Abstract
Purpose
Severity of hypoxemic events resulting from obstructive sleep apnea (OSA) is correlated with increased risk of complications and sudden death. We studied the use of a peripheral transcutaneous electrical stimulus (TES) on the magnitude and duration of sleep apnea associated hypoxemia in postoperative patients at high risk for OSA.
Methods
In this randomized, double-blind, controlled, single-centre trial, 106 adult patients undergoing elective surgery who were at medium to high risk for OSA (sleep apnea clinical scores of 18–35) were randomized to either TES (active stimulus group, n = 53) or control (non-stimulus group, n = 53) during their stay in the postanesthesia care unit. Transcutaneous electrical stimuli were delivered at threshold oxygen saturation measurements (SpO2) ≤ 93%. The primary endpoint was the SpO2 area under the curve (AUC) < 90%. Secondary endpoints included the percentage of patients with SpO2 < 90%, duration SpO2 < 90%, lowest SpO2 in the first hour, and adverse events associated with TES.
Results
Compared with controls (n = 45), those in the active group (n = 34) showed a decreased SpO2 AUC < 90% (median 0.0 vs 15.2 % sec, respectively; P = 0.009), a smaller percentage of subjects with SpO2 < 90% (47% active vs 71% control; P = 0.03), a shorter duration of SpO2 < 90% (median 0.0 vs 19.1 sec, respectively; P = 0.01), and a higher nadir of SpO2 recorded during the first hour (median 90.5% vs 87.9%, respectively; P = 0.04). Among patients with at least one SpO2 < 93%, there were fewer with SpO2 < 90% in the active group (55% vs 84%, respectively; P = 0.009). No adverse events related to TES were reported.
Conclusion
In postoperative surgical patients at risk for OSA, peripheral transcutaneous electrical stimulation applied during apneic episodes decreased the duration and magnitude of hypoxemia.
Trial registration
www.ClinicalTrials.gov (NCT02554110); registered 18 September, 2015.
Résumé
Objectif
La sévérité des incidents hypoxémiques résultant d’une apnée obstructive du sommeil (AOS) est corrélée à un risque accru de complications et de mort subite. Nous avons étudié l’utilisation d’un stimulus électrique transcutané (SET) périphérique sur l’ampleur et la durée de l’hypoxémie associée à l’apnée du sommeil chez des patients postopératoires courant un risque élevé d’AOS.
Méthode
Dans cette étude randomisée, à double insu, contrôlée et monocentrique, 106 patients adultes subissant une chirurgie non urgente et courant un risque modéré à élevé d’AOS (scores cliniques d’apnée du sommeil de 18-35) ont été randomisés à recevoir un SET (groupe stimulus actif, n = 53) ou aucune intervention (groupe sans stimulus, n = 53) pendant leur séjour en salle de réveil. Les stimuli électriques transcutanés ont été appliqués lorsque les mesures de saturation en oxygène atteignaient un seuil de SpO2 ≤ 93 %. Le critère d’évaluation principal était la surface sous la courbe (SSC) de la SpO2 < 90 %. Les critères d’évaluation secondaires comprenaient le pourcentage de patients présentant une SpO2 < 90 %, la durée de la SpO2 < 90 %, la SpO2 la plus basse au cours de la première heure, et les événements indésirables associés au SET.
Résultats
Par rapport au groupe témoin (n = 45), les patients dans le groupe actif (n = 34) ont affiché une SSC réduite de la SpO2 < 90 % (moyenne 0,0 vs 15,2 % sec, respectivement; P = 0,009), un nombre plus faible de patients ayant une SpO2 < 90 % (47 % dans le groupe actif vs 71 % groupe témoin; P = 0,03), une durée plus courte de la SpO2 < 90 % (moyenne 0,0 vs 19,1 sec, respectivement; P = 0,01), ainsi qu’un nadir plus élevé de SpO2 enregistré au cours de la première heure (moyenne 90,5 % vs 87,9 %, respectivement; P = 0,04). Parmi les patients ayant au moins une SpO2 < 93 %, il y avait moins de patients avec une SpO2 < 90 % dans le groupe actif (55 % vs 84 %, respectivement; P = 0,009). Aucun événement indésirable lié au SET n’a été rapporté.
Conclusion
Chez les patients chirurgicaux postopératoires courant un risque d’AOS, une stimulation électrique transcutanée périphérique appliquée pendant les épisodes d’apnée a réduit la durée et l’ampleur de l’hypoxémie.
