Abstract
Purpose
Flexible bronchoscopy with bronchoalveolar lavage (BAL) is commonly performed in immunocompromised patients. Nevertheless, it remains unclear whether bronchoscopy with BAL leads to changes in medical management or is associated with procedural complications among critically ill acute leukemia (AL) patients.
Methods
We evaluated 71 AL patients who underwent diagnostic bronchoscopy with BAL in the intensive care unit (ICU) between 1 January 2007 and 31 December 2012. We recorded baseline characteristics, vital signs (before, during, and after the procedure), changes in medical management following the procedure, and procedural complications. Using a multivariable logistic regression model, we explored the relationship between patient characteristics and whether bronchoscopy changed management or caused complications. Patient characteristics included as predictors in the regression model were age, sex, immunosuppression status (those undergoing active chemotherapy), and the Acute Physiology And Chronic Health Evaluation II score.
Results
The most common indication for ICU admission was respiratory failure (51 patients, 72%), followed by sepsis (14 patients, 20%). Overall, the results obtained from bronchoscopy with BAL were associated with a change in management in 32 patients (45%), most commonly a change in antimicrobial therapy as a result of an infectious pathogen being identified (17 patients, 24%). Complications were documented in nine patients (13%) and included post-procedural hypoxia (six patients, 8%), the need for intubation (one patient, 9% of non-intubated patients), and tracheal perforation (one patient, 1%). No clinically significant changes in patient vital signs were observed during or immediately following the procedure. Patient characteristics did not predict whether bronchoscopy was associated with changes in medical management or procedural complications in multivariable analyses.
Conclusions
Flexible bronchoscopy with BAL is relatively safe and helps to guide medical management among patients with AL admitted to the ICU.
Résumé
Objectif
La bronchoscopie flexible avec lavage broncho-alvéolaire (LBA) est une intervention de routine pratiquée chez les patients immunosupprimés. Toutefois, nous ne savons pas si la bronchoscopie avec LBA entraîne des modifications dans la prise en charge médicale ou si elle est associée à des complications liées à l’intervention chez les patients atteints de leucémie aiguë en phase critique.
Méthode
Nous avons évalué 71 patients atteints de leucémie aiguë et ayant subi une bronchoscopie diagnostique avec LBA à l’unité des soins intensifs (USI) entre le 1er janvier 2007 et le 31 décembre 2012. Leurs caractéristiques de base, leurs signes vitaux (avant, pendant et après l’intervention), les modifications de la prise en charge médicale après l’intervention et les complications liées à l’intervention ont été enregistrés. À l’aide d’un modèle de régression logistique multivarié, nous avons exploré la relation entre les caractéristiques des patients et la possibilité que la bronchoscopie ait modifié leur prise en charge ou provoqué des complications. Les caractéristiques des patients dont nous avons tenu compte en tant que prédicteurs dans le modèle de régression étaient l’âge, l’état d’immunosuppression (soit les patients sous traitement de chimiothérapie actif) et leur score APACHE II (Acute Physiology And Chronic Health Evaluation II).
Résultats
L’indication la plus fréquente pour une admission à l’USI était l’insuffisance respiratoire (51 patients, 72 %), suivie du sepsis (14 patients, 20 %). Globalement, les résultats obtenus grâce à la bronchoscopie avec LBA ont été associés à une modification de la prise en charge chez 32 patients (45 %), consistant la plupart du temps en une modification de la thérapie antimicrobienne suite à l’identification d’un agent pathogène infectieux (17 patients, 24 %). Des complications ont été documentées chez neuf patients (13 %) et comprenaient l’hypoxie suite à l’intervention (six patients, 8 %), le besoin d’intubation (un patient, 9 % des patients non intubés) et la perforation trachéale (un patient, 1 %). Aucun changement significatif d’un point de vue clinique n’a été observé dans les signes vitaux des patients pendant ou immédiatement après l’intervention. Dans les analyses multivariées, les caractéristiques des patients n’ont pas permis de prédire si la bronchoscopie serait associée à des modifications de la prise en charge médicale ou à des complications liées à l’intervention.
