Abstract
This narrative review discusses the role of thrombin generation in coagulation and bleeding in cardiac surgery, the laboratory methods for clinical detection of impaired thrombin generation, and the available hemostatic interventions that can be used to improve thrombin generation. Coagulopathy after cardiopulmonary bypass (CPB) is associated with excessive blood loss and adverse patient outcomes. Thrombin plays a crucial role in primary hemostasis, and impaired thrombin generation can be an important cause of post-CPB coagulopathy. Existing coagulation assays have significant limitations in assessing thrombin generation, but whole-blood assays designed to measure thrombin generation at the bed-side are under development. Until then, clinicians may need to institute therapy empirically for non-surgical bleeding in the setting of normal coagulation measures. Available therapies for impaired thrombin generation include administration of plasma, prothrombin complex concentrate, and bypassing agents (recombinant activated factor VII and factor eight inhibitor bypassing activity). In vitro experiments have explored the relative potency of these therapies, but clinical studies are lacking. The potential incorporation of thrombin generation assays into clinical practice and treatment algorithms for impaired thrombin generation must await further clinical development.
Résumé
Ce compte rendu narratif discute du rôle de la génération de thrombine dans la coagulation et le saignement en chirurgie cardiaque, des méthodes de laboratoire pour le dépistage clinique d’une génération de thrombine altérée et des interventions hémostatiques disponibles qui peuvent être utilisées pour améliorer la génération de thrombine. Une coagulopathie après la circulation extracorporelle (CEC) est associée à des pertes de sang excessives et à des complications pour les patients. La thrombine joue un rôle essentiel d’hémostase primaire, et une génération de thrombine altérée peut constituer une cause importante de coagulopathie post-CEC. Les analyses de coagulation existantes comportent d’importantes limites en ce qui touche à l’évaluation de la génération de thrombine, mais des analyses de sang complet conçues pour mesurer la génération de thrombine au chevet sont en cours d’élaboration. En attendant, les cliniciens pourraient devoir amorcer un traitement de manière empirique pour prendre en charge les saignements non chirurgicaux dans un contexte de valeurs de coagulation mesurées normales. Les traitements disponibles pour une génération de thrombine altérée comprennent l’administration de plasma, de concentrés de complexe prothrombinique, et d’agents de contournement (bypass) (facteur VII recombinant activé et activité de contournement de l’inhibiteur du facteur VIII). Des expériences in vitro ont exploré l’activité thérapeutique relative de ces traitements, mais les études cliniques manquent. L’intégration potentielle d’analyses de génération de thrombine dans la pratique clinique et d’algorithmes de traitement pour une génération de thrombine altérée doit attendre des développements cliniques plus poussés.
Similar content being viewed by others
Introduction
Patients undergoing cardiac surgery with cardiopulmonary bypass (CPB) frequently develop coagulopathy that can result in excessive blood loss and associated adverse outcomes.1,2,3,4 The causes of coagulopathy are usually multifactorial but can be categorized into four broad domains: impaired thrombin generation, platelet dysfunction, hypofibrinogenemia, and hyperfibrinolysis.5,6,7 Although there is a complex interplay between each of these domains, treatment guidelines have generally focused on the last three while thrombin generation has attracted relatively little attention.8,9 In light of emerging evidence highlighting the importance of impaired thrombin generation as a cause of cardiac surgery-associated coagulopathy,10,11,12 we conducted this narrative review focusing on: 1) the role of thrombin generation in coagulation and on bleeding in cardiac surgery; 2) laboratory methods for clinical detection of impaired thrombin generation; and 3) available hemostatic interventions that can be used to improve thrombin generation in bleeding cardiac surgery patients.
Role of thrombin generation in cell-based model of coagulation
Thrombin is a protease that has a critical role in all phases of clot formation and breakdown.13 The cell-based model of coagulation describes initiation, amplification, and propagation phases.14,15 Initiation begins when tissue factor is exposed to plasma at the site of endothelial injury, and forms a complex with circulating activated factor VII (FVIIa) to activate factors IX and X. In the ensuing amplification phase, activated factor X on the surface of TF-bearing cells generates trace (<1 nM) amounts of thrombin, which then activates platelets as well as factors V, VIII, and XI. The propagation phase takes place on a phosphatidylserine-rich membrane, often of activated platelets, which increases the binding of factors IXa, VIIIa, Xa, and Va, resulting in the thrombin burst necessary to convert fibrinogen to fibrin. Thrombin also activates thrombin-activated fibrinolysis inhibitor and factor XIII, producing a stable fibrin clot.13 Finally, thrombin activates protein C, which inactivates FVa and FVIIIa to stop thrombin generation.