Enregistrement de l’étude
www.ClinicalTrials.gov (NCT02554110); enregistrée le 18 septembre 2015.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Obstructive sleep apnea (OSA) is increasing throughout the world’s population1 in concordance with obesity rates.2 In the United States, depending on the subgroup, OSA has increased by an estimated 14–55% from 1988–1994 to 2007–2010.3 Data from Europe and North America indicate an overall prevalence of 9–38%.1
Obstructive sleep apnea in the surgical population mirrors the general population with a prevalence of 22%.4 Patients with OSA have a greater number of perioperative complications and adverse events than non-OSA patients.5,6 Anesthetics, opioids, and sedating medications increase the risk of complications,7 which range from mild hypoxemia8,9 to sudden death.10,11,12,13,14 Sudden postoperative death13,14 is believed to result from apnea-related hypoxemia,10,12,15 and the severity of hypoxemia during sleep strongly predicts sudden death independently of other risk factors.12
One strategy to limit OSA-related hypoxemic events is to restore ventilation when alarms indicating either apnea or oxygen desaturation are triggered. Indeed, this is the strategy commonly taken in the postanesthesia care unit (PACU), where cessation of breathing and/or hypoxemia are common in both OSA and non-OSA patients. In this case, algorithms that rely on alarm thresholds immediately prompt nursing interventions to encourage (or assist) patient ventilation. An evolving (and complementary) strategy is to use peripheral transcutaneous electrical stimulation (TES), triggered by similar alarm thresholds, to restore ventilation and so interrupt hypoxemic events. For example, TES applied to the submental area has been shown to decrease hypoxemic events in non-surgical OSA patients.16,17,18,19,20,21,22
In this randomized-controlled clinical trial, we evaluated the effectiveness of peripheral TES applied to the ventral surface of the wrist to reduce the duration and magnitude of oxygen desaturations in postoperative patients at risk for OSA.
Methods
This study was approved by the Mayo Clinic Institutional Review Board (IRB) and is registered in ClinicalTrials.gov (NCT02554110) as well as the registry for the World Health Organization and International Committee of Medical Journal Editors (ISRCTN 89522367). The study design is a prospective, randomized, double-blind, controlled clinical trial that assessed the ability of TES to limit the duration and magnitude of oxygen desaturation in postoperative patients recovering in the PACU. Patients assigned to the active group received low-level TES applied to the ventral surface of the wrist via electrodes, in response to oxygen saturation ≤ 93%. The control group had the TES device applied but did not receive electrical stimuli in response to oxygen saturation ≤ 93%.
In conjunction with Mayo Clinic’s Division of Engineering (DOE), a closed-loop system device (hereinafter “device”) was developed which monitors oxygen saturation (SpO2) and delivers low-level electrical stimuli when a programmed threshold SpO2 has been reached.23 The device consists of the following integrated components: a pulse oximeter (Radical-7®, Masimo Corporation, Irvine, CA, USA)24 to measure peripheral SpO2 via digital probe, a peripheral nerve stimulator (DS7A, Digitimer, Welwyn Garden City, Hertfordshire, UK)25 to deliver electrical stimulation via a bar electrode26 placed on the ventral surface of the wrist, a motion sensor (MotionNode, Motion Workshop, Seattle, WA, USA)27 connected to an accelerometer fixed to the same hand bearing the SpO2 probe to help identify spurious SpO2 measurements due to movement, and a nurse-operated foot pedal for recording nursing interventions during the study (Savant Elite, Kinesis Corporation, Bothell, WA, USA).28 Hardware components were controlled with a custom software application, developed in collaboration with Consistent Systems Engineering. The control software was installed on a Hewlett Packard 2570p computer (Hewlett Packard, Palo Alto, CA, USA). All study device components were mounted on a portable stand positioned next to the patients’ bed in the PACU (Fig. 1).
A safety assessment of the device was completed by the DOE safety engineer according to the International Organization for Standardization 14971-based safety risk management process, and the International Electrotechnical Commission, 60601-1.23 The device was approved for study and deemed to be a “non-significant risk” by the Mayo Clinic IRB.
