharmacol 2020; . https://doi.org/10.1016/j.ejphar.2019.172889.
Author contributions
Kimberley Chang, Donald E. G. Griesdale, Denise Foster, David Sweet, and Vinay K. Dhingra contributed to all aspects of this manuscript, including conception and design; acquisition, analysis, and interpretation of data and drafting the article. Megan Harbin contributed to the conception and design of the manuscript, acquisition of data and drafting of manuscript. Constantin Shuster and Michael D. Wood contributed to analysis, and interpretation of data and drafting the article.
Disclosures
None.
Funding statement
No funding was received in support of this work. Donald Griesdale received funding from Michael Smith Foundation for Health Research and is funded through a Health-Professional Investigator Award from the Michael Smith Foundation for Health.
Editorial responsibility
This submission was handled by Dr. Philip M. Jones, Associate Editor, Canadian Journal of Anesthesia.
Commercial or non-commercial affiliations
None.
Other associations
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chang, K., Harbin, M., Shuster, C. et al. Adding vitamin C to hydrocortisone lacks benefit in septic shock: a historical cohort study. Can J Anesth/J Can Anesth 67, 1798–1805 (2020). https://doi.org/10.1007/s12630-020-01814-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-020-01814-1