Conclusion
La bronchoscopie flexible avec LBA est relativement sécuritaire et guide la prise en charge médicale des patients atteints de leucémie aiguë admis à l’USI.
Similar content being viewed by others
Patients with acute leukemia (AL) are at high risk for developing pulmonary complications including infection, inflammatory drug reactions, tumours, alveolar hemorrhage, and cardiogenic pulmonary edema.1,2 These entities may present with non-specific symptoms (cough, fever, shortness of breath) and radiographic findings and can rapidly progress to acute respiratory failure. In patients with cancer, acute respiratory failure is the leading reason for admission to the intensive care unit (ICU) and carries a high mortality rate of up to 50%, particularly when the underlying cause cannot be identified.3,4,5 Diagnosis is critical to guide treatment, but is often challenging in this patient population. Available diagnostic tools include non-invasive techniques, such as computed tomography scans, and more invasive techniques, including flexible bronchoscopy with bronchoalveolar lavage (BAL) and lung biopsy. Flexible bronchoscopy with BAL is less invasive than lung biopsy and is used commonly in the immunocompromised population in an attempt to guide management.
Flexible bronchoscopy with BAL allows direct visualization of the airways and sampling of alveolar cells and fluid.6,7,8,9 It is considered safe and is of high diagnostic yield even in patients supported by mechanical ventilation and with underlying malignancy.1,10,11 Past studies evaluating its use in the immunocompromised population report a diagnostic yield between 32% and 66%.1,2,12,13,14 A recent systematic review comparing bronchoscopy with BAL to lung biopsy in patients with cancer or hematopoietic stem cell transplant found that bronchoscopy with BAL was superior to lung biopsy in diagnosing pulmonary infections and was associated with a lower complication risk including procedural mortality.15 In 2,792 procedures reviewed, BAL led to a diagnosis in 53% of patients and changes in management in 31%, with a low complication risk of 8%, including five cases of procedure-related death.8,15 The technique can be especially useful in diagnosing pulmonary infections, specifically opportunistic pathogens such as cytomegalovirus and Pneumocystis jiroveci.1,2,16 Identification of pathogens is important, as it allows for timely initiation of more targeted antimicrobial therapy that might not otherwise be prescribed and allows for discontinuation of unnecessary antibiotics.
Although the utility of flexibility bronchoscopy has been studied in immunocompromised populations including bone marrow transplant (BMT) recipients,17,18,19 patients with HIV,20,21 and thoracic malignancy,22 the diagnostic utility and safety of the technique among critically ill AL patients remain unclear. The primary objectives of this study were therefore to: 1) identify whether flexible bronchoscopy with BAL leads to changes in medical management and 2) describe the risk of procedure-related complications in patients with AL admitted to the ICU. Secondary objectives were to determine whether patient characteristics including age, sex, Acute Physiology And Chronic Health Evaluation (APACHE) II score, and immunosuppression status (patients undergoing active chemotherapy) were associated with the development of complications post-bronchoscopy.
Methods
This was a retrospective cohort study conducted at two academic teaching hospitals affiliated with the University of Toronto: Princess Margaret Cancer Center of the University Health Network and Sinai Health System. The Research Ethics Boards at both hospitals approved the study in October 2013.
Participants
We included consecutive patients who were 16 yr of age or older with a diagnosis of AL undergoing active medical management who were admitted to the ICU between 1 January 2007 and 31 December 2012 and underwent diagnostic flexible bronchoscopy with BAL. There were no exclusion criteria.