As indicated in the device’s functional algorithm (Fig. 2), when SpO2 reached ≤ 93%, a pre-determined, patient-specific mA current stimulus was administered for one second at a rate of 50 Hz and pulse duration of 500 µsec. A second stimulus of similar intensity was administered for three seconds if the SpO2 had not increased by a minimum of 1% within 30 sec. After a second stimulus, the device entered a “monitor only” mode for five minutes allowing for evaluation of response. Stimulation was withheld when spurious O2 saturation recording was flagged by hand movement detected by the accelerometer. Similarly, stimulation was withheld when poor oximetry signal quality was detected, as indicated by a signal identification and quality indicator (IQ) < 30 (manufacturer proprietary index of low signal reliability).29
Participant selection
The primary endpoint of interest was the area under the SpO2 curve below 90%. The sample size was determined using information from a prior study which evaluated patients with and without device activation and found hypoxemic episodes were less profound when the device was activated (91.0 ± 3.4% ON vs 88.5 ± 3.3% OFF).30 From these data, the difference between groups was approximately 0.75 standard deviation units. Although it was not known whether the primary endpoint would satisfy the assumption for using a parametric approach, we chose a sample size of n = 40 per group. For a parametric approach, this sample size would provide statistical power (two-tailed, alpha = 0.05) of 90% to detect a difference between groups of 0.75 standard deviation units. Target subject accrual was 100 participants. This number was selected to accommodate 20% attrition and so achieve a sample size of n = 40 per group.
Participants were recruited in the Preoperative Evaluation Clinic from October 2015 to January 2017. Patients who met inclusion criteria (Table 1) were invited to participate in the study and informed, written consent was obtained from all participants. The Sleep Apnea Clinical Score (SACS ≥ 15) was used to identify patients at risk for OSA31 (see Appendix). Of relevance, previous research has shown that PACU patients with SACS ≥ 15 are at increased risk of desaturation and apneic and hypopneic episodes.32 Patients with a pre-existing diagnosis of OSA were excluded because of possible confounding effect of continuous positive airway pressure use. Several exclusion criteria eliminated patients with cardiopulmonary and other conditions that would confound study data collection (Table 1).
Stimulus titration
Patient-specific TES thresholds were determined in the Preoperative Evaluation Clinic after informed written consent for study participation was obtained. A Digitimer E.SB010 bar surface electrode26 was affixed to the mid-ventral surface of the wrist and attached to a Digitimer DS7A stimulator. A graduated stimulus was delivered, starting at a 1 mA (500 µs width and frequency of 50 Hz), and titrated until the participant judged that the stimulus would likely awaken them without objectionable discomfort. Any participant who could not identify an appropriate intensity of stimulation between 1–4 mA was excluded from the study. Transcutaneous electrical stimulus thresholds for all study participants were recorded and the same apparatus was used to deliver stimuli during the active phase of investigation.
Randomization process
A randomization schedule, with sequentially numbered subject identifiers, was generated by the study statistician using blocks of size n = 4. This ensured that after every fourth subject was randomized, there was an equal number of subjects assigned to each group. This randomization schedule was provided to the software engineer who incorporated all subject number and treatment assignment information into the device controller. Based on the treatment assigned to a given participant, the device operated either in active mode, delivering algorithm-directed stimuli, or in control mode, monitoring, and recording data but withholding otherwise-qualifying stimulation. In accordance with the double-blind study design, the randomization schedule linking the subject identifier to treatment assignment was known only by the statistician and software engineer; the patient care team was blinded to the participant group.
Procedure
Anesthesia care was left entirely to the discretion of the attending anesthesiologist. The only provisions for this study were that the patient had to be awake and extubated, per institutional standards, prior to transfer from the operating room to the PACU, and that patients were normothermic (temperature ≥ 35.5°C). Upon arrival to the PACU, patients were connected to the device.
All participants received the same standardized supplemental oxygen therapy protocol in the PACU. Oxygen was delivered by nasal cannula at 2–4 L·min−1 to maintain SpO2 > 92% and was gradually weaned when SpO2 remained above 92%. All study patients were monitored per PACU routine using a Philips monitor (Koninklijke Philips N.V., Eindhoven, Netherlands) with audible alarms set for SpO2 ≤ 90% and respiratory rate < 8 per minute. Respiratory rate was measured through standard electrocardiogram leads and SpO2 with a separate SpO2 probe placed opposite to the hand of the study SpO2 probe. Nurses indicated any interventions (verbal prompts and/or tactile stimulation) that were made in response to audible desaturation monitor alarms (SpO2 ≤ 90%), apnea > ten seconds, or respiratory rate < 8 per minute by pressing the foot pedal of the device. With regard to the device, oxygen saturation was continuously monitored using a disposable finger probe (ipsilateral to arm without a blood pressure cuff). Transcutaneous electrical stimulation of the wrist, on the same side as the pulse oximeter, was achieved using currents of 1.0–4.0 mA based upon the pre-determined patient-specific thresholds (above).
