Abstract
Purpose
To investigate whether tracheostomy increases the risk of sternal wound infection (SWI) post cardiac surgery.
Methods
All patients undergoing cardiac surgery via median sternotomy from September 1997 to October 2010 were included in this retrospective observational study. Primary exposure was tracheostomy performed during admission to the cardiac surgical intensive care unit. The primary outcome was SWI during hospital admission. Multivariable logistic regression was used to determine if tracheostomy was an independent predictor of SWI. Restriction and propensity score analyses were then used to assess if tracheostomy is a causal risk factor for SWI.
Results
Four hundred and eleven of 18,845 patients (2.2%) were treated with tracheostomy. Incidences of SWI in tracheostomy and non-tracheostomy groups were 19.5% (80/411) and 0.8% (154/18,434), respectively. Using multivariable logistic regression analysis, tracheostomy was found to be an independent predictor of SWI (odds ratio [OR] 2.8; 95% confidence interval [CI] 1.9 to 4.2). In an analysis restricted to respiratory failure patients, tracheostomy was associated with sternal wound infection (OR 3.4; 95% CI 2.4 to 4.9). When the analysis was stratified by the risk of receiving tracheostomy as represented by propensity score (PS), 46 patients (12%) in the intermediate risk category (PS 0.2-0.4) had SWIs (adjusted OR 2.97; 95% CI 1.6 to 5.6), and 52 patients (14%) in the highest risk category (PS > 0.4) had SWIs (OR 1.52; 95% CI 0.85 to 2.87).
Discussion
Our single-centre observational study of cardiac surgery patients found tracheostomy to be an independent risk factor for SWI. Our analysis showed a robust association when restricted to patients with respiratory failure and after the population was stratified by the propensity to have a tracheostomy.
Résumé
Objectif
Déterminer si la trachéostomie augmente le risque d’infection de plaie sternale (IPS) après une chirurgie cardiaque.
Méthode
Tous les patients subissant une chirurgie cardiaque par sternotomie médiane entre septembre 1997 et octobre 2010 ont été inclus dans cette étude observationnelle rétrospective. La variable indépendante principale était une trachéostomie réalisée pendant l’admission à l’unité des soins intensifs de chirurgie cardiaque. Le critère d’évaluation principal était une IPS pendant le séjour à l’hôpital. La régression logistique multivariée a été utilisée pour déterminer si la trachéostomie était un prédicteur indépendant d’IPS. L’analyse des scores de restriction et de propension a ensuite été utilisée pour évaluer si la trachéostomie était un facteur de risque causal d’IPS.
Résultats
Quatre cents onze patients sur 18 845 (2,2 %) ont subi une trachéostomie. Les incidences d’IPS dans les groupes trachéostomie et non-trachéostomie étaient de 19,5 % (80/411) et 0,8 % (154/18,434), respectivement. L’analyse de régression logistique multivariée a permis de déterminer que la trachéostomie était un prédicteur indépendant d’IPS (rapport de cotes [RC] 2,8; intervalle de confiance [IC] 95 % 1,9 à 4,2). Dans une analyse ne portant que sur les patients en insuffisance respiratoire, la trachéostomie a été associée à une infection de plaie sternale (RC 3,4; IC 95 % 2,4 à 4,9). Lorsque l’analyse était stratifiée selon le risque de subir une trachéostomie tel que représenté par le score de propension (SP), 46 patients (12 %) de la catégorie de risque intermédiaire (SP 0,2-0,4) avaient une ISP (RC ajusté 2,97; IC 95 % 1,6 à 5,6), et 52 patients (14 %) de la catégorie de risque le plus élevé (SP > 0,4) avaient une ISP (RC 1,52; IC 95 % 0,85 à 2,87).
