Abstract
Background
This randomized trial aimed to evaluate combined infraclavicular-suprascapular blocks (ICB-SSBs) as a diaphragm-sparing alternative to interscalene blocks (ISBs) for arthroscopic shoulder surgery. We hypothesized that ICB-SSB would provide equivalent postoperative analgesia to ISB 30 min after surgery without the risk of hemidiaphragmatic paralysis.
Methods
Following research ethics board approval and written informed consent, participants in the ISB group received an ultrasound-guided ISB with 20 mL of levobupivacaine 0.25% and epinephrine 5 µg·mL−1. In the ICB-SSB group, ultrasound-guided ICB (20 mL) and SSB (10 mL) were carried out using the same local anesthetic. Thirty minutes after the block was performed, a blinded investigator assessed the presence of hemidiaphragmatic paralysis. Subsequently, all patients underwent general anesthesia. Postoperatively, a blinded investigator recorded pain scores at rest at 0.5, 1, 2, 3, 6, 12 and 24 hr. Consumption of intra- and postoperative narcotics was also tabulated.
Results
Compared to its ICB-SSB counterpart, the ISB group displayed non-equivalent (i.e., lower) postoperative pain scores at 30 min (difference of the medians, −4; 99% confidence interval [CI], −6 to −3), required less cumulative morphine iv at 24 hr (difference of the means, −6.1 mg; 95% CI, −10.5 to −1.6), and resulted in a higher incidence of hemidiaphragmatic paralysis (18/20 vs 0/20 patients, respectively; P < 0.001). Although postoperative pain scores at one, two, and three hours appeared lower in the ISB group, the upper bounds of the 99% CIs did not exceed the equivalence margin.
Conclusion
Compared with ICB-SSB, ISB provided non-equivalent (i.e., lower) postoperative pain scores 30 min after arthroscopic shoulder surgery. Thereafter, postoperative analgesia was comparable between the two groups. Further trials are required to compare ISB with ICB-SSB using a proximal (i.e., costoclavicular) technique for ICB.
Trial registration
www.clinicaltrials.gov, NCT02993939. Registered 12 December 2016.
Résumé
Contexte
Cette étude randomisée avait pour objectif d’évaluer l’efficacité des blocs combinés des nerfs infraclaviculaires et suprascapulaires (BIC-BSS), en tant qu’alternative épargnant le diaphragme, aux blocs interscaléniques (BIS), pour les chirurgies arthroscopiques de l’épaule. Nous avons émis l’hypothèse qu’un BIC-BSS procurerait une analgésie postopératoire équivalente à un BIS 30 min après la chirurgie et sans risque de paralysie hémidiaphragmatique.
Méthode
Après avoir obtenu l’approbation du comité d’éthique de la recherche et le consentement éclairé écrit, les participants du groupe BIS ont reçu un BIS échoguidé à l’aide de 20 mL de lévobupivacaïne 0,25 % et de 5 µg·mL−1 d’épinéphrine. Les participants du groupe BIC-BSS ont reçu un BIC (20 mL) et un BSS (10 mL) échoguidés avec les mêmes anesthésiques locaux. Trente minutes après la réalisation du bloc, un chercheur en aveugle a évalué la présence de paralysie hémidiaphragmatique. Par la suite, tous les patients ont reçu une anesthésie générale. Un chercheur en aveugle a enregistré les scores de douleur postopératoire au repos, à 30 min, et à une, deux, trois, six, 12 et 24 h. La satisfaction des patients à 24 h et la consommation d’agents narcotiques per- et postopératoires ont également été enregistrées.
Résultats
Par rapport au groupe BIC-BSS, le groupe BIS a manifesté des scores de douleur postopératoire non équivalents (c.-à-d. plus bas) à 30 min (différence médiane, −4; intervalle de confiance [IC] 99 %, −6 à −3; P = 0,001). Ils ont également nécessité une dose moindre de morphine iv cumulée à 24 h (différence moyenne, −6,1 mg; IC 95 %, −10,5 à −1,6; P = 0,010). En outre, comme nous l’avions anticipé, l’incidence de paralysie hémidiaphragmatique était plus élevée (18/20 vs 0/20 patients, respectivement; P < 0,001) dans le groupe BIS. Bien que les scores de douleur postopératoire à une, deux et trois heures ont semblé plus bas dans le groupe BIS, les limites supérieures des IC 99 % n’ont pas excédé la marge d’équivalence.
