Abstract
Background
Appropriate planning is crucial to avoid morbidity and mortality when difficulty is anticipated with airway management. Many guidelines developed by national societies have focused on management of difficulty encountered in the unconscious patient; however, little guidance appears in the literature on how best to approach the patient with an anticipated difficult airway.
Methods
To review this and other subjects, the Canadian Airway Focus Group (CAFG) was re-formed. With representation from anesthesiology, emergency medicine, and critical care, CAFG members were assigned topics for review. As literature reviews were completed, results were presented and discussed during teleconferences and two face-to-face meetings. When appropriate, evidence- or consensus-based recommendations were made, and levels of evidence were assigned.
Principal findings
Previously published predictors of difficult direct laryngoscopy are widely known. More recent studies report predictors of difficult face mask ventilation, video laryngoscopy, use of a supraglottic device, and cricothyrotomy. All are important facets of a complete airway evaluation and must be considered when difficulty is anticipated with airway management. Many studies now document the increasing patient morbidity that occurs with multiple attempts at tracheal intubation. Therefore, when difficulty is anticipated, tracheal intubation after induction of general anesthesia should be considered only when success with the chosen device(s) can be predicted in a maximum of three attempts. Concomitant predicted difficulty using oxygenation by face mask or supraglottic device ventilation as a fallback makes an awake approach advisable. Contextual issues, such as patient cooperation, availability of additional skilled help, and the clinician’s experience, must also be considered in deciding the appropriate strategy.
Conclusions
With an appropriate airway evaluation and consideration of relevant contextual issues, a rational decision can be made on whether an awake approach to tracheal intubation will maximize patient safety or if airway management can safely proceed after induction of general anesthesia. With predicted difficulty, close attention should be paid to details of implementing the chosen approach. This should include having a plan in case of the failure of tracheal intubation or patient oxygenation.
Résumé
Contexte
Une planification adaptée est essentielle afin d’éviter la morbidité et la mortalité lorsqu’on prévoit des difficultés dans la prise en charge des voies aériennes. De nombreuses recommandations émises par des sociétés nationales mettent l’emphase sur la gestion des difficultés rencontrées chez le patient inconscient. Toutefois, il n’existe dans la littérature que peu de suggestions sur la façon d’approcher le patient chez qui les difficultés sont prévisibles.
Méthode
Afin de passer en revue ce sujet et d’autres, le Canadian Airway Focus Group (CAFG), un groupe dédié à l’étude de la prise en charge des voies aériennes, a été reformé. Les membres du CAFG représentent diverses spécialités soit l’anesthésiologie, la médecine d’urgence et les soins intensifs. Chaque participant avait pour mission de passer en revue des sujets précis. Les résultats de ces revues ont été présentés et discutés dans le cadre de téléconférences et de deux réunions en personne. Lorsqu’indiqué, des recommandations fondées sur des données probantes ou sur un consensus ont été émises. Le niveau de confiance attribué à ces recommandations a aussi été défini.
Constatations principales
Plusieurs éléments permettant de prédire la laryngoscopie directe difficile sont connus. Des études plus récentes décrivent aussi les éléments permettant d’anticiper des difficultés lors de la ventilation au masque facial, de la vidéolaryngoscopie, de l’utilisation d’un dispositif supraglottique ou de la réalisation d’une cricothyrotomie. Tous ces éléments doivent être pris en compte lors de l’évaluation du patient chez qui des difficultés sont anticipées lors de la prise en charge des voies aériennes. De nombreuses études rapportent une morbidité accrue liée à des tentatives multiples d’intubation trachéale. Planifier de procéder à l’intubation trachéale après l’induction de l’anesthésie générale n’est donc recommandé que pour les patients chez qui la ou les techniques prévues ne nécessiteront pas plus de trois tentatives. Il est recommandé de prioriser d’emblée une approche vigile dans les cas où des difficultés reliées à l’utilisation du masque facial ou d’un dispositif supraglottique sont prévues. L’établissement d’une stratégie de prise en charge doit tenir compte d’éléments contextuels telles la collaboration du patient, la disponibilité d’aide supplémentaire et de personnel qualifié, et l’expérience du clinicien.
