Abstract
Purpose
Perioperative hypothermia is still a common occurrence, and it can be difficult to measure a patient’s core temperature accurately, especially during regional anesthesia, with placement of a laryngeal mask airway device, or postoperatively. We evaluated a new disposable double-sensor thermometer and compared the resulting temperatures with those of a distal esophageal thermometer and a bladder thermometer in patients undergoing general and regional anesthesia, respectively. Furthermore, we compared the accuracy of the thermometer between regional and general anesthesia, since forehead microcirculation might differ between the two types of anesthesia.
Methods
We assessed core temperature in 36 general anesthesia patients and 20 patients having regional anesthesia for orthopedic surgery. The temperatures obtained using the double-sensor thermometer were compared with those obtained with the distal esophageal thermometer in the general anesthesia population and those obtained with the bladder thermometer in regional anesthesia patients.
Results
In our general anesthesia patients, 90% (95% confidence interval [CI] 85 to 95) of all double-sensor values were within 0.5°C of esophageal temperatures. The average difference (bias) between the esophageal and double-sensor temperatures was −0.01°C. In patients undergoing regional anesthesia 89% (95% CI 80 to 97) of all double-sensor values were within 0.5°C of bladder temperatures. The average difference (bias) between the bladder and double-sensor temperatures was −0.13°C, limits of agreement were −0.65 to 0.40°C.
Conclusions
In a perioperative patient population undergoing general or regional anesthesia, the accuracy of the noninvasive disposable double-sensor thermometer is comparable with that of the distal esophageal and bladder thermometers in routine clinical practice. Furthermore, the sensor performed comparably in patients undergoing regional and general anesthesia.
Résumé
Objectif
L’hypothermie périopératoire est encore de survenue fréquente et il peut être difficile de mesurer exactement la température centrale d’un patient, en particulier au cours d’une anesthésie locorégionale, avec mise en place d’un dispositif pour les voies aériennes de type masque laryngé ou en postopératoire. Nous avons évalué un thermomètre jetable à double capteur et nous avons comparé les températures mesurées avec celles d’un thermomètre œsophagien distal et d’un thermomètre vésical chez des patients subissant, respectivement, une anesthésie générale et une anesthésie locorégionale. De plus, nous avons comparé l’exactitude du thermomètre entre les anesthésies locorégionales et les anesthésies régionales dans la mesure où la microcirculation peut être différente au niveau du front entre les deux types d’anesthésie.
Méthodes
Nous avons évalué la température centrale chez 36 patients subissant une anesthésie générale et chez 20 patients subissant une anesthésie locorégionale pour des chirurgies orthopédiques. Les températures obtenues au moyen du thermomètre à double capteur ont été comparées à celles qui ont été obtenues avec le thermomètre œsophagien distal dans la population d’anesthésie générale et avec ceux obtenus avec le thermomètre vésical chez les patients subissant une anesthésie locorégionale.
Résultats
Parmi nos patients ayant subi une anesthésie générale, 90 % (intervalle de confiance [IC] à 95 %: 85 à 95) de toutes les mesures des doubles capteurs se situaient dans une limite de 0,5 °C par rapport aux températures œsophagiennes. La différence moyenne (biais) entre la température œsophagienne et la température des doubles capteurs était −0,01 °C. Chez les patients subissant une anesthésie locorégionale, 89 % (IC à 95 %: 80 à 97) de toutes les valeurs des doubles capteurs se situaient dans une limite de 0,5 °C des températures vésicales. La différence moyenne (biais) entre la température vésicale et la température des doubles capteurs était de −0,13 °C; les limites de concordance étaient −0,65 °C à 0,40 °C.
Conclusions
Dans une population de patients périopératoires subissant une anesthésie générale ou une anesthésie locorégionale, l’exactitude du thermomètre jetable non invasif à doubles capteurs est comparable à celle du thermomètre œsophagien distal et du thermomètre vésical dans la pratique clinique régulière. De plus, le capteur s’est comporté de la même manière chez les patients sous anesthésie générale et les patients sous anesthésie locorégionale.