Conclusion
Par rapport à un BIC-BSS, un BIS a entraîné des scores de douleur postopératoire non équivalents (c.-à-d. plus bas) 30 min après une arthroscopie de l’épaule. À partir de ce moment-là, toutefois, l’analgésie postopératoire était comparable entre les deux groupes. Des études supplémentaires sont nécessaires pour comparer les BIS aux BIC-BSS en utilisant une technique proximale (soit costoclaviculaire) pour réaliser le BIC.
Enregistrement de l’étude
www.clinicaltrials.gov, NCT02993939. Enregistrée le 12 décembre 2016.
Similar content being viewed by others
Interscalene brachial plexus blocks (ISBs) constitute the criterion for analgesia after shoulder surgery, but may be contraindicated in patients with pulmonary pathology because of the inherent risk of phrenic nerve block and hemidiaphragmatic paralysis (HDP).1 In a recent review article, our team speculated that a possible diaphragm-sparing alternative to ISB lies in the combined use of infraclavicular brachial plexus block (ICB) and suprascapular nerve block (SSB).1 Theoretically, the ICB targets the posterior and lateral cords, thereby anesthetizing the axillary nerve (which supplies the anterior and posterior shoulder joint) as well as the subscapular and lateral pectoral nerves (both of which supply the anterior shoulder joint), while the SSB anesthetizes the posterior shoulder.1 Although combined ICB-SSB has been successfully used for proximal humeral surgery,2 its benefits for shoulder surgery require investigation.
Thus, in this randomized trial, we compared ISB with ICB-SSB for patients undergoing arthroscopic shoulder surgery. We hypothesized that ICB-SSB would provide equivalent postoperative analgesia to ISB at 30 min (without the risk of HDP), and therefore, designed the study as an equivalence trial.
Methods
Following approval of the Ethics Board of the Hospital Clínico Universidad de Chile (December 12th, 2016), we enrolled 40 patients undergoing arthroscopic shoulder surgery. Inclusion criteria were patients aged 18-75 yr, American Society of Anesthesiologists (ASA) physical status I-III, and body mass index 20-30 kg·m−2. Exclusion criteria were an inability to consent to the study, preexisting (obstructive or restrictive) pulmonary disease, coagulopathy, sepsis, hepatic or renal failure, pregnancy, allergy to local anesthetic, chronic pain condition requiring the intake of opioids at home, and prior surgery in the neck or infraclavicular/suprascapular fossa. Written informed consent was obtained from all participants.
After arrival in the induction room, an 18- or 20G intravenous catheter was placed in the upper limb contralateral to the surgical site, and intravenous premedication (midazolam 2 mg and fentanyl 50 µg) was administered to all patients. Supplemental oxygen (nasal cannulae at 4 L·min−1) and standard ASA monitoring were applied throughout the procedure.
The same 5-13 MHz LOGIQ™ e linear ultrasound (US) transducer (GE Healthcare, Wauwatosa, WI, USA) and local anesthetic solution (0.25% levobupivacaine with epinephrine 5 µg·mL−1) were used in all patients. Stimuplex® Ultra 360® 22G block needles (B. Braun Medical AG, Melsungen, Germany) of varying lengths were employed in both groups: a 10-cm needle in the ICB-SSB group and a 5-cm needle in the ISB group. Two investigators (J.A. or D.B.) supervised all blocks. We used a computer-generated sequence of random numbers and a sealed envelope technique to allocate patients randomly to receive ISB (n = 20) or ICB-SSB (n = 20).
Prior to performing ISB or ICB-SSB, all patients received an US-guided intermediate cervical plexus block to anesthetize the cutaneous “cape” area of the shoulder.1 This step was undertaken to ensure that postoperative pain from arthroscopic port insertion and skin closure would not constitute a confounding variable. Using a previously described technique, 5 mL of the local anesthetic solution were injected into the intermuscular plane between the sternocleidomastoid and scalene muscles at the level of the thyroid cartilage.3
In the ISB group, the US transducer was applied in a sterile fashion on the lateral side of the neck at the level of the cricoid cartilage in order to obtain a view of the three hypoechoic structures which represent the roots/trunks of the brachial plexus.4 A skin wheal was raised with 3 mL of lidocaine 1.0%. Using an in-plane technique and a lateral-to-medial direction, the block needle was advanced until its tip was positioned under the prevertebral fascia between the two most superficial hypoechoic structures.4,5 Twenty mL of the local anesthetic solution were deposited in this location (Fig. 1).