Discussion
Notre étude observationnelle unicentrique portant sur des patients de chirurgie cardiaque a découvert que la trachéostomie constituait un facteur de risque indépendant d’ISP. Notre analyse a montré une association importante lorsqu’elle se limitait aux patients en insuffisance respiratoire et après stratification de la population par propension à subir une trachéostomie.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Tracheostomy is commonly employed as an alternative means of airway management in critically ill patients.1-3 In medical, trauma, and neurological critical care, tracheostomy has been shown to improve patient comfort, reduce the requirement for sedation, and decrease intensive care unit (ICU) and hospital lengths of stay.1,3,4 Nevertheless, in the postoperative cardiac surgery setting, the utility of tracheostomy is questioned in view of the idea that a soiled surgical site resulting from a tracheal stoma increases the risk of sternal wound infection (SWI). There continues to be uncertainty as to the true nature of the relationship between tracheostomy and SWI, with recent publications both supporting and refuting tracheostomy as a cause of SWI post cardiac surgery.5-11
Three large observational studies support tracheostomy as a risk factor for SWI. In a study of 6,057 patients undergoing isolated coronary artery bypass, Curtis et al.5 reported an 8.6% risk of mediastinitis, defined as positive culture of mediastinal tissue or fluid, in tracheostomy patients and a 0.7% risk in non-tracheostomy patients. In a study of 16,277 cardiac surgery patients, Force et al. 6 found tracheostomy to be one of four predictors of SWI in their multivariable regression analysis (odds ratio [OR] 5.65; 95% confidence interval [CI] 2.76 to 11.54). Ngaage et al. 11 reported a 0.8% incidence of SWI in 7,002 patients, and tracheostomy was identified in multivariable regression analysis as one of four determinants of SWI (OR 3.22; 95% CI 1.11 to 9.31).
Two previous studies that failed to detect an association between tracheostomy and SWI were limited by small numbers of events, which precluded robust risk adjustment.7,8
It is obvious that patients in the group receiving tracheostomy differ greatly from the non-tracheostomy group. Only patients with poor postoperative course would likely ever be offered a tracheostomy. The clinical question is whether the observed increased risk of SWI in the tracheostomy group is a result of the tracheostomy or simply a result of the underlying complicated postoperative course. The primary purpose of this study was to determine whether tracheostomy is a causal risk factor for SWI after cardiac surgery.
Methods
Design and selection criteria
The Research Ethics Board at the Ottawa Hospital approved this protocol on February 11, 2011. Written consent from individual study participants was not required because the study represented a secondary use of non-identifiable data (TCPS Article 5.5). All patients undergoing cardiac surgery via full median sternotomy from September 1, 1997 to October 31, 2010 were included in this historical observational study. We excluded patients with preoperative tracheostomy in situ, those patients receiving tracheostomy following documented SWI, as well as patients undergoing surgeries via non-median sternotomy approaches.
Description of database
Data were extracted from the University of Ottawa Heart Institute (UOHI) perioperative database. This is a multi-modular database containing comprehensive pre-, intra-, and postoperative information prospectively collected on all patients undergoing major cardiac surgery at the UOHI. Since its inception, data have been prospectively collected on more than 21,000 patient encounters. More than 400 variables are available to capture baseline patient characteristics, surgical type, intraoperative details, postoperative interventions, and organ-specific morbidities. The database is managed by the UOHI Perioperative Database Unit consisting of data managers and information technology support and is overseen by a committee with representation from Cardiac Anesthesiology, Cardiac Surgery, and Epidemiology that regulates data access, ethics, and confidentiality requirements.
Variable definitions
All captured variables were pre-defined in accordance with commonly employed expert consensus definitions and kept up to date, i.e., where possible, preoperative variable definitions or specific postoperative cardiac surgical outcomes, such as “re-opening”, are in keeping with definitions employed by EuroSCORE12 and/or the STS database.13 Our database definition of SWI is in concordance with the definition used for the Society of Thoracic Surgery database,13 i.e., occurring within 30 days of surgery if no implant is left in place, or within one year of surgery if implant is in place. It must involve the skin, subcutaneous tissue, muscle, or tissue below the fascial layer, and comprise any of the following:
-
a)
Purulent drainage from the incision or drain located above or beneath the fascial layer.