In vivo, thrombin concentration is highly dynamic during the coagulation process and can be categorized into three distinct phases. In phase 1, which lasts two to six minutes and corresponds to the initiation and amplification phases of coagulation, a trace (< 1 nM) amount of thrombin is produced on TF-bearing cells. Phase 2 is the maximum rate of increase (i.e., maximum speed) in thrombin generation that corresponds to the thrombin burst on activated platelets, which lasts for approximately ten minutes. In phase 3, thrombin generation reaches its peak (> 500 nM) before dropping back to baseline because of inhibition by antithrombin, with the area under the curve representing the total amount of free thrombin generated (phase 4).13 Thrombin generation capacity is a key determinant of the structure, stability, and strength of the fibrin clot.13,16 Low thrombin generation capacity leads to generation of clots that are composed of loosely woven fibrin strands that are permeable and prone to fibrinolysis, whereas higher thrombin generation capacity leads to clots that are composed of a dense network of fibrin strands that are less permeable and more resistant to fibrinolysis.13,16
Adequate thrombin generation capacity is therefore crucial for successful primary hemostasis and can be influenced by many factors in the coagulation cascade. As such, thrombin generation dynamics may be a good indicator of global hemostatic function,17,18,19 allowing for targeted, individualized bleeding management in the perioperative setting.
Impaired thrombin generation in cardiac surgery
Cardiac surgery can lead to impaired thrombin generation via several mechanisms (Fig. 1). To prevent catastrophic clotting during CPB, unfractionated heparin is administered to block thrombin generation during CPB and is then neutralized with protamine after CPB. Heparin inhibits thrombin generation during CPB primarily by binding to antithrombin and accelerating (by approximately 1,000-fold) the inactivation of thrombin and other serine proteases, including factors IXa and Xa, by antithrombin.20 Thrombin generation, however, is not completely inhibited by heparin during CPB, which can lead to a progressive consumption of coagulation factors during and after CPB.20,21,22,23 This consumption, in conjunction with perioperative blood loss and CPB-associated hemodilution, causes a substantial (30–40%) drop in most coagulation factor levels (including prothrombin) and platelets,1 and thus impairs thrombin generation. Heparin also causes a substantial increase in tissue factor pathway inhibitor levels that remain elevated even after heparin is neutralized with protamine.11 Tissue factor pathway inhibitor is a reversible inhibitor of the TF-FVIIa-FXa complex and thus a potent inhibitor of thrombin generation at the site of endothelial injury.11,20,24 Further contributing to impaired thrombin generation during cardiac surgery is the progressive platelet dysfunction that occurs during CPB1 and after protamine administration,25 impairing platelet-induced thrombin generation.26
Measurement of thrombin generation capacity
Currently, no assays that specifically measure thrombin generation are available for clinical use. There is, however, increasing experience with research-based assays, such as the calibrated automated thrombogram (CAT),19,27,28,29,30,31 that can reliably measure thrombin concentration during the distinct phases of coagulation outlined above.
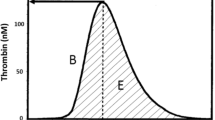
The CAT uses automated fluorometres, microplate technology, and a slow-acting thrombin-specific fluorogenic substrate (that allows for continuous measurement of cleaved substrate) to simultaneously measure thrombin concentration in multiple samples.28,29,30 The resulting “thrombogram”, or thrombin generation curve, measures the dynamic thrombin concentration changes during the phases of coagulation outlined earlier and illustrated in Fig. 2.