In the ICB-SSB group, the paracoracoid ICB was first performed according to a previously described technique.6 The US transducer was applied in a sterile fashion in the lateral infraclavicular fossa, medially to the coracoid process, in order to obtain a short-axis view of the axillary artery. A skin wheal was raised with 3 mL of lidocaine 1.0%. Using an in-plane technique and a cephalad-to-caudad direction, the block needle was advanced until its tip was positioned dorsal to the axillary artery. Twenty mL of the local anesthetic solution were injected in this location6 (Fig. 2A). Subsequently, the patient was placed in the lateral decubitus position with the surgical limb uppermost. The US transducer was applied in a sterile fashion cephalad and parallel to the scapular spine in order to obtain a view of the suprascapular fossa. A skin wheal was raised with 3 mL of lidocaine 1.0%. Using an in-plane technique and a lateral-to-medial direction, the block needle was advanced until its tip was located in the floor of the suprascapular fossa, ventral to the fascia of the supraspinatus muscle. Ten mL of the local anesthetic solution were injected7 (Fig. 2B).
Subsequently, whether the sensorimotor blocks were complete or incomplete at 30 min (see below), all patients underwent general anesthesia with endotracheal intubation using intravenous propofol 1.5-2 mg·kg−1, fentanyl 1 ug·kg−1, rocuronium 0.6 mg·kg−1, dexamethasone 4 mg, and sevoflurane (end-tidal minimal alveolar concentration = 1.0). All subjects were placed in the beach chair position. Intraoperatively, if the patient’s heart rate or blood pressure exceeded 20% of the preoperative value, a 25-µg bolus dose of fentanyl was administered. At the end of the case, prior to extubation, all patients received further nausea prophylaxis (ondansetron 4 mg). The surgical duration (defined as the interval from skin incision to closure) was recorded.
Postoperatively, in the postanesthesia care unit (PACU), all patients received intravenous acetaminophen 1 g, ketoprofen 100-mg iv, as well as patient-controlled analgesia (1-mg bolus doses of morphine with a lockout interval of eight minutes). On the surgical ward, all subjects continued to receive acetaminophen 1 g per os every eight hours and ketoprofen 100 mg per os every 12 hr as well as patient-controlled morphine. All nursing personnel (PACU and surgical ward) were blinded to group allocation. All patients were discharged in the afternoon of the first postoperative day.
Primary outcome measurement
In the PACU, a blinded investigator recorded pain scores at 30 min using a 0-10 numerical rating scale (0 = no pain; 10 = worst imaginable pain). Time zero was defined as the patient’s admission to the PACU. Only pain at rest was assessed as our surgical colleagues requested that the surgical limb not be mobilized in the postoperative period. Thereafter, the blinded assessor evaluated pain scores at rest at one, two, three, six, 12, and 24 hr. The primary outcome was the postoperative pain score in the PACU at 30 min. All blocks, whether complete or incomplete (see below), were included in the primary outcome analysis (i.e., intent-to-treat analysis).
Secondary outcomes measurement
During the performance of ISBs and ICBs-SSBs, the coauthor supervising the blocks (J.A. or D.B.) recorded the performance time using a stopwatch. Performance time was defined as the temporal interval between the start of skin disinfection and the end of local anesthetic injection through the block needle; it encompassed the time required to reposition the patient as well as to disinfect and drape the skin for the second block (ICB-SSB group). The supervising coauthor (J.A. or D.B.) also recorded potential adverse events (e.g., vascular puncture, local anesthetic toxicity, paresthesia) occurring during the performance of the blocks.