The device programmed algorithm (Fig. 2) used a threshold SpO2 ≤ 93%. Data collection for the control group was identical to the active group: by design, control subjects did not receive TES triggered by oxygen desaturation. All participants remained in the PACU for a minimum of 60 min during which time the data were collected. Given the possibility that TES could mask respiratory depression (hypoxemia, apnea) in the active group, which is normally a criterion for an extended PACU stay, all participants received 24 hr of remote pulse oximetry monitoring.
Data collection
Data were recorded once each second during the 60-min interval in the PACU. Data collected included: SpO2, signal IQ, delivery of stimuli, accelerometer motion, and nursing interventions. In accordance with Masimo recommendations, SpO2 data were considered to be unreliable when the pulse oximetry signal IQ was less than 30 (signal IQ range 0–100).29 As such, these values were programmed to be “missing” and not further evaluated. After eliminating data with a low signal IQ, the SpO2 recordings still included obvious outliers (e.g., SpO2 < 25%).33 Therefore, additional data scrutiny was performed by visual inspection of de-identified subject-specific plots of SpO2 over time. The SpO2 outliers frequently occurred immediately before and after the signal IQ measured less than 30. To address these outliers, all SpO2 values were set to “missing” for three seconds before and after the pulse oximetry signal IQ was less than 30.
Endpoints
The primary endpoint was the SpO2 area under the curve (AUC) < 90%. Secondary endpoints included the percentage of patients with SpO2 < 90%, duration SpO2 < 90%, lowest SpO2 in the first hour, and adverse events associated with the device—specifically TES (burns, unresolved numbness or tingling of extremity, or new onset cardiac arrhythmia). Each participant was interviewed, in-hospital or by phone, 24–72 hr after their participation in the study. Any issues possibly related to the device were documented.
Statistical analysis
Data are presented as median [interquartile range] for continuous variables and frequency percentages for categorical variables. The SpO2 AUC below 90% was calculated using the trapezoidal rule, a mathematical technique for approximating integrals (i.e., the area under the graph of a given function). Continuous endpoints between groups were compared using the rank sum test and binary endpoints were compared using the Chi-square test. The rank sum test assesses whether one group tends to have higher values than another. Since higher values could result from differences in either location or shape, it should be noted that the rank sum test does not specifically compare medians. Two-tailed P values < 0.05 were considered statistically significant.
Results
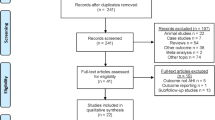
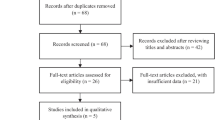
Of the 114 patients assessed for eligibility, 106 were randomized: 53 to the active group and 53 to the control group (Fig. 3). Of these, 27 patients (19 active, eight control) were eliminated because of problems encountered in the Preoperative Evaluation Clinic (technical difficulties), or on the day of surgery (procedure rescheduled as outpatient or cancelled, technical difficulties, hypothermia, patient withdrawal), leaving 79 subjects (34 active, 45 control) who completed the study.
Baseline characteristics of the participants, summarized in Table 2, indicate that those in the active group were younger compared with controls. Oxygen saturation data and nursing interventions recorded during the 60-min trial in the PACU are presented in Table 3. While the percentage of patients with SpO2 below 93% did not differ between groups, those in the active group showed a decreased SpO2 AUC below 90%. To account for the possible effect of age (actives were younger than controls), a post-hoc analysis was done using a least squares regression with a Van der Waerden rank transformation of AUC below 90% included as the dependent variable, and treatment (active vs control) and age as explanatory variables. From this analysis, we determined that the effect of treatment persisted (P = 0.006) even after adjusting for age. A post hoc age-adjusted analysis of the AUC below 90% was also performed for the subset of those who had at least one measure below 90%. Among this subset, the difference in the AUC below 90% did not differ significantly between groups (P = 0.09) after adjusting for age.
With regard to the secondary endpoints, compared with controls, the active group showed a decrease in the percentage of patients with any SpO2 below 90%, a shorter duration of time with SpO2 below 90%, and a higher nadir of pulse oximetry reading. Among those who had at least one SpO2 below 93%, the percentage of patients proceeding to an SpO2 below 90% was lower in the active group. Among those who experienced at least one SpO2 measurement below 90%, the total time below 90% and the AUC below 90% did not differ between actives vs controls. Examples of subject-specific SpO2 plots from a control and active participant illustrate stimuli and nursing interventions to threshold SpO2 and subsequent improvement in SpO2 recordings (Fig. 4).