Thrombogram parameters. A = lag time (min); B = maximal rate (nM·min-1); C = peak height (nM); D = time to peak (min); E = endogenous thrombin potential (ETP) which is equivalent to the area under the curve. Modified with permission from: Hemker HC, Al Dieri R, De Smedt E, Beguin S. Thrombin generation, a function test of the haemostatic-thrombotic system. Thromb Haemost 2006; 96: 553-61. The parameters correspond to the following phases of thrombin generation: phase 1 (initiation and amplification phases of coagulation) ≈ A; phase 2 (maximum rate of increase in thrombin generation) ≈ B; phase 3 (time to and peak thrombin generation) ≈ C and D; and phase 4 (shaded area: total amount of thrombin generation) ≈ E.13
After activation (usually with low concentrations of tissue factor), the CAT can measure thrombin generation in citrated plasma samples with or without platelets (platelet-poor samples require addition of procoagulant phospholipids to augment tissue factor), but not in whole blood.30 Thus, it measures thrombin generation in the presence of the full spectrum of plasma coagulation proteins close to their physiologic concentrations and, in platelet-rich samples, captures the influence of platelets on thrombin generation.29 Its results, however, are highly dependent on sample preparation (citrate or other anticoagulants) and activation.28,29 Moreover, because of technical issues, CAT cannot measure thrombin generation in whole blood, and thus the important effects of red cells (which may promote thrombin generation by exposing coagulant phospholipids on their surfaces) and white cells (which express tissue factor) are not captured in the resulting thrombogram.30 Next-generation assays currently in development are designed to address the limitations of CAT and may be able to provide reliable, point-of-care measures of thrombin generation capacity in whole blood.32,33
Meanwhile, to diagnose impaired thrombin generation capacity, clinicians have to rely on traditional coagulation assays that measure fibrin clot formation rather than thrombin generation specifically. These include the end-point assays activated clotting time (ACT), prothrombin time (PT), and partial thromboplastin time (PTT), which measure the time from coagulation activation (after addition of relevant triggers) to initiation of fibrin clot formation. Since blood and plasma start to clot in vitro after only a small (< 5%) amount of prothrombin is converted to thrombin, these assays provide very little information on overall thrombin generation capacity.34
Quantitative assays of thrombin generation that measure the by-products of thrombin formation (e.g., prothrombin fragment 1.2 and thrombin-antithrombin complexes) provide more information on how much thrombin has been generated, and have been used in studies examining coagulation after CPB to indicate thrombin formation.1,35,36 These assays, however, require specialized laboratory expertise and equipment, have long analysis times, and do not fully describe the kinetics or tensile strength of an in vivo clot.
Another group of assays are viscoelastic coagulation assays, such as rotational thromboelastometry (ROTEM) and thromboelastography (TEG), which provide a global measure of fibrin clot formation in whole blood.17,37 While the clinical utility of these assays for managing perioperative coagulopathy has been shown,37,38,39 they suffer from the same limitation of end-point assays in that they do not measure thrombin generation specifically.30 Nevertheless, several parameters derived from the clotting curves of these assays indirectly reflect the course of thrombin generation.40 One parameter is the measure of the time from coagulation activation to initiation of fibrin clot formation (clotting time in ROTEM, and R in TEG). Similar to PT and PTT, however, this parameter only measures the initiation phase of thrombin generation. Other parameters that reflect the velocity profile (i.e., slope) of the clotting curve have been correlated with thrombin generation measures and bleeding in various settings.40,41,42,43,44 These include the time from first fibrin clot formation until the clot is 20 mm wide (clot formation time in ROTEM, and K in TEG), and the first derivative plot of the clot formation curve to generate the “thrombus generation velocity curve”. As noted, however, these parameters do not actually measure thrombin generation; rather, they measure kinetics of fibrin clot formation.
Prevalence and clinical relevance of impaired thrombin generation in cardiac surgery
Despite the central role of thrombin generation in coagulation and conduct of CPB, data on the prevalence and clinical relevance of impaired thrombin generation in cardiac surgery is limited to a few small observational studies, which is not surprising given the limitations in diagnostic assays noted above.