After the performance of the ISB and ICB-SSB, a blinded investigator assessed the blocks every five minutes until 30 min using a sensorimotor composite scale. Sensory function was tested on the skin overlying the clavicle (supraclavicular nerves)1,3 and the lateral surface of the deltoid (axillary nerve).1 Each territory was graded according to a three-point scale using a cold test: 0 = no block; 1 = analgesia (patient can feel touch, not cold); 2 = anesthesia (patient cannot feel touch).3,6 Motor function was tested using shoulder abduction (axillary and suprascapular nerves)4,7 and external shoulder rotation (suprascapular nerve)7 according a three-point scale: 0 = no block; 1 = paresis; 2 = paralysis.6 We considered the blocks complete if, at 30 min, a global composite score ≥ six points (out of a maximum of eight points) was achieved. Thus onset time was defined as the time required to reach a minimal composite score of six points.
The investigator who evaluated the blocks also assessed the presence of HDP at 30 min. A 2-5 MHz curvilinear LOGIQ™ e US transducer (GE Healthcare, Wauwatosa, WI, USA) and the M-mode were employed in all subjects. The liver and spleen served as acoustic windows on the right and left side, respectively. Patients were scanned along the anterior axillary line and the US probe was angled cranially.8 Hemidiaphragmatic paralysis was defined as the absence of diaphragmatic motion during normal respiration, coupled with absent or (paradoxical) cranial diaphragmatic movement when the patient forcefully sniffed.8
The blinded investigator also tabulated demographic data (sex, age, weight, and height), the type of surgery, as well as the incidence of hoarseness and Horner syndrome (30 min after the performance of the blocks) prior to assessing HDP. Postoperatively, the same investigator recorded the total intra- and postoperative opioid consumption, opioid-related side effects (e.g., postoperative nausea/vomiting and pruritus), and patient satisfaction at 24 hr using a 0-10 scale (0 = not satisfied; 10 = very satisfied). The investigator also contacted all patients one week after the surgery to inquire about complications such as persistent numbness/paresthesia or motor deficit. All blocks, whether complete or incomplete, were included in the analysis of secondary outcomes.
Statistical analysis
A pilot study (n = 15) conducted at the Hospital Clinico Universidad de Chile revealed that patients undergoing ISB for arthroscopic shoulder surgery reported a mean (standard deviation [SD]) pain score of 1.0 (1.7) on a 0-10 scale at 30 min in the PACU (unpublished data). Although postoperative pain at 30 min may seem less important than pain at one week,9 we reasoned that, for patients with pulmonary pathology undergoing shoulder surgery, the greatest risk lies in the immediate postoperative period because uncontrolled pain and parenteral opioid administration could lead to hypoventilation and oxygen desaturation. Therefore, our research hypothesis was that ICB-SSB would provide equivalent postoperative analgesia to ISB at 30 min (without the risk of HDP). We elected to set the equivalence margin at two points in terms of pain scores because Tashijian et al.10 have previously reported that a difference < 1.4 points carries minimal clinical significance for patients afflicted with rotator cuff disease. Thus, a calculated sample size of 40 patients was required for a statistical power of 0.90 and a type I error of 0.025.
Statistical analysis was performed using SPSS® version 21 statistical software (IBM, Armonk, NY, USA). Postoperative pain scores did not follow a normal distribution (Lilliefors test, P < 0.05 for all time periods except 24 hr); thus, equivalency was assessed by examining the 99% confidence intervals (CIs) of the differences of the medians using the Hodges-Lehmann method. For other data, normality was first assessed with the Lilliefors test and then analyzed with the Student’s t test. Data that did not have a normal distribution, as well as ordinal data, were analyzed with the Mann-Whitney U test. For categorical data, the Chi square or Fisher’s exact test was used. All P values presented were two-sided and values inferior to 0.05 were considered significant.
Results
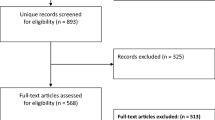
The 40 subjects were recruited over a period of 4.5 months (mid-December 2016 to April 2017) (Fig. 3). Anthropometric data, surgical procedures, and surgical duration are presented in Table 1.
The ICB-SSB and ISB groups were found to be non-equivalent in terms of the primary outcome; the ISB group displayed significantly lower PACU pain scores at 30 min (difference of the medians, −4; 99% CI, −6 to −3). Although pain scores at one, two, and three hours appeared lower in the ISB group, the upper bounds of the 99% CIs did not exceed the equivalence margin (Fig. 4).