-
b)
Organism isolated from culture of fluid taken from primarily closed wound.
-
c)
Surgeon deliberately opens wound, unless wound culture is negative.
-
d)
Wound spontaneously dehisces or is deliberately opened by surgeon when patient has fever (> 38°C) and/or localized pain or tenderness, unless culture is negative.
-
e)
Other evidence seen on direct or histopathologic examination or during surgery.
-
f)
Surgeon’s or attending physician’s diagnosis of infection.
The Cardiac Anesthesia Risk Assessment (CARE) score is a simple risk-ranking system based on clinical judgement, comorbid conditions (classified as controlled or uncontrolled), complexity, and urgency of cardiac procedure and resembles the well-known American Society of Anesthesiologists’ physical status classification.14 It was developed within our institution as a model for predicting mortality risk and has been shown to perform as well as other better known multifactorial risk indices such as the EuroSCORE.15 The CARE score has eight categories representing increasing risk of predicting operative mortality, i.e., CARE 1, 2, 3, 3E, 4, 4E, 5, and 5E, where E designates an emergency case requiring immediate surgery. Respiratory failure is defined as pulmonary insufficiency requiring intubation and mechanical ventilation for a period exceeding 72 hr at any time during the postoperative period or if re-intubation is required. Cardiogenic shock is defined as a state of low cardiac output requiring more than two inotropes for more than a 24-hr period or needing an intra-aortic balloon pump. Stroke is defined as new deficit or abnormality occurring postoperatively but not present in the preoperative assessment.
Primary exposure
The primary risk factor of interest was tracheostomy performed during admission in the cardiac surgical intensive care unit (CSICU). Tracheostomy is one of the predefined variables in the database. Patients with tracheostomy in situ or de novo tracheostomy can be discriminated and the date of the tracheostomy procedure can be captured. Throughout the study inclusion period, clinical practice for performing tracheostomies remained stable in accordance with the following description: all tracheostomies were performed via an open technique by one of three cardiac surgeons, and most tracheostomies were performed in the patient’s room within the CSICU, using appropriately sized Shiley™ cuffed tracheostomy tubes. The procedures were done with an anesthesiologist at the bedside as well as operating room nursing support and the usual sterile precautions, including antiseptic skin preparation and full body drape. When difficulty was anticipated due to complex airway anatomy (infrequently, in < 2% of cases), an otolaryngologist would be consulted to perform open tracheostomy in the operating room.
Outcomes
The outcome of interest was the incidence of SWI during the same postoperative hospital admission. All patients were followed for the duration of their hospital admission or until the time of in-hospital death.
Statistical analysis
Continuous normally distributed variables are presented as mean (standard deviation [SD]), and continuous variables with non-normal distributions are presented as median (interquartile range [IQR]). Categorical variables are presented as number (proportion). Unadjusted odds ratios were determined using univariable logistic regression. To investigate if tracheostomy is an independent predictor of SWI, we fitted a multivariable model that included other known predictors of SWI. We identified predictors a priori based on a structured PubMed search using the MESH terms “Cardiac Surgical Procedures”, “Coronary Artery Bypass”, “Cardiopulmonary Bypass”, “Surgical Wound Infection”, “Risk Factors”, and “Epidemiologic Methods”. For our regression analyses, we included baseline, intraoperative, and postoperative risk factors that were identified by this search strategy in at least two publications during 1995-2010. All of these identified predictors were available within our database. Adjusted odds ratios (OR) and 95% confidence intervals (CI) were used to assess the independent relationship between tracheostomy and SWI. The CARE score was included as a general measure of preoperative mortality risk as well as a measure of procedural complexity. No patients were excluded from the study because of missing data; however, we did not perform imputations for missing covariate data for the multivariable model, and the sample size was allowed to vary with the analysis.