Percy et al.45 studied 102 patients undergoing cardiac valve surgery with CPB and found that, compared with baseline levels, there was a greater than 50% drop in thrombin generation potential after CPB. In similar studies, Coakley et al.10 (n = 77) and Bosch et al.11 (n = 29) also found that CPB led to similar drops in thrombin generation potential. These studies, however, were not equipped to delineate the risk factors for impaired thrombin generation nor were they able to elucidate its relationship with postoperative coagulopathy or bleeding. An important consideration when exploring the role of impaired thrombin generation is the natural variability in baseline thrombin generation, as studies have found an inverse relationship between patients’ baseline thrombin generation potential and postoperative bleeding.10,46
Studies have also assessed the relationship of thrombin generation with assessment of hemostasis and potential for guiding hemostatic therapy outside of the cardiac surgery setting (e.g., von Willebrand disease, hemophilia).47,48,49 There are, however, scant data on the potential clinical applications of thrombin generation tests or the clinical impact of impaired thrombin generation on coagulation. Until a reliable, whole blood-based point-of-care assay that directly measures thrombin generation becomes available, it seems reasonable to consider impaired thrombin generation as a cause of cardiac surgery-associated bleeding by a process of elimination. That is, after exclusion of other identifiable causes of coagulopathy—e.g., non-specific causes such as hypothermia or specific causes such as thrombocytopenia, platelet dysfunction, or hypofibrinogenemia—one could consider therapeutic interventions aimed at improving thrombin generation. As an example, a coagulation management algorithm that uses such a step-wise approach to diagnosis and therapy has proven successful in reducing bleeding and transfusions in clinical trials.38,50 For management of non-surgical bleeding during or after cardiac surgery, this algorithm first targets correction of thrombocytopenia, platelet dysfunction, and hypofibrinogenemia. If bleeding continues after these factors have been corrected, the algorithm recommends targeting impaired thrombin generation, either confirmed by relevant assays (e.g., elevated ROTEM clotting time or international normalized ratio) or empirically. In a cluster randomized trial, the algorithm significantly reduced surgical re-exploration, and red-cell and platelet transfusions.38 Clearly, however, one cannot determine which component of the algorithm (if any) was responsible for improving the outcomes. Nevertheless, it would be reasonable to expect that the addition of a quantifiable parameter that can reliably identify impaired thrombin generation could improve the management of coagulopathy and further improve outcomes.
Available hemostatic interventions for improving thrombin generation
Current options for improving thrombin generation include the administration of plasma, prothrombin complex concentrates (PCCs), and bypassing agents (recombinant activated factor VIIa [rFVIIa] and factor eight inhibitor bypassing activity [FEIBA]).
Plasma contains the full complement of coagulation factors, fibrinogen, plasma proteins such as albumin, as well as naturally occurring anticoagulant proteins (i.e., protein C, protein S, antithrombin, tissue factor pathway inhibitor). Thus, plasma delivers a balanced array of coagulation factors and can thereby improve thrombin generation.45 Nevertheless, the concentration of factor concentrates in plasma varies from unit to unit, and the recommended dose of 10–15 mL·kg−1 in bleeding patients is aimed at increasing plasma factor concentrates in general rather than improving thrombin generation per se.51
Prothrombin complex concentrates are derived from plasma and contain factors II, VII, IX, and X, as well as anticoagulants protein C, protein S, and heparin. There are 3-factor and 4-factor PCCs available, with the difference being the presence of factor VII (only in the 4-factor PCC).52 While primarily developed for reversal of vitamin K antagonists, their use for management of perioperative bleeding is increasing.53,54,55,56 There is gathering evidence that PCCs augment thrombin generation in the setting of oral anticoagulation,57 in models of dilutional coagulopathy,54,58 and post-CPB.45,59,60 Animal models have shown that this effect is correlated with reductions in diffuse bleeding,55 but clinical data connecting improved thrombin generation to reduced bleeding and transfusions are lacking. While PCCs do not have the full complement of coagulation factors that plasma has, the relative advantages of PCCs over plasma include a lower volume of infusion, more predictable pharmacodynamic profile, and lower risk of transmission of pathogens. There are, however, theoretical concerns regarding the potential for late thromboembolic events due to an imbalance between procoagulant and anticoagulant proteins in bleeding patients who receive repeated doses of PCCs.53
Recombinant activated factor VII (rFVIIa) is a potent pro-hemostatic agent that is approved for use in hemophiliac patients with inhibitors, factor VII deficiency, and Glanzmann’s thrombasthenia. It acts by binding to the surface of platelets and directly activating factors IX and X, resulting in a dose-dependent increase in thrombin generation that leads to the formation of a well-structured fibrin clot that is resistant to fibrinolysis.61 Outside of approved indications, rFVIIa is used in cardiac surgery to treat bleeding patients in whom other hemostatic agents have failed.62 In a placebo-controlled double-blinded randomized trial in patients bleeding after cardiac surgery, rFVIIa reduced transfusions and the need for surgical re-exploration, but the number of critical adverse events was higher in the rFVIIa group.63 If considering the off-label use of rFVIIa to control refractory bleeding, clinicians are advised to consider the potential for thromboembolic events, to administer lower doses than those used in hemophiliac patients (a dose of 35–70 µg·kg−1 has been shown to be effective),64 and correct other hemostatic parameters before administering rFVIIa.65
Factor eight inhibitor bypassing activity (FEIBA) is another procoagulant agent that has been used in managing refractory bleeding. It is an anti-inhibitor coagulation complex that contains primarily non-activated but also activated factors II, VII, IX, and X.66 Similar to rFVIIa, FEIBA acts on platelets to increase thrombin generation.66 As its name suggests, FEIBA is almost exclusively used as a bypassing agent in hemophiliac patients with inhibitors, and published experience in cardiac surgery for patients without hemophilia is scarce.67,68 To our knowledge, no guidelines currently recommend the use of FEIBA for management of bleeding in cardiac surgery.