Conclusion
Une évaluation adaptée des voies aériennes ainsi que les éléments contextuels propres à chaque situation sont les bases qui permettent de déterminer de manière rationnelle si l’intubation trachéale vigile est apte à optimiser la sécurité du patient, ou si la prise en charge des voies aériennes peut être réalisée de manière sécuritaire après l’induction de l’anesthésie générale. Lorsqu’on prévoit des difficultés, une attention particulière doit être portée aux détails nécessaires au succès de l’approche envisagée. De plus, il convient d’avoir un plan en cas d’échec de l’intubation trachéale ou si l’oxygénation du patient s’avérait difficile.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
What other recommendation statements are available on this topic?
Many developed countries have published national guidelines and recommendations on management of the difficult airway. Most of these recommendations emphasize management of the already unconscious patient in whom difficulty has been encountered.
Why were these recommendations developed?
Little guidance is provided by many of the existing guidelines on planning and decision-making for the patient with an anticipated difficult airway. These recommendations were developed to help address this gap.
How do these statements differ from existing recommendations?
These statements aim to address situations where the patient with a predicted difficult tracheal intubation can be safely managed after induction of general anesthesia or where an awake approach should be considered.
Why do these statements differ from existing recommendations?
These recommendations differ from existing consensus guidelines to reflect the widespread availability of recent innovations in airway management equipment and clinicians' increasing familiarity with these newer devices.
Contents
Methods
Airway evaluation: anticipating the difficult airway
Options when difficult tracheal intubation is anticipated
Avoiding tracheal intubation
Proceeding with tracheal intubation: options
Deciding on awake or post-induction tracheal intubation
The elective surgical patient with a difficult airway
The uncooperative patient with a difficult airway
The emergency patient with a difficult airway
Implementation: proceeding with anticipated difficult tracheal intubation
Awake tracheal intubation
Failed awake intubation
Inadvertent loss of airway during attempted awake intubation
Post-induction tracheal intubation when difficulty is predicted
Preparation
Pre- and peri-intubation oxygenation
Equipment choice
Ablation or maintenance of spontaneous ventilation
Checking for efficacy of face mask ventilation after induction, before administration of a neuromuscular blocking agent (NMBA)
Use of a short- or intermediate-acting NMBA
Cricoid pressure
Difficult tracheal intubation encountered in the unconscious patient
Obstructing airway pathology
Inhalational induction with obstructing airway pathology
The “double setup airway intervention”
The morbidly obese patient
Tracheal extubation in the patient with a difficult airway
Summary of recommendations
References
DISCLAIMER: | |
Care has been taken to confirm the accuracy of the information presented and to describe generally accepted practices. The authors accept that medical knowledge is an ever-changing science that continually informs, improves, and alters attitudes, beliefs, and practices. | |
Recommendations are not intended to represent or be referred to as a standard of care in the management of the difficult or failed airway. | |
Application of the information provided in a particular situation remains the professional judgement and responsibility of the practitioner. |
When planning how to approach the anticipated difficult airway, the primary focus should be on ensuring adequate oxygenation and ventilation and not simply on intubating the trachea. Management of the anticipated difficult airway follows an assessment of the probable success of ventilation by face mask or supraglottic device (SGD) as well as direct or indirect (e.g., video) laryngoscopy, tracheal intubation and surgical airway access.1 Unfortunately, predicting difficulty with these measures remains an imperfect science. Furthermore, surveys suggest that clinicians’ management choices vary widely even when significant difficulty is predicted.2-4
There is agreement in many national consensus guidelines on the importance of performing an airway evaluation to predict difficulty with airway management.5-10 Unfortunately, once identified, some guidelines fail to provide sufficient guidance on how to proceed, simply implying that tracheal intubation should be performed awake when difficulty is anticipated. Certainly, as highlighted by the 4th National Audit Project (NAP4) from the United Kingdom, airway-related patient morbidity and mortality can occur following induction of general anesthesia when difficult tracheal intubation is predicted.11 Sponsored by the Difficult Airway Society and the Royal College of Anaesthetists, the NAP4 study reported complications of airway management associated with nearly three million airway interventions in the United Kingdom during a 12-month period. Difficulty had been anticipated in most of the 43 operative patients in whom the initial attempts at tracheal intubation failed. The most common problem identified was the “failure to plan for failure”.11 When difficulty is anticipated, airway management after induction of general anesthesia can be justified only when the risk of failure to oxygenate is low and when an appropriate backup plan can be quickly implemented.