To investigate if tracheostomy is a causal risk factor for SWI, we conducted a separate logistic regression analysis restricted to patients with respiratory failure, aiming to achieve better covariate balance between the exposed and unexposed groups. We also calculated a propensity score (PS) estimating each patient’s likelihood to receive a tracheostomy, and we examined the relationship between tracheostomy and SWI within each of the three predetermined categories of the propensity score (PS < 0.2, PS 0.2-0.4, PS > 0.4); in addition, we adjusted for propensity score as a continuous variable within these analyses to attempt a further reduction in the potential for residual confounding within each category. The propensity score was calculated by constructing a nonparsimonious logistic regression model that included but was not limited to the reported risk factors, including age, sex, left ventricular ejection fraction < 50%, case urgency, body mass index, diabetes, CARE score, glomerular filtration rate, previous cerebrovascular accident, chronic obstructive pulmonary disease on medication, awake intubation, re-sternotomy, aortic cross-clamp time, postoperative ventilation > 72 hr, re-intubation, postoperative re-opening, dialysis, stroke, low cardiac output syndrome, ICU length of stay > ten days, ICU re-admission, and pneumonia.4
All statistical tests were two-sided and performed using SAS® 9.2 (SAS Institute, Cary, NC, USA), with statistical significance defined as P < 0.05.
Results
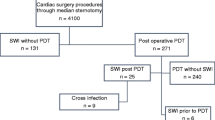
Overall, 18,845 patients met the criteria for inclusion in the study, and no subject was lost to follow up. Tracheostomy prior to any identified SWI occurred in 411 patients (2.2%), and the median [IQR] time from post-surgical ICU admission to tracheostomy was 14 [10-19] days. Table 1 summarizes the perioperative characteristics of included patients with and without tracheostomy.
There were 234 SWI cases documented, with incidences of 19.5% (80/411) in the tracheostomy group and 0.8% (154/18,434) in the non-tracheostomy group. The results of the univariable and multivariable regression analyses are presented in Table 2. Tracheostomy was an independent predictor of SWI (adjusted OR 2.8; 95% CI 1.9 to 4.2). An additional ten variables were also identified as independent predictors of SWI from our multivariable logistic regression model.
There were 15,953 patients included in the multivariable logistic regression analysis. In order to ascertain that missing covariate data did not introduce bias for our effect estimate, we conducted a sensitivity analysis by re-running our multivariable model using an indicator variable for each categorical variable with an added category for “missingness”. Median values of each of the continuous variables were assigned to their missing points. This was done as a conservative measure to bias the adjusted odds ratio towards the null. The association between tracheostomy and SWI was essentially unaltered when calculated using this approach (adjusted OR 2.8; 95% CI 1.9 to 4.0).
When the cohort was restricted to patients with respiratory failure (n = 1,866), 406 underwent a tracheostomy, and 77 (19%) of those patients developed SWI. Table 3 describes the characteristics of the patients with respiratory failure who underwent a tracheostomy. Tracheostomy was found to be an independent predictor of SWI in this subgroup (OR 3.4; 95% CI 2.4 to 4.9) (Table 3). Only five of the patients without respiratory failure (n = 16,979) had a tracheostomy; three of the five patients developed SWI. Due to the low number of events in this subgroup, we were not able to conduct a stable multivariable analysis.
The association between tracheostomy and SWI in each predetermined propensity score category is presented in Table 4. There were < 20 cases of SWI in the lowest risk category, and a stable regression model was not possible. In the intermediate and high-risk groups, the risk of SWI was increased in patients with a tracheostomy (OR 2.97; 95% CI 1.58 to 5.58 and OR 1.56 95% CI 0.85 to 2.87, respectively).
Discussion
This study supports a positive association between tracheostomy and sternal wound infection. Using a multivariable regression model that included all patients, we found tracheostomy to be an independent predictor of SWI. Nevertheless, the finding based on traditional multivariable regression cannot be considered causal, as the differences between the two groups may be too large to rely on statistical adjustment alone to eliminate confounding. In a propensity score analysis that is designed to reduce the imbalances between tracheostomy and non-tracheostomy patients, a robust association exists in all but the highest risk population.