Postoperative pain scores at rest. Median values for the time periods are indicated inside their respective boxes. The 99% confidence intervals for the differences of the medians were 30 min (−6 to −3); one hour (−5 to −1); two hours (−3 to 0); three hours (−2 to 0); six hours (−2 to 0); 12 hr (−1 to +2); 24 hr (−1 to +2). ISB = interscalene brachial plexus block; ICB-SSB = combined infraclavicular-suprascapular nerve blocks
Expectedly, the ISB resulted in a shorter mean (SD) performance time than the ICB-SSB [9.9 (4.6) vs 17.9 (10.1) min, respectively; P = 0.003] and a higher incidence of HDP at 30 min (18/20 vs 0/20 patients, respectively; P < 0.001). Patients in the ISB group also required less cumulative intravenous morphine at 24 hr (difference of the means, −6.1; 95% CI, −10.5 to −1.6) (Table 2). However, no intergroup differences were found in terms of the proportions of patients with complete blocks at 30 min (95-100%), onset time, procedural pain, intraoperative opioid consumption, adverse events, or patient satisfaction at 24 hr (Table 2).
Patient follow-up at one week revealed no sensory or motor deficit.
Discussion
In this randomized trial, we compared US-guided ISB with ICB-SSB in patients undergoing arthroscopic shoulder surgery. Our results suggest that ISB provides non-equivalent (i.e., improved) pain control during the first 30 min. Thereafter, although pain scores at one, two, and three hours appeared lower in the ISB group, the upper bounds of the 99% CIs did not exceed the equivalence margin. Since the ISB and ICB-SSB groups displayed similar proportions of patients with complete blocks at 30 min (95-100%), we conclude that axillary and suprascapular nerve blocks can be achieved with both strategies. However, we speculate that the initial analgesic difference between the two groups stems from ICB-SSB’s incomplete coverage of the lateral pectoral and subscapular nerves, which originate from the lateral and posterior cord, respectively, and conjointly supply the anterior shoulder joint with the axillary nerve.1 We hypothesize that limited local anesthetic spread proximally with paracoracoid ICB may contribute to the partial block of the lateral pectoral and subscapular nerves. In the current protocol, we selected a 20-mL injectate for ICB in order to avoid HDP altogether. We concede that a higher volume may provide more complete coverage of the lateral and posterior cords; however, 30-mL injectates can result in a 13% risk of complete or partial HDP.11 Thus, future trials are required to compare ISB with ICB-SSB using volumes ranging between 20 and 30 mL for ICB. Alternately, the costoclavicular technique could be employed for ICB in order to target the lateral and posterior cords more proximally.12,13
Shoulder surgery in patients with pulmonary pathology represents an interesting anesthetic challenge. These subjects benefit most from regional anesthesia because systemic opioids can hinder ventilation. Paradoxically, the standard nerve block for shoulder surgery (i.e., ISB) virtually guarantees hypoventilation due to HDP.1 Besides ICB-SSB, diaphragm-sparing alternatives to ISB include C7 nerve root blocks (with a local anesthetic volume < 6 mL), supraclavicular brachial plexus blocks (with local anesthetic injection confined to the “corner pocket” as well as the posterolateral aspect of the brachial plexus), and combined axillary-suprascapular nerve blocks (AXB-SSB).1 Of the three options, only AXB-SSB has been formally investigated. Two randomized-controlled trials (combined n = 129) have compared ISB with AXB-SSB in patients undergoing arthroscopic shoulder surgery (mainly rotator cuff repair).14,15 Compared with ISB, AXB-SSB resulted in higher intraoperative opioid requirements,13 increased pain/opioid consumption in the PACU,14,15 and decreased patient satisfaction at six hours.14 Although ICB-SSB can be expected to provide better coverage of the lateral pectoral and subscapular nerves than AXB-SSB, further investigation is required to compare these two diaphragm-sparing strategies.