Active (lower panel) vs control sample SpO2 plots (upper panel). Stimuli were administered for SpO2 ≤ 93% measurements in active but withheld for SpO2 ≤ 93% readings in control participant. Blue dots indicate when 93% O2 saturation thresholds (upper horizontal line) were reached to trigger transcutaneous electrical stimulus. Green dots indicate nursing interventions that were triggered by 90% O2 saturation thresholds (lower horizontal line), apnea, or hypoventilation (see text). SpO2 = oximeter saturation measurement
Interviews with all participants 24–72 hr after the study indicated that there were no adverse effects or reported pain related to use of the device.
Discussion
This randomized-controlled, double-blind clinical trial illustrates the potential benefits of TES for diminishing apnea-induced oxygen desaturation. The device we tested operated as a closed-loop system, constantly monitoring oxygen saturation and delivered (active) or withheld (control) TES according to algorithm criteria. The primary outcome, SpO2 AUC below 90%, was improved in the patients allocated to the active group compared with controls. As such, patients selectively recruited for their propensity to experience OSA had reduced depth and duration of hypoxemic episodes following TES. These results suggest that timely application of low-level peripheral TES can prevent or minimize hypoxemia related to OSA, presumably by arousing the patient to breathe. The findings also show the potential for a well-designed algorithm to prevent stimulation in response to erroneous oximeter measurements related to movement, poor signal quality, and self-limited desaturation. This may be an important feature to minimize disruption of normal sleep patterns secondary to the unnecessary triggering of TES.
During OSA, SpO2 can fall at a rapid rate,34 decreasing as much as 1.5% saturation per second.35 In fact, arterial oxygen saturation can quickly decrease to a point where the brain no longer receives sufficient oxygen to generate a survival-dependent central arousal.11,15,36 This dangerous condition has been termed “Lights Out Saturation” (LOS).15 Once LOS is reached, without immediate intervention or resuscitation, patients may progress to respiratory failure.15 During apnea-induced hypoxemia, mere seconds delay in treatment may result in dramatic increases in morbidity and even mortality.
Contemporary practice for preventing OSA-related complications relies primarily upon screening, pulse oximetry monitoring, and increased levels of provider surveillance.37,38 Recent research has revealed serious limitations with these practices. One study, using the STOP-BANG screening tool, failed to identify 53% of patients with severe OSA and 65% of patients with moderate OSA during a preoperative anesthesia assessment.39 Another investigation found patients who were first screened for OSA on the day of surgery had significantly worse postoperative outcomes.40 Intermittent nursing assessments, as typically performed in general care units, can fail to capture abrupt onset of airway obstruction, apnea, and hypoxemia.8 Pulse oximetry-driven nurse interventions require particular consideration. Nurse response times to pulse oximeter alarms have been observed to range from 34-66 sec, with higher response times for those patients with more frequent alarms.41
Pulse oximetry is associated with frequent false alarms, and “alarm fatigue” has been well documented in healthcare providers,42,43 which may directly impact patient safety.44 One investigation found that 26% of desaturation events failed to trigger notification pages in patients with high rates of desaturations, suggesting that alarm fatigue may be associated with alarm disablement.41 Another study attempted to reduce alarm fatigue by increasing the threshold alarm trigger to SpO2 < 80% and adding a 30-sec notification delay.45 Nevertheless, this may have been at the expense of being able to identify, in a timely manner, patients in respiratory distress.45 In point of fact, many “false positive” alarms, thought to be triggered from motion artifact, may really be “true positives” originating from repetitive sleep apnea-related desaturations.15 A closed-loop “smart” system, such as that developed for this study, has the ability to monitor SpO2, minimize false alarm triggers, and deliver appropriate stimuli immediately in response to concerning levels of hypoxemia.
Transcutaneous electrical stimulus intervention therapy may be applied in a wide range of environments. The PACU is uniquely challenging with regard to the potential for airway obstruction because of the synergistic respiratory depressive effects of anesthetics, analgesics, anxiolytics, and sedating anti-emetics that are administered to patients with a variable (and often unknown) potential for OSA. As such, PACU patients receive continual nursing observations and are closely monitored for respiratory failure. At our institution, PACU nursing protocols require intervention when SpO2 < 90%, apnea > ten seconds, or respiratory rate < 8/minute. Because such interventions could also impact on the endpoints of this study, we attempted to minimize this by using the device TES algorithm trigger set to ≤ 93% saturation. The oxygen saturation threshold for device activation was above the standard of care prompt for nursing intervention and so eliminated the confounding effect that this would otherwise have incurred. Assuming that active and control patients experienced a similar degree of respiratory depression, one would predict a similar percentage of patients with SpO2 ≤ 93% in both groups, as indeed was the case (Table 3).