Comparative clinical studies between treatment options for impaired thrombin generation are lacking. In vitro exploratory studies have shown that addition of plasma, PCC, rFVIIa, and FEIBA to post-CPB blood samples all significantly increase thrombin generation.45,60 Clearly, however, clinical trials are needed to determine the effect of these therapies on thrombin generation in vivo and to compare their relative clinical effectiveness in controlling bleeding caused by impaired thrombin generation.
Conclusions
Postoperative bleeding remains a significant source of morbidity and mortality for patients undergoing cardiac surgery. Given the importance of thrombin generation in coagulation, further work is needed to improve our understanding of the role of impaired thrombin generation in perioperative coagulopathy and how best to diagnose and treat impaired thrombin generation as a cause of perioperative bleeding. To date, no thrombin measurement assays can be used clinically, and current transfusion algorithms generally address impaired thrombin generation as a diagnosis of exclusion. In the future, thrombography may be useful in assessing the specific contribution of impaired thrombin generation to the coagulopathy, allowing us to advance the use of targeted hemostatic therapy for bleeding patients.
References
Karkouti K, McCluskey SA, Syed S, Pazaratz C, Poonawala H, Crowther MA. The influence of perioperative coagulation status on postoperative blood loss in complex cardiac surgery: a prospective observational study. Anesth Analg 2010; 110: 1533-40.
Karkouti K, Wijeysundera DN, Beattie WS, et al. Variability and predictability of large-volume red blood cell transfusion in cardiac surgery: a multicenter study. Transfusion 2007; 47: 2081-8.
Hofer J, Fries D, Solomon C, Velik-Salchner C, Ausserer J. A snapshot of coagulopathy after cardiopulmonary bypass. Clin Appl Thromb Hemost 2016; 22: 505-11.
Karkouti K, Wijeysundera DN, Yau TM, et al. The independent association of massive blood loss with mortality in cardiac surgery. Transfusion 2004; 44: 1453-62.
Johansson PI, Solbeck S, Genet G, Stensballe J, Ostrowski SR. Coagulopathy and hemostatic monitoring in cardiac surgery: an update. Scand Cardiovasc J 2012; 46: 194-202.
Despotis G, Eby C, Lublin DM. A review of transfusion risks and optimal management of perioperative bleeding with cardiac surgery. Transfusion 2008; 48(1 Suppl): 2S-30S.
Karkouti K, Ho LT. Preventing and managing catastrophic bleeding during extracorporeal circulation. Hematology Am Soc Hematol Educ Program 2018; 1: 522-9.
Task Force on Patient Blood Management for Adult Cardiac Surgery of the European Association for Cardio-Thoracic Surgery (EACTS) and the European Association of Cardiothoracic Anaesthesiology (EACTA); Boer C, Meesters MI, Milojevic M, et al. 2017 EACTS/EACTA guidelines on patient blood management for adult cardiac surgery. J Cardiothorac Vasc Anesth 2018; 32: 88-120.