Similar content being viewed by others
Perioperative hypothermia is associated with adverse outcomes, including increased risk for surgical wound infections, cardiac morbidity, coagulopathy, impaired drug metabolism, shivering, and increased use of hospital resources.1-3 In light of the many complications caused by hypothermia, the Surgical Care Improvement Project generated guidelines which mandate that surgical patients should have a core temperature of at least 36.0°C near the end of surgery or should be actively warmed intraoperatively.4 These new standards of thermal management increase the importance of accurate intraoperative core temperature measurements.
Current methods of measuring core temperature are either invasive or inaccurate. Esophageal temperature monitoring is used primarily in patients whose lungs are intubated, but this invasive technique is difficult or impossible in the rapidly increasing population of patients with a laryngeal mask airway device or undergoing regional anesthesia, as well as those under sedation or in the postanesthesia care unit. In addition, the noninvasive temperature monitoring devices, such as the infrared thermometer5 and those measuring axillary6-9 or skin temperature,10 are fairly unreliable. Thus, there is a need for an accurate noninvasive core temperature monitoring system that can be used in both general and regional anesthesia.
The first-generation non-disposable Draeger temperature monitoring device accurately measured core temperature in patients undergoing abdominal surgery.11 The current version of this device is a disposable sensor which is secured to the patient’s forehead via adhesive tape. The sensor contains two temperature probes that are separated by a known thermal resistance (double-sensor). While based on the same technology, there are substantial differences between the initial prototype11 and the current double-sensor version. Originally, the prototype was tested in patients undergoing general anesthesia; however, the real advantage of the noninvasive sensor is in patients undergoing regional anesthesia or in patients in whom an esophageal probe cannot be placed. It is possible that the technology might be less accurate in patients undergoing regional anesthesia. Vasodilation during general anesthesia effects vasomotion on the forehead, and thus, the forehead temperature is slightly higher in patients undergoing general anesthesia than in patients undergoing regional anesthesia.12
Consequently, the larger temperature difference between the forehead and the true core temperature during regional anesthesia might affect the performance of the double-sensor thermometer. We thus determined the accuracy of the new disposable device and compared the results with those obtained with the bladder thermometer in patients undergoing regional anesthesia as well as with those obtained with the esophageal thermometer in-patients undergoing general anesthesia. Furthermore, we compared the accuracy of the sensor in patients having general vs regional anesthesia.
Methods
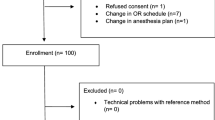
After approval by the Ethics Committees of the Medical University of Vienna, Austria (March 2008) and the Ethics Committee of the Cleveland Clinic, Ohio, USA (August 2008) and written informed consent, we studied trauma surgery patients having general anesthesia at the University of Vienna and orthopedic surgery patients having regional anesthesia at the Cleveland Clinic from January 2009 to June 2011. All general anesthesia patients had balanced anesthesia with tracheal intubation and ventilated lungs. After intubation, an esophageal temperature probe was inserted into the lower third of the esophagus. Regional anesthesia patients received spinal anesthesia, and a bladder catheter containing a temperature probe was placed after induction of anesthesia. All patients were 18-80 yr of age.
Protocol
We used a standardized warming protocol for patients at both institutions that consisted of forced-air warming (Bair Hugger®, Arizant, MN, USA). For patients undergoing general anesthesia, we used either an upper- or lower-body forced-air blanket depending on the location of surgery. For patients undergoing regional anesthesia, we used upper-body forced-air warming. In either case, we avoided warming the face or the proximity to the temperature probe.
Measurements
We recorded demographic and morphologic patient characteristics. Anesthetic management was at the discretion of the attending anesthesiologist. An experienced researcher trained in the use of the double-sensor thermometer performed the placement and measurements with the device. The disposable sensor was secured to the patient’s forehead with adhesive tape after a small amount of contact gel was applied between the sensor and the skin. Subsequently, we allowed a minimum of ten minutes for thermal equilibration before recording the first measurements.