The combined use of ICB and SSB deserves special mention. In two recent trials, single-injection and continuous SSB have been found to provide similar analgesia to ISB for outpatient arthroscopic shoulder surgery between 0 and 24 hr16 and for shoulder arthroplasty at 24 hr,17 respectively. Thus, one could question why, in our study, we combined SSB and ICB. A review of the literature reveals that the benefits of SSB for shoulder surgery remain controversial. Singelyn et al.18 have previously concluded that SSB was inferior to ISB. Furthermore, Lee et al. 19 reported that, compared with SSB alone, combined AXB-SSB resulted in lower pain scores (until 24 hr) as well as improved satisfaction in patients undergoing arthroscopic rotator cuff repair. Thus, we reasoned that, if AXB-SSB outperforms SSB, the same would also be true for ICB-SSB. Therefore, in the current trial, we erred on the side of caution and elected to compare ISB with ICB-SSB instead of SSB alone.
Our patient population requires discussion. The current trial sought to investigate a diaphragm-sparing alternative to ISB for patients in whom HDP represents a prohibitive risk. Therefore, logic dictates that only subjects with documented pulmonary pathology should be recruited. However, we discarded this possibility for safety reasons, because of the documented risk of HDP with ISB.1 Furthermore, although we suspected that our ICB-SSB technique would not result in phrenic nerve block, we could not ignore the fact that Petrar et al.11 had reported possible HDP with ICBs. Thus, exclusion of patients with pulmonary compromise constituted an ethical fail-safe precaution.
Our protocol contains some limitations. First, because our standard of care involved general anesthesia (to minimize intraoperative discomfort due to the beach chair position), we were unable to measure the rate of surgical anesthesia. Second, we employed the posterior approach for SSB. We concede that the anterior approach20 could have circumvented patient repositioning after ICB and decreased block performance time. We elected to use the posterior approach because its sonographic target (i.e., the suprascapular fossa) is easily identifiable, whereas the suprascapular nerve can escape detection in 18% of patients with the anterior approach.16 Third, our composite scale encompassed sensory and/or motor functions of the axillary and suprascapular nerves, but not those of the lateral pectoral and subscapular nerves. The subscapular nerve, which supplies the subscapularis muscle and mediates internal rotation of the humeral head, would have been difficult to evaluate in patients with rotator cuff tears. Finally, we performed ISB at the level of the cricoid cartilage using a 20-mL injectate because this method represents the standard of care in our centre. We recognize that a lower volume (e.g., 5 mL) and an injection point closer to C7 could have decreased the incidence of phrenic nerve block in the ISB group.1
In conclusion, compared with ICB-SSB, ISB results in non-equivalent (i.e., lower) postoperative pain scores 30 min after arthroscopic shoulder surgery. Although pain scores at one, two, and three hours appeared lower in the ISB group, the upper bounds of the 99% CIs did not exceed the equivalence margin. Expectedly, the incidence of hemidiaphragmatic paralysis was higher with ISB. Further trials are required to compare ISB with ICB-SSB using a proximal (i.e., costoclavicular) technique for ICB or supraclavicular brachial plexus blocks (with local anesthetic injection confined to the “corner pocket” as well as the posterolateral aspect of the brachial plexus).
References
Tran DQ, Elgueta MF, Aliste J, Finlayson RJ. Diaphragm-sparing nerve blocks for shoulder surgery. Reg Anesth Pain Med 2017; 42: 32-8.
Martinez J, Sala-Blanch X, Ramos I, Gomar C. Combined infraclavicular plexus block with suprascapular nerve block for humeral head surgery in a patient with respiratory failure: an alternative approach. Anesthesiology 2003; 98: 784-5.
Tran DQ, Dugani S, Finlayson RJ. A randomised comparison between ultrasound-guided and landmark-based superficial cervical plexus block. Reg Anesth Pain Med 2010; 35: 539-43.
Spence BC, Beach ML, Gallagher JD, Sites BD. Ultrasound-guided interscalene blocks: understanding where to inject the local anaesthetic. Anaesthesia 2011; 66: 509-14.
Franco CD, Williams JM. Ultrasound-guided interscalene block: reevaluation of the “stoplight” sign and clinical implications. Reg Anesth Pain Med 2016; 41: 452-9.
Tran DQ, Bertini P, Zaouter C, Munoz L, Finlayson RJ. A prospective, randomized comparison between single- and double-injection ultrasound-guided infraclavicular brachial plexus block. Reg Anesth Pain Med 2010; 35: 16-21.
Chan CW, Peng PW. Suprascapular nerve block: a narrative review. Reg Anesth Pain Med 2011; 36: 358-73.