Data collection
Data were collected from paper and electronic hospital records. Patient demographics, baseline characteristics, details of the ICU admission, bronchoscopy procedural details, and clinical outcomes were collected for the entire cohort. The respiratory therapist assisting in each bronchoscopy recorded the respiratory rate, heart rate (HR), blood pressure, oxygenation, and ventilator settings at set intervals throughout the procedure and recorded one value per time period (before, during, and after the procedure). We recorded changes in medical management following bronchoscopy including a diagnosis of alveolar hemorrhage and identification of an infectious organism resulting in a change in antimicrobials or a therapeutic intervention. We searched for specific procedural complications including hypoxia (oxygen saturation ≤ 92% immediately post-bronchoscopy), the need for noninvasive ventilation or intubation within 48 hr and other complications that were directly related to the procedure. No data were missing for the data points of interest.
Data analysis
Descriptive statistics were calculated for all variables. Continuous variables were not normally distributed (Shapiro-Wilk statistic < 0.05) and were expressed as median (interquartile range [IQR]). Categorical variables were calculated as proportions. Vital signs immediately before, during, and after the procedure were compared using Friedman tests given the paired nature of the data. We explored the relationship between patient characteristics and whether bronchoscopy changed management or caused complications by developing a multivariable logistic regression model. Predictors included in the regression model were age, sex, immunosuppression status, and APACHE II score. These were selected a priori based on clinical experience and previous studies. Included predictors were also limited by the smallest category of the outcome variable (change in medical management, n = 32) and were not collinear (tolerance > 0.9). The Hosmer-Lemeshow test was not significant for either regression model, indicating adequate model fit (P > 0.97 and 0.92, respectively).
All statistical analyses were performed using SAS® Studio 9.3 University Edition (SAS® Institute; Cary, NC, USA). The type I error probability was 0.05 for two-sided tests of statistical significance when comparisons were made.
Results
Seventy-one patients with AL underwent bronchoscopy in the ICU during the study period. Baseline characteristics are shown in Table 1. Patients had a median [IQR] age of 55 [46-63] yr, median [IQR] APACHE II score of 22 [17-29], and 37 (52%) were female. The types of AL during various stages of treatment were: acute myeloid leukemia (61%), acute lymphoid leukemia (21%), acute promyelocytic leukemia (6%), and myelodysplastic syndrome (4%), and 8% had an alternate malignant hematologic diagnosis.
The most common indication for ICU admission was respiratory failure (51 patients, 72%), followed by sepsis (14 patients, 20%). On the day of ICU admission, patients had a median [IQR] absolute neutrophil count of 0.34 [0.02-1.80] × 109 cells·L−1 and platelet count of 26 [11-47] × 109 cells·L−1. Of the 71 patients, 28 (39%) were receiving steroids, and 32 (45%) were receiving chemotherapy. The median [IQR] ICU length of stay was 11 [6-22] days, and 36 (58%) patients survived their ICU admission. Survival at one year was 31%.
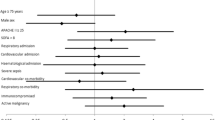
The median [IQR] day that bronchoscopy with BAL was performed was day 2 [2-6] of ICU admission, and 60 (85%) patients were intubated at the time of bronchoscopy. The median [IQR] oxygen saturation and F i O2 on the day of bronchoscopy were 96 [94-98] % and 45 40-60] %, respectively. Vital signs immediately before, during, and after bronchoscopy are represented in Table 5 and the Figure. We found there was a significant difference with HR during the procedure being the highest; the median [IQR] HR before, during, and after bronchoscopy was 107 [97-122] beats·min−1, 113 [98-124] beats·min−1, and 109 [93-121] beats·min−1, respectively (P = 0.03). Other vital signs including oxygen saturation and mean arterial pressure remained stable throughout the procedure (Table 5, Figure).