Calculation of core temperature
We estimated core temperature (Tcore) from the double-sensor system using the following variables: Th1 (skin temperature under the insulator), Th2 (temperature above the insulator), the heat conduction coefficient (Ks) of the insulator (calibrated at Draeger AG for each double sensor), and an empirically estimated heat transfer coefficient of human tissue (Kg). As in our previous study, we used the following formula for calculating core temperature with the empiric human tissue heat transfer coefficient:
We measured all temperatures (esophageal, bladder, double-sensor) at five-minute intervals and stored the data in a data logger (Eltek 400 Series Squirrel Model 401/451, Eltek Limited, Haslingfield, Cambridge, UK). We subsequently calculated double-sensor core temperatures derived from temperatures Th1 and Th2 after the experiment offline using R software (R Project for Statistical Computing) and the formula above.
Statistical analysis
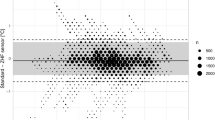
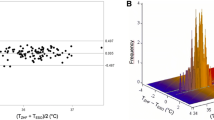
Our primary outcome was agreement between the esophageal or bladder temperatures and the double-sensor thermometer temperatures with a difference of ≤ 0.5°C. Two hundred measurement pairs determined an approximate accuracy of ± 0.24 standard errors as 95% confidence intervals (CI) for the limits of agreement. We aimed to obtain a minimum of 200 measurement pairs in both groups – except that convenience sampling was performed for an additional increase in the accuracy of the limits of agreement. Footnote 1 , 13 Furthermore, we calculated sensitivity and specificity for detection of hypothermia (core temperature < 36.0°C). As in other studies, we defined 0.5°C a priori as an acceptable limit of agreement.14-16 These limits reflect the normal magnitude of human circadian temperature variation.17,18 We performed a Bland-Altman analysis to evaluate agreement between the temperature measurement methods, assuming that the true value changes from measurement to measurement.13 In a Bland-Altman analysis, the bias and 95% limits of agreement are determined. The bias, which is estimated by the average difference, summarizes the lack of agreement between the two methods, whereas the 95% limits of agreement represent where 95% of future differences between the two methods would be expected.
We calculated bias (esophageal temperature minus double-sensor temperature or bladder temperature minus double-sensor temperature) for each outcome and for each time point, and we also calculated the mean bias across all pairs of measurements. We created a Bland-Altman plot of bias vs the average of each pair of measurements for visual representation of the agreement with the standard or reference core temperature measurements over the range of temperatures. For each patient group, we fit a mixed-effects regression model; differences between measurement pairs were modelled with a constant bias, the subject by methods interaction term, and a random error within the subject for the pair of observations. We also used the Bland-Altman limits of agreement for repeated measurements to estimate where approximately 95% of future differences from the standard core temperatures for each method were to be expected. Confidence intervals for proportions were calculated using a bootstrap sampling method. To account for repeated measurements,19 we used a proprietary R function (agreement.rma.R) for comparison analysis of all methods.
Lin’s concordance correlation coefficient was used to obtain a single-number agreement summary between the double-sensor vs the esophageal or bladder temperature.20 We considered P values < 0.05 statistically significant; results are presented as mean (SD) or mean (95% CI), and R 2.4.1 (R Project for Statistical Computing) was used for all analyses.
Results
We studied 36 patients having trauma surgery with general anesthesia at the University of Vienna and 20 patients having orthopedic surgery with regional anesthesia at the Cleveland Clinic. All patient demographic, morphometric, and thermal management data were recorded (Table 1). About 110 hours of data were obtained from 1,305 measurement pairs (n = 36 general anesthesia patients; n = 20 regional anesthesia patients).
General anesthesia patients
One thousand forty-seven measurement pairs were obtained. The range of esophageal temperatures was comparable with the range of double-sensor temperatures (34.4-37.8°C vs 34.5-38.0°C, respectively). The bias of measurements did not change systematically with the mean core temperature (r = −0.02). The estimated bias between temperatures using the esophageal thermometer vs temperatures using the double-sensor thermometer was −0.01°C with 95% limits of agreement of −0.61 to 0.59°C (Fig. 1), and 90% (95% CI 85 to 95) of all double-sensor values were within 0.5°C of the esophageal values.