Certain limitations of this study should be noted. Patients in the active group may have been cognizant of the TES, thus un-blinding participants to group allocation. A higher number of participant dropouts occurred in the active group (Fig. 3), which may have introduced a bias to group allocation. Despite the participant randomization process, patients were somewhat younger in the active group (Table 2). Nevertheless, additional analysis, adjusting for age, suggested that age did not impact the primary outcome (AUC < 90%) of the study. Nevertheless, we cannot definitely exclude an age-related effect on the outcomes of interest.
In this study, there were relatively few female participants (6% active; 9% controls). This stems, in part, from our institution’s Research Ethics Board recommendation to exclude females of childbearing potential without a negative pregnancy test (Table 1). In addition, inclusion criteria mandated participants at risk for OSA, which may bias towards selection of males.32,46,47 In point of fact, the Sleep Apnea Clinical Score that was used to identify patients at risk for OSA relies on assessment of hypertension, snoring, and neck circumference, which are features that are biased towards higher scoring in males.47,48 As such, the applicability of our findings to females remains to be shown.
Although the results from this study are promising, further investigation is needed. Future studies should explore the usefulness of this technology in a wider range of patients (e.g., females, younger patients, OSA-identified patients), over a wider time interval (e.g. hours/days following surgical or medical interventions), and in different environments (e.g., critical care locations, general hospital wards, home). Endpoints other than hypoxemia to consider include sleep quality, patient well-being, and satisfaction scores. The technology can be expanded to assess the efficacy of graduated increases in intensity of stimulation in response to failed interventions, and to variable oxygen saturation triggers for TES.
References
Senaratna CV, Perret JL, Lodge CJ, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev 2017; 34: 70-81.
Garvey JF, Pengo MF, Drakatos P, Kent BD. Epidemiological aspects of obstructive sleep apnea. J Thorac Dis 2015; 7: 920-9.
Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177: 1006-14.
Finkel KJ, Searleman AC, Tymkew H, et al. Prevalence of undiagnosed obstructive sleep apnea among adult surgical patients in an academic medical center. Sleep Med 2009; 10: 753-8.
Opperer M, Cozowicz C, Bugada D, et al. Does obstructive sleep apnea influence perioperative outcome? A qualitative systematic review for the Society of Anesthesia and Sleep Medicine Task Force on Preoperative Preparation of Patients with Sleep-Disordered Breathing. Anesth Analg 2016; 122: 1321-34.
Kaw R, Chung F, Pasupuleti V, Mehta J, Gay PC, Hernandez AV. Meta-analysis of the association between obstructive sleep apnoea and postoperative outcome. Br J Anaesth 2012; 109: 897-906.
Davis MP, Behm B, Balachandran D. Looking both ways before crossing the street: assessing the benefits and risk of opioids in treating patients at risk of sleep -disordered breathing for pain and dyspnea. J Opioid Manag 2017; 13: 183-96.
Sun Z, Sessler DI, Dalton JE, et al. Postoperative hypoxemia is common and persistent: a prospective blinded observational study. Anesth Analg 2015; 121: 709-15.
Kaw R, Pasupuleti V, Walker E, Ramaswamy A, Foldvary-Schafer N. Postoperative complications in patients with obstructive sleep apnea. Chest 2012; 141: 436-41.
Carr GE, Mokhlesi B, Gehlbach BK. Acute cardiopulmonary failure from sleep-disordered breathing. Chest 2012; 141: 798-808.
Dyken ME, Yamada T, Glenn CL, Berger HA. Obstructive sleep apnea associated with cerebral hypoxemia and death. Neurology 2004; 62: 491-3.
Gami AS, Olson EJ, Shen WK, et al. Obstructive sleep apnea and the risk of sudden cardiac death: a longitudinal study of 10,701 adults. J Am Coll Cardiol 2013; 62: 610-6.
Benumof JL. Mismanagement of obstructive sleep apnea may result in finding these patients dead in bed. Can J Anesth 2016; 63: 3-7.