Patient vital signs before, during, and after bronchoscopy. Data points represent the median and 95% confidence interval for the readings during each time period. Pre = vital signs immediately before bronchoscopy; Bronch = vital signs during bronchoscopy; Post = vital signs immediately after bronchoscopy; *heart rate was highest during the procedure (P = 0.03)
Overall, bronchoscopy with BAL was associated with a change in medical management in 32 of the 71 cases (45%). Changes to management included changes to antimicrobial therapy (including starting, discontinuing, or changing antimicrobials) based on culture results, the establishment of an alternative diagnosis, or a local therapeutic intervention based on bronchoscopy findings. An organism was identified from BAL in 19 cases (27%). The most common organisms identified included Pseudomonas aeruginosa (n = 3), Herpes simplex virus-1 (n = 2), Klebsiella pneumoniae (n = 2), Aspergillus fumigatus (n = 2), Aspergillus flavus (n = 2), and Candida albicans (n = 2) (Table 2). Of these 19 cases, 17 (89%) had changes to their antimicrobials to target the causative organism, while in two cases the organisms identified were presumed to be contaminants and no changes to antimicrobials were made. In three cases, changes to antimicrobial therapy were made despite negative cultures—two cases were treated as bacterial pneumonia based on the presence of pus and thick secretions in the airways, while a third was treated as a fungal pneumonia based on the appearance of black lesions in the airways. Empiric antimicrobials were discontinued in an additional 12 (17%) patients with negative BAL culture results who had alternative diagnoses. Of these 12 patients, nine were diagnosed with alveolar hemorrhage, one was diagnosed with mucous plugging, and one was diagnosed with laryngeal edema. These final two cases underwent additional therapeutic interventions: pulmonary hygiene (suctioning of secretions from the airways) and intubation within 48 hr of the procedure.
The final patient was a case of refractory leukemia undergoing active chemotherapy with known invasive pulmonary fungal lesions. This patient experienced a tracheal perforation as a complication of the procedure, likely as a result of friable mucosa in the airways, and later underwent an elective tracheostomy and was discharged from the ICU five days later in stable condition.
Complications were rare and reported in only nine (13%) patients, with four major complications. These complications included the need for intubation within 48 hr post-procedure (one of 11 non-intubated patients), endotracheal tube (ETT) change (one of 60 intubated patients), post-bronchoscopy hypoxia with an oxygen saturation of < 92% (six patients, three of whom required adjustment of ventilator settings immediately following the procedure), and tracheal perforation (one of 71 patients) (Table 3).
The indications for intubation and ETT change were laryngeal edema secondary to an anaphylactic reaction to a penicillin antibiotic and diffuse pulmonary hemorrhage, respectively. Both patients survived to ICU discharge, but were dead at one-month post-ICU discharge from other causes. Age, sex, APACHE II score, and active chemotherapy were not significantly associated with 1) changes in management or 2) the development of complications post-bronchoscopy in multivariable analyses (Table 4).
Discussion
In patients with AL admitted to the ICU who underwent bronchoscopy with BAL, the results were associated with changes to management in 32 cases (45%). An infectious pathogen was identified in 19 of 71 cases (27%) and resulted in a change in antimicrobial therapy in 17 of the 19 cases (89%). In cases of negative BAL cultures, empiric antimicrobials were discontinued in all cases when an alternative diagnosis was made (12 of 71 cases, 17%) such as pulmonary hemorrhage, mucous plugging, tracheal perforation, or laryngeal edema. Complications were documented in nine patients (13%) and included intubation within 48 hr of the procedure, ETT change, post-procedural hypoxia, and tracheal perforation. There were no clinically significant changes in patient vital signs during or immediately following the procedure. These results suggest that among leukemia patients admitted to the ICU, flexible bronchoscopy with BAL was relatively safe, had a high diagnostic yield, and helped to guide medical management (Table 5).
There have been few studies evaluating the clinical utility and safety of bronchoscopy with BAL in patients with hematologic malignancies23,24,25,26 and fewer looking specifically at AL patients.27,28 Only one of these studies looked specifically at patients admitted to the ICU.28 In general, they reported a low risk of complications ranging from 0-10%,23,24,25,26,27,28 and a diagnostic yield for infectious pathogens of between 15-66%.