Sensitivity for detection of hypothermia was 0.70 (95% CI 0.48 to 1.00) and specificity was 0.78 (95% CI 0.68 to 0.95). Lin’s concordance correlation for the two methods of temperature measurement showed a coefficient of 0.89.
Regional anesthesia patients
Overall, we obtained 258 measurement pairs over ≈24.5 hr (n = 20 patients). The range of temperatures using the bladder thermometer was comparable with the range of double-sensor temperatures (34.7-36.9°C vs 34.9-36.9°C, respectively). As in the general anesthesia patients, the bias of measurements did not change systematically with the mean core temperature (r = −0.14). The estimated bias between temperatures using the bladder thermometer vs temperatures with the double-sensor thermometer was −0.13°C with 95% limits of agreement of −0.65 to 0.40°C (Fig. 2). Eighty-nine percent (95% CI 80 to 97) of all double-sensor values were within 0.5°C of bladder values. Sensitivity was 0.83 (95% CI 0.68 to 1.00), specificity was 0.69 (95% CI 0.34 to 1.00), and Lin’s concordance correlation coefficient was 0.76. There was no significant difference between general anesthesia and regional anesthesia regarding the proportion of measurement error within ± 0.5°C.
Discussion
Maintenance of perioperative normothermia improves patient outcome.1-3 Considering the negative consequences of perioperative hypothermia, temperature monitoring and thermal management, i.e., active warming, are currently standard of care in many countries. Furthermore, the National Institute for Health and Clinical Excellence’s (NICE) guidelines recommend perioperative thermal management as well as accurate perioperative core temperature measurement.21 In the United States, the Surgical Care Improvement Project requires core temperatures to be ≥ 36.0°C at the end of surgery or at arrival in the postanesthesia care unit.4 The demands on thermal management make measurement and monitoring of intraoperative core temperature essential for patients undergoing general and regional anesthesia.
Although accurate core temperature values are easy to obtain when using invasive methods, such as esophageal temperature monitoring, access to these areas is not possible in the rapidly growing population of sedated patients, patients ventilated with a laryngeal mask airway device, or patients undergoing neuraxial anesthesia.22 Even so, patients undergoing neuraxial anesthesia are just as likely or even more likely to become hypothermic23-25 than patients undergoing general anesthesia, and they most likely suffer similar hypothermia-related complications. Thus, a noninvasive device that can accurately represent core temperature will help the clinician determine a course of thermal management.
Our results show that the most recent generation of the Draeger double-sensor thermometer secured on the patient’s forehead obtains temperatures that are comparable with esophageal and bladder temperatures obtained in patients undergoing general and regional anesthesia, respectively. Ninety percent of the double-sensor values were within 0.5°C of the reference esophageal temperatures, and approximately 89% of the double-sensor values were within 0.5°C of the bladder temperatures. Although the sensor did not completely fulfill our pre-specified limits of agreement, in our view, it agrees sufficiently enough for clinical practice. Furthermore, several studies have shown that even more invasive core thermometry methods (e.g., rectal or bladder thermometry) perform outside of the ≤ 0.5°C limits of agreement range.26,27 Our results are consistent with our previous research using a non-disposable sensor in intensive care patients as well as patients undergoing abdominal surgery.11 Despite the fact that forehead temperatures are usually slightly lower in the regional anesthesia patients, the performance of the double-sensor in these patients was comparable with its performance in patients undergoing general anesthesia. We consider it important to show the accuracy of the new double-sensor thermometer in patients undergoing regional anesthesia, as accurate measurement of core temperature is currently not possible in this patient population. Many hospitals use skin temperature, or infrared tympanic or temporal artery thermometers in this patient population, all of which do not accurately reflect core temperature.28 The only other noninvasive method of temperature measurement that could be used in patients undergoing regional anesthesia is oral temperature. It is fairly accurate but provides only intermittent measurements and is difficult to perform correctly as continuous measurement in sedated or sleeping patients.28 We thus propose that the new disposable double-sensor thermometer provides continuous core temperature measurements that are sufficiently accurate to reflect core temperature in patients undergoing either general or regional anesthesia.