Sternal wound infection following cardiac surgery is a life-threatening complication associated with major increases in morbidity and mortality.16 As a result, many clinicians are concerned about proceeding with tracheostomy, as direct soiling of the healing sternal wound could result. Tracheostomy in critically ill patients does not lend itself easily to a randomized interventional trial. Furthermore, as both the exposure and outcome are relatively rare events, whether a tracheostomy is associated with an increased incidence of SWI can likely be answered only through an observational study.
It is obvious that patients in the group receiving a tracheostomy are very different from those in the non-tracheostomy group, and previous studies are limited by their lack of adjustment for potential confounders. Although Rahmanian et al. employed perhaps the most rigorous methodology to date in their study on the subject of SWI, the small number of events (13 in the respiratory failure patients) meant the OR (0.7) was imprecisely estimated (95% CI 0.2 to 2.4) and an increased risk could not be ruled out. The major problem with risk adjustment is the baseline differences between patients needing a tracheostomy and those who don’t, which is clearly illustrated in Table 1. It is almost impossible to overcome these large imbalances in patient characteristics with statistical modelling.17 We attempt to reduce these large imbalances in our secondary analyses, which are restricted to those patients most likely to receive a tracheostomy. When we employed these rigorous statistical methods to provide minimally biased estimates of the treatment effect, we continued to find that tracheostomy is associated with an increased risk of SWI in postoperative cardiac surgery patients.
Limitations
Our study is limited by several factors. First, although our data were prospectively collected, the study remained retrospective in nature as treatment allocation was not random. As a result, we cannot rule out the existence of confounding by indication. Furthermore, we are limited by missing data. For instance, we could not easily delineate the causative organisms for all documented cases of SWI, e.g., if the causative organisms were identical to those responsible for concurrent respiratory tract infections, this would strengthen any argument for causality. We also lack information on the surgical service performing the tracheostomy (cardiac surgery vs ear nose and throat), and on the location where the tracheostomy was performed (operating room vs patient’s room in the CSICU), which is almost certainly a surrogate for increased risk of infection. Such misclassification could have shifted our treatment effect.
Second, the data on the timing of the onset of SWI were unreliable, and this may have influenced our ability to risk adjust for the treatment effect. Obviously a tracheostomy performed the day before the onset of a SWI is not likely to be causal. Third, the cohort being studied is limited to a single centre, which limits the generalizability of our findings. A multicentre prospective observational database may be the ideal way to evaluate this question.
Finally, analyses restricted to the highest risk patients by using propensity score methods are still subject to the effects of unknown confounders, and thus residual confounding is still possible.
In conclusion, this large single-centre observational study of cardiac surgery patients reports tracheostomy as an independent risk factor for SWI. Although tracheostomy offers several potential benefits, our analysis suggests these benefits may be outweighed by the risk of surgical site infection. With the high mortality experienced by patients with complicated and prolonged treatment following cardiac surgery, further studies on risk vs benefit of tracheostomy are warranted and specifically with regard to the timing of the procedure.
References
Blot F, Similowski T, Trouillet JL, et al. Early tracheotomy versus prolonged endotracheal intubation in unselected severely ill ICU patients. Intensive Care Med 2008; 34: 1779-87.
Arabi Y, Haddad S, Shirawi N, Al Shimemeri A. Early tracheostomy in intensive care trauma patients improves resource utilization: a cohort study and literature review. Crit Care 2004; 8: R347-52.
Shirawi N, Arabi Y. Bench-to-bedside review: early tracheostomy in critically ill trauma patients. Crit Care 2006; 10: 201.
Trouillet JL, Combes A, Vaissier E, et al. Prolonged mechanical ventilation after cardiac surgery: outcome and predictors. J Thorac Cardiovasc Surg 2009; 138: 948-53.