Raphael J, Mazer CD, Subramani S, et al. Society of Cardiovascular Anesthesiologists Clinical Practice Improvement Advisory for management of perioperative bleeding and hemostasis in cardiac surgery patients. Anesth Analg 2019; 129: 1209-21.
Coakley M, Hall JE, Evans C, et al. Assessment of thrombin generation measured before and after cardiopulmonary bypass surgery and its association with postoperative bleeding. J Thromb Haemost 2011; 9: 282-92.
Bosch YP, Al Dieri R, ten Cate H, et al. Measurement of thrombin generation intra-operatively and its association with bleeding tendency after cardiac surgery. Thromb Res 2014; 133: 488-94.
Kremers RM, Bosch YP, Bloemen S, et al. A reduction of prothrombin conversion by cardiac surgery with cardiopulmonary bypass shifts the haemostatic balance towards bleeding. Thromb Haemost 2016; 116: 442-51.
Wolberg AS. Thrombin generation and fibrin clot structure. Blood Rev 2007; 21: 131-42.
Hoffman M, Monroe DM 3rd. A cell-based model of hemostasis. Thromb Haemost 2001; 85: 958-65.
Roberts HR, Hoffman M, Monroe DM. A cell-based model of thrombin generation. Semin Thromb Hemost 2006; 32(Suppl 1): 32-8.
Wolberg AS, Campbell RA. Thrombin generation, fibrin clot formation and hemostasis. Transfus Apher Sci 2008; 38: 15-23.
Brummel-Ziedins KE, Wolberg AS. Global assays of hemostasis. Curr Opin Hematol 2014; 21: 395-403.
Dinkelaar J, Patiwael S, Harenberg J, Leyte A, Brinkman HJ. Global coagulation tests: their applicability for measuring direct factor Xa- and thrombin inhibition and reversal of anticoagulation by prothrombin complex concentrate. Clin Chem Lab Med 2014; 52: 1615-23.
Castoldi E, Rosing J. Thrombin generation tests. Thromb Res 2011; 127(Suppl 3): S21-5.
Ferraris VA, Ferraris SP. Thrombin and cardiopulmonary bypass: a paradigm for evaluation of the regulation of hemostasis. Int J Angiol 2005; 14: 193-210.
Sniecinski RM, Chandler WL. Activation of the hemostatic system during cardiopulmonary bypass. Anesth Analg 2011; 113: 1319-33.
Chandler WL, Velan T. Estimating the rate of thrombin and fibrin generation in vivo during cardiopulmonary bypass. Blood 2003; 101: 4355-62.
Boisclair MD, Lane DA, Philippou H, et al. Mechanisms of thrombin generation during surgery and cardiopulmonary bypass. Blood 1993; 82: 3350-7.
Tanaka KA, Key NS, Levy JH. Blood coagulation: hemostasis and thrombin regulation. Anesth Analg 2009; 108: 1433-46.
Boer C, Meesters MI, Veerhoek D, Vonk AB. Anticoagulant and side-effects of protamine in cardiac surgery: a narrative review. Br J Anaesth 2018; 120: 914-27.
Monroe DM, Hoffman M, Roberts HR. Platelets and thrombin generation. Arterioscler Thromb Vasc Biol 2002; 22: 1381-9.
Hemker HC, Giesen P, AlDieri R, et al. The calibrated automated thrombogram (CAT): a universal routine test for hyper- and hypocoagulability. Pathophysiol Haemost Thromb 2002; 32: 249-53.
Hemker HC, Al Dieri R, Beguin S. Thrombin generation assays: accruing clinical relevance. Curr Opin Hematol 2004; 11: 170-5.
Hemker HC, Al Dieri R, De Smedt E, Beguin S. Thrombin generation, a function test of the haemostatic-thrombotic system. Thromb Haemost 2006; 96: 553-61.
Tripodi A. The long-awaited whole-blood thrombin generation test. Clin Chem 2012; 58: 1173-5.
Lance MD. A general review of major global coagulation assays: thrombelastography, thrombin generation test and clot waveform analysis. Thromb J 2015. Doi: https://doi.org/10.1186/1477-9560-13-1.
Ninivaggi M, Apitz-Castro R, Dargaud Y, de Laat B, Hemker HC, Lindhout T. Whole-blood thrombin generation monitored with a calibrated automated thrombogram-based assay. Clin Chem 2012; 58: 1252-9.