Loyd T, Tang YM, Benson MD, King S. Diaphragmatic paralysis: the use of M mode ultrasound for diagnosis in adults. Spinal Cord 2006; 44: 505-8.
Salviz EA, Xu D, Frulla A, et al. Continuous interscalene block in patients having outpatient rotator cuff repair surgery: a prospective randomised trial. Anesth Analg 2013; 117: 1485-92.
Tashijian RZ, Deloach J, Porucznick CA, Powell AP. Minimal clinically important differences (MCID) and patient acceptable symptomatic pass (PASS) for visual analog scales (VAS) measuring pain in patients treated for rotator cuff disease. J Shoulder Elbow Surg 2009; 18: 927-32.
Petrar SD, Seltenrich ME, Head SJ, Schwarz SK. Hemidiaphragmatic paralysis following ultrasound-guided supraclavicular versus infraclavicular brachial plexus blockade: a randomized clinical trial. Reg Anesth Pain Med 2015; 40: 133-8.
Karmakar MK, Sala-Blanch X, Songthamwat B, Tsui BC. Benefits of the costoclavicular space for ultrasound-guided infraclavicular brachial plexus block: description of a costoclavicular approach. Reg Anesth Pain Med 2015; 40: 287-8.
Leurcharusmee P, Elgueta MF, Tiyaprasertkul W, et al. A randomized comparison between costoclavicular and paracoracoid ultrasound-guided infraclavicular block for upper limb surgery. Can J Anesth 2017; 64: 617-25.
Dhir S, Sondekoppam RV, Sharma R, Ganapathy S, Athwal GS. A comparison of combined suprascapular and axillary nerve blocks to interscalene nerve block for analgesia in arthroscopic shoulder surgery: an equivalence study. Reg Anesth Pain Med 2016; 41: 564-71.
Pitombo PF, Barros RM, Matos MA, Modolo NS. Selective suprascapular and axillary nerve block provides adequate analgesia and minimal motor block. Comparison with interscalene block. Braz J Anesthesiol 2013; 63: 45-51.
Wiegel M, Morrigl B, Schwarzkopf P, Petroff D, Reske AW. Anterior suprascapular nerve block versus interscalene brachial plexus block for shoulder surgery in the outpatient setting: a randomized controlled patient- and assessor-blinded trial. Reg Anesth Pain Med 2017; 42: 310-8.
Auyong DB, Yuan SC, Choi DS, Pahang JA, Slee AE, Hanson NA. A double-blind randomized comparison of continuous interscalene, supraclavicular, and suprascapular blocks for total shoulder arthroplasty. Reg Anesth Pain Med 2017; 42: 302-9.
Singelyn FJ, Lhotel L, Fabre B. Pain relief after arthroscopic shoulder surgery: a comparison of intraarticular analgesia, suprascapular nerve block, and interscalene brachial plexus block. Anesth Analg 2004; 99: 589-92.
Lee JJ, Kim DY, Hwang JT, et al. Effect of ultrasonographically guided axillary nerve block combined with suprascapular nerve block in arthroscopic rotator cuff repair: a randomized controlled trial. Arthroscopy 2014; 30: 906-14.
Siegenthaler A, Moriggl B, Mlekusch S, et al. Ultrasound-guided suprascapular nerve block, description of a novel supraclavicular approach. Reg Anesth Pain Med 2012; 37: 325-8.
Declaration of interest
The authors have no interests to declare. The content is solely the responsibility of the authors.
Editorial responsibility
This submission was handled by Dr. Gregory L. Bryson, Deputy Editor-in-Chief, Canadian Journal of Anesthesia.
Author contributions
De Q. Tran and Roderick J. Finlayson were involved in the design of the study protocol. Julian Aliste and Daniela Bravo were involved in the recruitment of all study subjects. They also conducted the clinical trial and collected the data. All authors were involved in the data analysis, reviewing the first draft of the manuscript, and reading and approving the manuscript.
Funding
None of the authors received funding for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aliste, J., Bravo, D., Finlayson, R.J. et al. A randomized comparison between interscalene and combined infraclavicular-suprascapular blocks for arthroscopic shoulder surgery. Can J Anesth/J Can Anesth 65, 280–287 (2018). https://doi.org/10.1007/s12630-017-1048-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-017-1048-0