Similar to these studies, the overall risk of post-procedural complications in our cohort was low at 13%. This is in contrast to previous studies of bronchoscopy with BAL in ICU patients29 and BMT recipients,17,18,19 which quote a much higher risk of complications of 49% and between 0-27%, respectively. The relative safety of the procedure in our patients may be related to the mechanical ventilator support in 85% of cases. The relatively low risk of post-procedural hypoxia (oxygen saturation < 92%) may also be due to a selection bias regarding which patients underwent the procedure. Pre-procedural hypoxia is a known risk factor for respiratory decompensation,30,31 and none of our patients were hypoxic prior to the procedure, although many were supported with moderate oxygen therapy. Therefore, the low incidence of hypoxia may be a reflection of the clinicians’ selection of patients expected to tolerate the procedure without complications. Additionally, the cases requiring intubation and ET tube change secondary to laryngeal edema and pulmonary hemorrhage may not have been direct complications of the bronchoscopy itself and may in fact overestimate the actual procedure-related complications. Both oxygen saturation and blood pressure remained stable throughout the procedure. Although HR was significantly higher during the procedure, the increase was small and unlikely to be of clinical significance.
Despite the low risk of complications, it should be noted that complications such as the need for intubation and tracheal perforation may have serious repercussions for the patient, including prolonged time spent on the ventilator, the need for a tracheostomy, and even an increased risk of death. Correspondingly, consideration must be given to the utility of the procedure, and patients and families should be made aware of these potential complications as part of the informed consent process.
The diagnostic yield in our cohort is similar to previous studies evaluating bronchoscopy with BAL in immunocompromised hosts.1,2,8,12,13,14,15 Our results suggest that the procedure is most useful for the diagnosis of infectious complications, specifically opportunistic pathogens such as aspergillus and herpes simplex virus. Identification of these opportunistic pathogens is important as they may portend poor clinical outcomes when left untreated; however, because treatment can have toxic side effects, it may only be initiated once these organisms are identified. Furthermore, identification of the causative organism can lead to a more timely initiation of targeted antimicrobial therapy or discontinuation of unnecessary broad-spectrum empiric antibiotics. Negative BAL cultures can also lead to the discontinuation of empiric antibiotics and seem to be particularly useful in cases where an alternative diagnosis (e.g., pulmonary hemorrhage) is made on bronchoscopy.
Age, sex, APACHE II score, and active chemotherapy were not significantly associated with changes in management or the development of complications post-bronchoscopy (Table 4). Age may not play a large role in predicting outcomes of bronchoscopy in this population given that the extremes of age (very old and very young) are largely absent (median age 55, IQR 46-63). Furthermore, while the APACHE II score reflects severity of illness and predicts patient mortality, it may not predict patient morbidity such as complications associated with a given procedure. Finally, chemotherapy status alone may not impact clinical outcomes, as leukemia patients are already severely immunocompromised as shown by the relative degree of neutropenia amongst our patients and the use of steroids alone or in addition to chemotherapy in 39%. In a larger population, other patient or procedural characteristics may better predict adverse outcomes.
The strengths of this study are the homogeneous population of AL patients and complete procedural, microbiologic, and outcome data. Limitations include the relatively small number of patients who underwent bronchoscopy during our study period (n = 71) and our inability to comment on the diagnostic yield and safety of the procedure in non-mechanically ventilated patients, given that 85% were intubated prior to the procedure. Furthermore, because of the observational study design, results may be influenced by residual or unmeasured confounders. Finally, the diagnostic yield and clinical utility of bronchoscopy in our study may be overestimated secondary to: 1) confounding by indication—i.e., the total number of AL patients admitted to the ICU during our study period, including those that did not undergo bronchoscopy is not known, and the procedure is more likely to be performed in cases of diagnostic ambiguity; 2) we cannot be sure whether the changes in management following the procedure were based on the results alone or took other factors such as the patients’ clinical status into account.