Curtis JJ, Clark NC, McKenney CA, et al. Tracheostomy: a risk factor for mediastinitis after cardiac operation. Ann Thorac Surg 2001; 72: 731-4.
Force SD, Miller DL, Petersen R, et al. Incidence of deep sternal wound infections after tracheostomy in cardiac surgery patients. Ann Thorac Surg 2005; 80: 618-21.
Rahmanian PB, Adams DH, Castillo JG, Chikwe J, Filsoufi F. Tracheostomy is not a risk factor for deep sternal wound infection after cardiac surgery. Ann Thorac Surg 2007; 84: 1984-91.
Bacchetta MD, Girardi LN, Southard EJ, et al. Comparison of open versus bedside percutaneous dilatational tracheostomy in the cardiothoracic surgical patient: outcomes and financial analysis. Ann Thorac Surg 2005; 79: 1879-85.
Stamenkovic SA, Morgan IS, Pontefract DR, Campanella C. Is early tracheostomy safe in cardiac patients with median sternotomy incisions? Ann Thorac Surg 2000; 69: 1152-4.
Gaudino M, Losasso G, Anselmi A, Zamparelli R, Schiavello R, Possati G. Is early tracheostomy a risk factor for mediastinitis after median sternotomy? J Card Surg 2009; 24: 632-6.
Ngaage DL, Cale AR, Griffin S, Guvendik L, Cowen ME. Is post-sternotomy percutaneous dilatational tracheostomy a predictor for sternal wound infections? Eur J Cardiothorac Surg 2008; 33: 1076-9.
EuroSCORE. European System for Cardiac Operative Risk Evaluation. Available from URL: http://www.euroscore.org/ (accessed March 2013).
The Society of Thoracic Surgeons National Database. Available from URL: http://www.sts.org/quality-research-patient-safety/quality/quality-performance-measures (accessed March 2013).
Dupuis JY, Wang F, Nathan H, Lam M, Grimes S, Bourke M. The cardiac anesthesia risk evaluation score: a clinically useful predictor of mortality and morbidity after cardiac surgery. Anesthesiology 2001; 94: 194-204.
Tran DT, Dupuis JY, Mesana T, Ruel M, Nathan HJ. Comparison of the EuroSCORE and Cardiac Anesthesia Risk Evaluation (CARE) score for risk-adjusted mortality analysis in cardiac surgery. Eur J Cardiothorac Surg 2012; 41: 307-13.
Singh K, Anderson E, Harper JG. Overview and management of sternal wound infection. Semin Plast Surg 2011; 25: 25-33.
Rubin DB. The design versus the analysis of observational studies for causal effects: parallels with the design of randomized trials. Stat Med 2007; 26: 20-36.
Disclosures
This work was presented in part by Dr. Louise Sun at the Resident Research Competition, Canadian Anesthesiologists’ Society Annual Meeting held at Quebec City, June 15-19, 2012. This study was supported by the research funds of the Cardiac Surgical Intensive Care Unit and the Division of Cardiac Anesthesiology of the University of Ottawa Heart Institute, Ottawa, Ontario, Canada.
None of the authors have any financial or academic competing interest in this work.
Conflicts of interest
None of the authors have any financial or academic competing interest in this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is accompanied by an editorial. Please see Can J Anesth 2013; 60: this issue.
Author contributions
Louise Sun and Bernard McDonald were involved in study conception and the acquisition of data. Louise Sun and Munir Boodhwani were involved in the analysis of data. Louise Sun was involved in drafting the manuscript. Louise Sun, Bernard McDonald, Munir Boodhwani, and Heather Baer were involved in the study design, interpretation of data, and critical revision of the manuscript.
Rights and permissions
About this article
Cite this article
Sun, L., Boodhwani, M., Baer, H. et al. The association between tracheostomy and sternal wound infection in postoperative cardiac surgery patients. Can J Anesth/J Can Anesth 60, 684–691 (2013). https://doi.org/10.1007/s12630-013-9950-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-013-9950-6