Subramani Y, Nagappa M, Wong J, Patra J, Chung F. Death or near-death in patients with obstructive sleep apnoea: a compendium of case reports of critical complications. Br J Anaesth 2017; 119: 885-99.
Lynn LA, Curry JP. Patterns of unexpected in-hospital deaths: a root cause analysis. Patient Saf Surg 2011; 5: 3.
Miki H, Hida W, Chonan T, Kikuchi Y, Takishima T. Effects of submental electrical stimulation during sleep on upper airway patency in patients with obstructive sleep apnea. Am Rev Respir Dis 1989; 140: 1285-9.
Edmonds LC, Daniels BK, Stanson AW, Sheedy PF 3rd, Shepard JW Jr. The effects of transcutaneous electrical stimulation during wakefulness and sleep in patients with obstructive sleep apnea. Am Rev Respir Dis 1992; 146: 1030-6.
Decker MJ, Haaga J, Arnold JL, Atzberger D, Strohl KP. Functional electrical stimulation and respiration during sleep. J Appl Physiol 1993; 75: 1053-61.
Hida W, Okabe S, Miki H, et al. Effects of submental stimulation for several consecutive nights in patients with obstructive sleep apnoea. Thorax 1994; 49: 446-52.
Guilleminault C, Powell N, Bowman B, Stoohs R. The effect of electrical stimulation on obstructive sleep apnea syndrome. Chest 1995; 107: 67-73.
Pengo MF, Steier J. Emerging technology: electrical stimulation in obstructive sleep apnoea. J Thorac Dis 2015; 7: 1286-97.
Bisogni V, Pengo MF, De Vito A, et al. Electrical stimulation for the treatment of obstructive sleep apnoea: a review of the evidence. Expert Rev Respir Med 2017; 11: 711-20.
Kuhlmann J, Deick S. Design of an Oxistimulator system for use in clinical trials - 2017. Available from URL: https://ieeexplore.ieee.org/document/7985871/ (accessed April 2019).
Masimo Corporation. Radical-7 pulse CO-Oximeter®. Available from URL: http://www.masimo.com/products/continuous/radical-7/ (accessed April 2019).
Digitimer. DS7A. Available from URL: https://digitimer.com/constant-current-stimulators/ (accessed April 2019).
Digitimer. Bar Stimulating Electrode. Available from URL: https://digitimer.com/products/human-neurophysiology/neurodiagnostic-accessories/bar-stimulating-electrode/ (accessed April 2019).
Motion Workshop. MotionNode. Available from URL: https://www.motionnode.com/imu.html (accessed April 2019).
Kinesis Corporation. Savant Elite Foot Pedal. Available from URL: https://kinesis-ergo.com/shop/1-pedal-for-savant-elite2-jsb/ (accessed April 2019).
Masimo C. Signal IQ Technology - 2008. Available from URL: http://www.masimo.co.jp/pdf/whitepaper/LAB3412C.pdf (accessed April 2019).
Zornow MH. Clinical testing of the apnea prevention device: proof of concept data. Anesth Analg 2011; 112: 582-6.
Flemons WW, Whitelaw WA, Brant R, Remmers JE. Likelihood ratios for a sleep apnea clinical prediction rule. Am J Respir Crit Care Med 1994; 150(5 Pt 1): 1279-85.
Gali B, Whalen FX, Schroeder DR, Gay PC, Plevak DJ. Identification of patients at risk for postoperative respiratory complications using a preoperative obstructive sleep apnea screening tool and postanesthesia care assessment. Anesthesiology 2009; 110: 869-77.
Webb RK, Ralston AC, Runciman WB. Potential errors in pulse oximetry. II. Effects of changes in saturation and signal quality. Anaesthesia 1991; 46: 207-12.
Sands SA, Edwards BA, Kelly VJ, et al. Mechanism underlying accelerated arterial oxygen desaturation during recurrent apnea. Am J Respir Crit Care Med 2010; 182: 961-9.
Wilkinson MH, Berger PJ, Blanch N, Brodecky V. Effect of venous oxygenation on arterial desaturation rate during repetitive apneas in lambs. Respir Physiol 1995; 101: 321-31.
Hlavac MC, Catcheside PG, McDonald R, Eckert DJ, Windler S, McEvoy RD. Hypoxia impairs the arousal response to external resistive loading and airway occlusion during sleep. Sleep 2006; 29: 624-31.