Dai Y, Gross P. A point-of-care assay measuring thrombin activity in finger-prick whole blood. Thromb Res 2019; 182 (Suppl 1): 4-5 (abstract).
Mann KG, Brummel K, Butenas S. What is all that thrombin for? J Thromb Haemost 2003; 1: 1504-14.
Knudsen L, Hasenkam JM, Kure HH, et al. Monitoring thrombin generation with prothrombin fragment 1.2 assay during cardiopulmonary bypass surgery. Thromb Res 1996; 84: 45-54.
Bannan S, Danby A, Cowan D, Ashraf S, Gesinde M, Martin P. Cell activation and thrombin generation in heparin bonded cardiopulmonary bypass circuits using a novel in vitro model. Eur J Cardiothorac Surg 1997; 12: 268-75.
Curry NS, Davenport R, Pavord S, et al. The use of viscoelastic haemostatic assays in the management of major bleeding: a British Society for Haematology Guideline. Br J Haematol 2018; 182: 789-806.
Karkouti K, Callum J, Wijeysundera DN, et al. Point-of-care hemostatic testing in cardiac surgery: a stepped-wedge clustered randomized controlled trial. Circulation 2016; 134: 1152-62.
Sniecinski RM. Viscoelastic testing: it’s not the measurement, it’s what you do with it! Can J Anesth 2018; 65: 1283-7.
Sorensen B, Johansen P, Christiansen K, Woelke M, Ingerslev J. Whole blood coagulation thrombelastographic profiles employing minimal tissue factor activation. J Thromb Haemost 2003; 1: 551-8.
Rivard GE, Brummel-Ziedins KE, Mann KG, Fan L, Hofer A, Cohen E. Evaluation of the profile of thrombin generation during the process of whole blood clotting as assessed by thrombelastography. J Thromb Haemost 2005; 3: 2039-43.
Tafur LA, Taura P, Blasi A, et al. Rotation thromboelastometry velocity curve predicts blood loss during liver transplantation. Br J Anaesth 2016; 117: 741-8.
Johansson PI, Svendsen MS, Salado J, Bochsen L, Kristensen AT. Investigation of the thrombin-generating capacity, evaluated by thrombogram, and clot formation evaluated by thrombelastography of platelets stored in the blood bank for up to 7 days. Vox Sang 2008; 94: 113-8.
Faraoni D, Fenger-Eriksen C, Gillard S, Willems H, Levy H, Van der Linden P. Evaluation of dynamic parameters of thrombus formation measured on whole blood using rotational thromboelastometry in children undergoing cardiac surgery: a descriptive study. Paediatr Anaesth 2015; 25: 573-9.
Percy CL, Hartmann R, Jones RM, et al. Correcting thrombin generation ex vivo using different haemostatic agents following cardiac surgery requiring the use of cardiopulmonary bypass. Blood Coagul Fibrinolysis 2015; 26: 357-67.
Bosch Y, Al Dieri R, ten Cate H, et al. Preoperative thrombin generation is predictive for the risk of blood loss after cardiac surgery: a research article. J Cardiothorac Surg 2013. Doi: https://doi.org/10.1186/1749-8090-8-154.
Duarte RC, Ferreira CN, Rios DR, Reis HJ, Carvalho MD. Thrombin generation assays for global evaluation of the hemostatic system: perspectives and limitations. Rev Bras Hematol Hemoter 2017; 39: 259-65.
Rugeri L, Beguin S, Hemker C, et al. Thrombin-generating capacity in patients with von Willebrand’s disease. Haematologica 2007; 92: 1639-46.
Young G, Sorensen B, Dargaud Y, Negrier C, Brummel-Ziedins K, Key NS. Thrombin generation and whole blood viscoelastic assays in the management of hemophilia: current state of art and future perspectives. Blood 2013; 121: 1944-50.
Karkouti K, McCluskey SA, Callum J, et al. Evaluation of a novel transfusion algorithm employing point-of-care coagulation assays in cardiac surgery: a retrospective cohort study with interrupted time-series analysis. Anesthesiology 2015; 122: 560-70.
American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Practice guidelines for perioperative blood transfusion and adjuvant therapies: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology 2006; 105: 198-208.