Manufacturer guidelines specify that the thermometer needs a minimum of ten minutes to equilibrate; therefore, the double-sensor thermometer is not accurate for short-term core temperature screening. Furthermore, the double-sensor thermometer may not be appropriate or practical for usage during cranial or facial surgery as the sensor may interfere with the surgical procedure. The manufacturer has stated, that the sensor can be used simultaneously with BIS monitoring. This sentence was applicable only to previous versions of the sensor not tested in this study.
A limitation of our study is that we studied only the perioperative period, and thus, we did not have a wide range of temperatures; temperatures ranged from 34.5-37°C. We did not include intensive care unit patients, feverish patients, or patients with moderate to severe hypothermia into the present study. Nevertheless, the previous version of the disposable double-sensor thermometer has been evaluated in these patient populations and showed sufficient accuracy over a wide range of core temperatures. Furthermore, the sensor may have to be modified for pediatric patients, as the forehead of these patients is smaller in size. Hence, pediatric patients were not included in the study, and the results may not be applicable to these patient populations. Another limitation of our study is that we used esophageal temperature as the reference temperature in our general anesthesia group and bladder temperature as the reference temperature in the regional anesthesia group. Since bladder and esophageal temperature are highly correlated, we consider it acceptable to compare the two groups.27
In conclusion, the latest generation of the Draeger double-sensor thermometer was sufficiently accurate and comparable in patients undergoing general and regional anesthesia. We thus suggest that this sensor can replace the esophageal or other invasive temperature probes in patient populations where placement of invasive probes is not possible.
Notes
Bland JM. Available from URL: http://www.martinbland.co.uk/ (accessed September 2013).
References
Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med 1996; 334: 1209-15.
Schmied H, Kurz A, Sessler DI, Kozek S, Reiter A. Mild hypothermia increases blood loss and transfusion requirements during total hip arthroplasty. Lancet 1996; 347: 289-92.
Lenhardt R, Marker E, Goll V, et al. Mild intraoperative hypothermia prolongs postanesthetic recovery. Anesthesiology 1997; 87: 1318-23.
The Joint Commission. SCIP Inf-10: Surgery Patients with Perioperative Temperature Management. Available from URL; http://www.jointcommission.org/specifications_manual_for_national_hospital_inpatient_quality_measures.aspx (accessed September 2013).
Jean-Mary MB, Dicanzio J, Shaw J, Bernstein HH. Limited accuracy and reliability of infrared axillary and aural thermometers in a pediatric outpatient population. J Pediatr 2002; 141: 671-6.
Kresch MJ. Axillary temperature as a screening test for fever in children. J Pediatr 1984; 104: 596-9.
Morley CJ, Hewson PH, Thornton AJ, Cole TJ. Axillary and rectal temperature measurements in infants. Arch Dis Child 1992; 67: 122-5.
Muma BK, Treloar DJ, Wurmlinger K, Peterson E, Vitae A. Comparison of rectal, axillary, and tympanic membrane temperatures in infants and young children. Ann Emerg Med 1991; 20: 41-4.
Wilshaw R, Beckstrand R, Waid D, Schaalje GB. A comparison of the use of tympanic, axillary, and rectal thermometers in infants. J Pediatr Nurs 1999; 14: 88-93.
Cattaneo CG, Frank SM, Hesel TW, El-Rahmany HK, Kim LJ, Tran KM. The accuracy and precision of body temperature monitoring methods during regional and general anesthesia. Anesth Analg 2000; 90: 938-45.
Kimberger O, Thell R, Schuh M, Koch J, Sessler DI, Kurz A. Accuracy and precision of a novel non-invasive core thermometer. Br J Anaesth 2009; 103: 226-31.
Hatano Y, Nakamura K, Yakushiji T, Nishiwada M, Mori K, Anaes FC. Comparison of the direct effects of halothane and isoflurane on large and small coronary arteries isolated from dogs. Anesthesiology 1990; 73: 513-7.