Chung F, Memtsoudis SG, Ramachandran SK, et al. Society of Anesthesia and Sleep Medicine Guidelines on Preoperative Screening and Assessment of Adult Patients With Obstructive Sleep Apnea. Anesth Analg 2016; 123: 452-73.
Lockhart EM, Willingham MD, Abdallah AB, et al. Obstructive sleep apnea screening and postoperative mortality in a large surgical cohort. Sleep Med 2013; 14: 407-15.
Singh M, Liao P, Kobah S, Wijeysundera DN, Shapiro C, Chung F. Proportion of surgical patients with undiagnosed obstructive sleep apnoea. Br J Anaesth 2013; 110: 629-36.
Fernandez-Bustamante A, Bartels K, Clavijo C, et al. Preoperatively screened obstructive sleep apnea is associated with worse postoperative outcomes than previously diagnosed obstructive sleep apnea. Anesth Analg 2017; 125: 593-602.
Voepel-Lewis T, Parker ML, Burke CN, et al. Pulse oximetry desaturation alarms on a general postoperative adult unit: a prospective observational study of nurse response time. Int J Nurs Stud 2013; 50: 1351-8.
Rheineck-Leyssius AT, Kalkman CJ. Influence of pulse oximeter settings on the frequency of alarms and detection of hypoxemia: theoretical effects of artifact rejection, alarm delay, averaging, median filtering or a lower setting of the alarm limit. J Clin Monit Comput 1998; 14: 151-6.
Gross B, Dahl D, Nielsen L. Physiologic monitoring alarm load on medical/surgical floors of a community hospital. Biomed Instrum Technol 2011; Suppl: 29-36.
Wilken M, Hüske-Kraus D, Klausen A, Koch C, Schlauch W, Röhrig R. Alarm fatigue: causes and effects. Stud Health Technol Inform 2017; 243: 107-11.
Taenzer AH, Pyke JB, McGrath SP, Blike GT. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology 2010; 112: 282-7.
Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med 2001; 163(3 Pt 1): 608-13.
Quintana-Gallego E, Carmona-Bernal C, Capote F, et al. Gender differences in obstructive sleep apnea syndrome: a clinical study of 1166 patients. Respir Med 2004; 98: 984-9.
Basoglu OK, Tasbakan MS. Gender differences in clinical and polysomnographic features of obstructive sleep apnea: a clinical study of 2827 patients. Sleep Breath 2018; 22: 241-9.
Acknowledgements
The authors would like to recognize the following people who contributed in various ways to ensure a successful project: Joel Kuhlmann, M.S. and Steven Deick, M.H.A. Division of Engineering Mayo Clinic; Richard Hinds, R.R.T., Bradley Narr, M.D, Linda Weise, R.R.T., Brenda Anderson, RN., and David Plevak, M.D. Mayo Clinic Department of Anesthesiology and Perioperative Medicine; Bruce Walters, Consistent Systems software development; Randall Newman, Mayo Clinic Division of Engineering safety engineer; and Albert J. Kilger, B.S., Graphics.
Declaration of interest
Mayo Clinic owns and has licensed the intellectual property for technology described in this research. This license agreement, with MediPines, was made after the study protocol and study prototypes were fully developed. Mayo Clinic and investigators Joan Kilger, M.S. and Richard Hinds, R.R.T. have a financial interest in technology used in this research. Mayo Clinic and these investigators may stand to gain financially from the successful outcome of this research. Kilger (author) and Hinds (contributor) did not participate in enrollment of study participants or participate in the analysis and interpretation of study data.
Conflicts of interest
None declared.
Editorial responsibility
This submission was handled by Dr. Steven Backman, Associate Editor, Canadian Journal of Anesthesia.
Author contributions
Hugh M. Smith, Joan Kilger, Christopher M. Burkle, Darrell R. Schroeder, and Bhargavi Gali certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript.
Funding
Mayo Clinic, “Discovery, Translation, Program” internal funding grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is accompanied by an editorial. Please see Can J Anesth 2019; 66: this issue.
Appendix: Sleep Apnea Clinical Score
Appendix: Sleep Apnea Clinical Score
OSA Questionnaire


Rights and permissions
About this article
Cite this article
Smith, H.M., Kilger, J., Burkle, C.M. et al. Peripheral electrical stimulation reduces postoperative hypoxemia in patients at risk for obstructive sleep apnea: a randomized-controlled trial. Can J Anesth/J Can Anesth 66, 1296–1309 (2019). https://doi.org/10.1007/s12630-019-01451-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-019-01451-3