Conclusion
Our study suggests that while bronchoscopy with BAL is a safe procedure in the critically ill leukemia patient, serious complications including the need for intubation and tracheal perforation do occur. Furthermore, the results of the procedure can alter patient management in some cases, particularly when an infectious pathogen is identified or an alternative diagnosis to infection is made. More work is needed to better understand patient and procedural characteristics that will help predict the diagnostic utility and risk of complications related to flexible bronchoscopy.
References
Stover DE, Zaman MB, Hajdu SI, Lange M, Gold J, Armstrong D. Bronchoalveolar lavage in the diagnosis of diffuse pulmonary infiltrates in the immunosuppressed host. Ann Intern Med 1984; 101: 1-7.
Tenholder MF, Hooper RG. Pulmonary infiltrates in leukemia. Chest 1980; 78: 468-73.
Azoulay E, Schlemmer B. Diagnostic strategy in cancer patients with acute respiratory failure. Intensive Care Med 2006; 32: 808-22.
Azoulay E, Thiery G, Chevret S, et al. The prognosis of acute respiratory failure in critically ill cancer patients. Medicine (Baltimore) 2004; 83: 360-70.
Chaoui D, Legrand O, Roche N, et al. Incidence and prognostic value of respiratory events in acute leukemia. Leukemia 2004; 18: 670-5.
Costabel U, Bross KJ, Matthys H. Diagnosis by bronchoalveolar lavage of cause of pulmonary infiltrates in haematological malignancies. Br Med J (Clin Res Ed) 1985; 290: 1041.
Crystal RG, Reynolds HY, Kalica AR. Bronchoalveolar lavage. The report of an international conference. Chest 1986; 90: 122-31.
Olopade CO, Prakash UB. Bronchoscopy in the critical-care unit. Mayo Clin Proc 1989; 64: 1255-63.
Reynolds HY. Bronchoalveolar lavage. Am Rev Respir Dis 1987; 135: 250-63.
Martin WJ 2nd, Smith TF, Sanderson DR, Brutinel WM, Cockerill FR 3rd, Douglas WW. Role of bronchoalveolar lavage in the assessment of opportunistic pulmonary infections: utility and complications. Mayo Clin Proc 1987; 62: 549-57.
Azoulay E, Mokart D, Lambert J, et al. Diagnostic strategy for hematology and oncology patients with acute respiratory failure: randomized controlled trial. Am J Respir Crit Care Med 2010; 182: 1038-46.
Abramson MJ, Stone CA, Holmes PW, Tai EH. The role of bronchoalveolar lavage in the diagnosis of suspected opportunistic pneumonia. Aust N Z J Med 1987; 17: 407-12.
Cordonnier C, Bernaudin JF, Fleury J, et al. Diagnostic yield of bronchoalveolar lavage in pneumonitis occurring after allogeneic bone marrow transplantation. Am Rev Respir Dis 1985; 132: 1118-23.
Pisani RJ, Wright AJ. Clinical utility of bronchoalveolar lavage in immunocompromised hosts. Mayo Clin Proc 1992; 67: 221-7.
Chellapandian D, Lehrnbecher T, Phillips B, et al. Bronchoalveolar lavage and lung biopsy in patients with cancer and hematopoietic stem-cell transplantation recipients: a systematic review and meta-analysis. J Clin Oncol 2015; 33: 501-9.
Springmeyer SC, Hackman RC, Holle R, et al. Use of bronchoalveolar lavage to diagnose acute diffuse pneumonia in the immunocompromised host. J Infect Dis 1986; 154: 604-10.
Dunagan DP, Baker AM, Hurd DD, Haponik EF. Bronchoscopic evaluation of pulmonary infiltrates following bone marrow transplantation. Chest 1997; 111: 135-41.