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1: 307-10.
Suleman MI, Doufas AG, Akca O, Ducharme M, Sessler DI. Insufficiency in a new temporal-artery thermometer for adult and pediatric patients. Anesth Analg 2002; 95: 67-71.
Moran JL, Peter JV, Solomon PJ, et al. Tympanic temperature measurements: are they reliable in the critically ill? A clinical study of measures of agreement. Crit Care Med 2007; 35: 155-64.
Kimberger O, Cohen D, Illievich U, Lenhardt R. Temporal artery versus bladder thermometry during perioperative and intensive care unit monitoring. Anesth Analg 2007; 105: 1042-7.
Tayefeh F, Plattner O, Sessler DI, Ikeda T, Marder D. Circadian changes in the sweating-to-vasoconstriction interthreshold range. Pflugers Arch 1998; 435: 402-6.
Sessler DI, Lee KA, McGuire J. Isoflurane anesthesia and circadian temperature cycles in humans. Anesthesiology 1991; 75: 985-9.
Bland JM, Altman DG. Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat 2007; 17: 571-82.
Lin LI. A Concordance correlation coefficient to evaluate reproducibility. Biometrics 1989; 45: 255-68.
National Institute for Health and Care Excellence (NICE). Perioperative hypothermia (inadvertent) (CG65) 2008 [cited 05/23/2012]. Available from URL: www.nice.org.uk/CG065 (accessed September 2013).
Frank SM, Nguyen JM, Garcia C, Barnes RA. Temperature monitoring practices during regional anesthesia. Anesth Analg 1999; 88: 373-7.
Leslie K, Sessler DI. Reduction in the shivering threshold is proportional to spinal block height. Anesthesiology 1996; 84: 1327-31.
Ozaki M, Kurz A, Sessler DI, et al. Thermoregulatory thresholds during epidural and spinal anesthesia. Anesthesiology 1994; 81: 282-8.
Saito T, Sessler DI, Fujita K, Ooi Y, Jeffrey R. Thermoregulatory effects of spinal and epidural anesthesia during cesarean delivery. Reg Anesth Pain Med 1998; 23: 418-23.
Brauer A, Martin JD, Schuhmann MU, Braun U, Weyland W. Accuracy of intraoperative urinary bladder temperature monitoring during intra-abdominal operations (German). Anasthesiol Intensivmed Notfallmed Schmerzther 2000; 35: 435-9.
Lefrant JY, Muller L, de La Coussaye JE, et al. Temperature measurement in intensive care patients: comparison of urinary bladder, oesophageal, rectal, axillary, and inguinal methods versus pulmonary artery core method. Intensive Care Med 2003; 29: 414-8.
Langham GE, Maheshwari A, Contrera K, You J, Mascha E, Sessler DI. Noninvasive temperature monitoring in postanesthesia care units. Anesthesiology 2009; 111: 90-6.
Funding
This work was supported by Draegerwerk AG & Co. KGaA (Luebeck, Germany).
Declaration of interests
Oliver Kimberger consulted for Draegerwerk AG & Co. KGaA and received research funding and travel support from Draegerwerk AG & Co. KGaA. Sebahat Dizili received research funding from Draegerwerk AG & Co. KGaA. Jochim Koch works for Draegerwerk AG & Co. KGaA and reported a conflict of interest with Draegerwerk AG & Co. KGaA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
Oliver Kimberger, Jochim Koch, and Andrea Kurz helped design the study. Oliver Kimberger, Jochim Koch, Andrea Kurz, Leif Saager, Cameron Egan, Ivan Parra Sanchez, and Sebahat Dizili helped conduct the study, analyze the data, and write the manuscript.
Rights and permissions
About this article
Cite this article
Kimberger, O., Saager, L., Egan, C. et al. The accuracy of a disposable noninvasive core thermometer. Can J Anesth/J Can Anesth 60, 1190–1196 (2013). https://doi.org/10.1007/s12630-013-0047-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-013-0047-z