Ghadimi K, Levy JH, Welsby IJ. Prothrombin complex concentrates for bleeding in the perioperative setting. Anesth Analg 2016; 122: 1287-300.
Grottke O, Levy JH. Prothrombin complex concentrates in trauma and perioperative bleeding. Anesthesiology 2015; 122: 923-31.
Mitterlechner T, Innerhofer P, Streif W, et al. Prothrombin complex concentrate and recombinant prothrombin alone or in combination with recombinant factor X and FVIIa in dilutional coagulopathy: a porcine model. J Thromb Haemost 2011; 9: 729-37.
Kaspereit F, Hoffmann S, Pragst I, Dickneite G. Prothrombin complex concentrate mitigates diffuse bleeding after cardiopulmonary bypass in a porcine model. Br J Anaesth 2010; 105: 576-82.
Fitzgerald J, Lenihan M, Callum J, et al. Use of prothrombin complex concentrate for management of coagulopathy after cardiac surgery: a propensity score matched comparison to plasma. Br J Anaesth 2018; 120: 928-34.
Schulman S, Gross PL, Ritchie B, et al. Prothrombin complex concentrate for major bleeding on factor Xa inhibitors: a prospective cohort study. Thromb Haemost 2018; 118: 842-51.
Mitrophanov AY, Szlam F, Sniecinski RM, Levy JH, Reifman J. A step toward balance: thrombin generation improvement via procoagulant factor and antithrombin supplementation. Anesth Analg 2016; 123: 535-46.
Guzzetta NA, Szlam F, Kiser AS, et al. Augmentation of thrombin generation in neonates undergoing cardiopulmonary bypass. Br J Anaesth 2014; 112: 319-27.
Franklin SW, Szlam F, Fernandez JD, Leong T, Tanaka KA, Guzzetta NA. Optimizing thrombin generation with 4-factor prothrombin complex concentrates in neonatal plasma after cardiopulmonary bypass. Anesth Analg 2016; 122: 935-42.
Hedner U. Recombinant activated factor VII: 30 years of research and innovation. Blood Rev 2015; 29(Suppl 1): S4-8.
Karkouti K, Beattie WS, Arellano R, et al. Comprehensive Canadian review of the off-label use of recombinant activated factor VII in cardiac surgery. Circulation 2008; 118: 331-8.
Gill R, Herbertson M, Vuylsteke A, et al. Safety and efficacy of recombinant activated factor VII: a randomized placebo-controlled trial in the setting of bleeding after cardiac surgery. Circulation 2009; 120: 21-7.
Karkouti K, Beattie WS, Crowther MA, et al. The role of recombinant factor VIIa in on-pump cardiac surgery: proceedings of the Canadian Consensus Conference. Can J Anesth 2007; 54: 573-82.
Vincent JL, Rossaint R, Riou B, Ozier Y, Zideman D, Spahn DR. Recommendations on the use of recombinant activated factor VII as an adjunctive treatment for massive bleeding–a European perspective. Crit Care 2006. Doi: https://doi.org/10.1186/1749-8090-8-154.
Turecek PL, Varadi K, Gritsch H, Schwarz HP. FEIBA: mode of action. Haemophilia 2004; 10(Suppl 2): 3-9.
Balsam LB, Timek TA, Pelletier MP. Factor eight inhibitor bypassing activity (FEIBA) for refractory bleeding in cardiac surgery: review of clinical outcomes. J Card Surg 2008; 23: 614-21.
Rao VK, Lobato RL, Bartlett B, et al. Factor VIII inhibitor bypass activity and recombinant activated factor VII in cardiac surgery. J Cardiothorac Vasc Anesth 2014; 28: 1221-6.
Author contributions
All authors contributed to the conception or design of the study.
Conflicts of interest
None.
Funding statement
None.
Editorial responsibility
This submission was handled by Dr. Hilary P. Grocott, Editor-in-Chief, Canadian Journal of Anesthesia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fitzgerald, J., McMonnies, R., Sharkey, A. et al. Thrombin generation and bleeding in cardiac surgery: a clinical narrative review. Can J Anesth/J Can Anesth 67, 746–753 (2020). https://doi.org/10.1007/s12630-020-01609-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-020-01609-4