Murray PV, O’Brien ME, Padhani AR, et al. Use of first line bronchoalveolar lavage in the immunosuppressed oncology patient. Bone Marrow Transplant 2001; 27: 967-71.
White P, Bonacum JT, Miller CB. Utility of fiberoptic bronchoscopy in bone marrow transplant patients. Bone Marrow Transplant 1997; 20: 681-7.
Salzman SH. Bronchoscopic techniques for the diagnosis of pulmonary complications of HIV infection. Semin Respir Infect 1999; 14: 318-26.
Narayanswami G, Salzman SH. Bronchoscopy in the human immunodeficiency virus-infected patient. Semin Respir Infect 2003; 18: 80-6.
Zhang X, Kuang Y, Zhang Y, et al. Shifted focus of bronchoalveolar lavage in patients with suspected thoracic malignancy: an analysis of 224 patients. J Thorac Dis 2016; 8: 3245-54.
Hummel M, Rudert S, Hof H, Hehlmann R, Buchheidt D. Diagnostic yield of bronchoscopy with bronchoalveolar lavage in febrile patients with hematologic malignancies and pulmonary infiltrates. Ann Hematol 2008; 87: 291-7.
Kim SW, Rhee CK, Kang HS, et al. Diagnostic value of bronchoscopy in patients with hematologic malignancy and pulmonary infiltrates. Ann Hematol 2015; 94: 153-9.
Marra R, Pajano L, Pagliari G, et al. The yield of bronchoalveolar lavage in the etiological diagnosis of pneumonia in leukemia and lymphoma patients. Eur J Haematol 1993; 51: 256-8.
von Eiff M, Zuhlsdorf M, Roos N, Thomas M, Buchner T, van de Loo J. Pulmonary infiltrates in patients with haematologic malignancies: clinical usefulness of non-invasive bronchoscopic procedures. Eur J Haematol 1995; 54: 157-62.
Rabbat A, Chaoui D, Lefebvre A, et al. Is BAL useful in patients with acute myeloid leukemia admitted in ICU for severe respiratory complications? Leukemia 2008; 22: 1361-7.
Saito H, Anaissie EJ, Morice RC, Dekmezian R, Bodey GP. Bronchoalveolar lavage in the diagnosis of pulmonary infiltrates in patients with acute leukemia. Chest 1988; 94: 745-9.
Azoulay E, Mokart D, Rabbat A, et al. Diagnostic bronchoscopy in hematology and oncology patients with acute respiratory failure: prospective multicenter data. Crit Care Med 2008; 36: 100-7.
Verra F, Hmouda H, Rauss A, et al. Bronchoalveolar lavage in immunocompromised patients. Clinical and functional consequences. Chest 1992; 101: 1215-20.
Anonymous. Technical recommendations and guidelines for bronchoalveolar lavage (BAL). Report of the European Society of Pneumology Task Group. Eur Respir J 1989; 2: 561-85.
Conflicts of interest
The authors have no funding sources or conflicts of interest to declare.
Editorial responsibility
This submission was handled by Dr. Hilary P. Grocott, Editor-in-Chief, Canadian Journal of Anesthesia.
Author contributions
Uday Deotare was involved in the conception and design of study, data collection, and manuscript preparation. Erica Merman was involved in data analysis and manuscript preparation. Sangeeta Mehta was involved in the conception and design of the study, data analysis, and manuscript preparation. Daniel Pincus was involved in data analysis and manuscript preparation. Alan P. Kraguljac, Danielle Croucher, Vikram Kumar, Narmin Ibrahimova, Mark D. Minden, and Christie Lee were involved in the manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deotare, U., Merman, E., Pincus, D. et al. The utility and safety of flexible bronchoscopy in critically ill acute leukemia patients: a retrospective cohort study. Can J Anesth/J Can Anesth 65, 272–279 (2018). https://doi.org/10.1007/s12630-017-1041-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-017-1041-7