Historically, airway assessment has focused mainly on predictive tests of successful direct laryngoscopy. These tests had limited sensitivity and specificity, resulting in both unanticipated failures to obtain a view of the larynx12 and unnecessary awake tracheal intubations. Patient safety was assigned a higher priority than comfort so awake intubations were appropriately advocated when uncertainty existed. Nevertheless, with recent innovations (e.g., video laryngoscopes) and alternative methods of providing oxygenation (e.g., supraglottic airways), it may be that more patients can be safely managed after induction of general anesthesia.
This article, the second of two publications, seeks to address the approach to a patient with an anticipated difficult airway as well as implementation of the chosen approach. The first article in the series addressed difficult tracheal intubation encountered in the already unconscious patient.13 The two publications aim to provide recommendations and a cognitive framework to inform clinician decision-making in the interest of patient safety, regardless of specialty or practice environment.
Methods
The methods presented are identical to those described in the companion article13 and are reproduced here for the benefit of the reader. The Canadian Airway Focus Group (CAFG) was originally formed in the mid-1990s and published recommendations for the management of the unanticipated difficult airway in 1998.5 Four of the original CAFG members rejoined the current iteration, and the first author invited an additional 14 clinicians with an interest in airway management to participate. The current Focus Group includes representatives from anesthesiology, emergency medicine, and critical care.
Topics for review were divided among the members, and participants conducted a literature review on their topic(s). Electronic literature searches were not conducted according to a strict protocol, but participants were instructed to search, at a minimum, Medline and EMBASE databases together with the Cochrane Central Register of Controlled Trials (CENTRAL). Search strings were determined by individual participants. A worksheet was completed for each topic with details of the search strategy, a synopsis of the relevant studies, an overall summary of findings, the perceived quality of evidence, and the author’s suggestion(s) for strength of recommendation (see below). Once finished, worksheets were made available to the CAFG membership on a file hosting service.
The Focus Group convened regularly by teleconference, and face-to-face meetings occurred on two occasions during the 24 months taken to complete the process. Worksheet authors presented their topics to the members, who then arrived at consensus on overall quality of evidence and any recommendations. In the event that evidence was of low quality or altogether lacking, “expert opinion” by consensus was sought. Finally, a draft of the completed manuscripts was distributed to all members for review prior to submission.
The strength of a recommendation and assignment of level of evidence were modelled after the GRADE system, as per previously published criteria.14,15 When made, formal strength of recommendations adhere to the following descriptors:
-
Strong recommendation for – most patients should receive the intervention; most patients in this situation would want the recommended course of action;
-
Weak recommendation for – most patients would want the suggested course of action, but some would not; the appropriate choice may vary for individual patients.
-
Strong recommendation against – most patients should not receive the intervention; most patients in this situation would not want the suggested course of action;
-
Weak recommendation against – most patients would not want the suggested course of action, but some would; the appropriate choice may vary for individual patients.
Three levels of evidence were applied,14 as follows:
-
Level of evidence A (High) – systematic reviews of randomized controlled trials (RCTs), RCTs without important limitations, or observational studies providing overwhelming evidence;
-
Level of evidence B (Moderate) – RCTs with limitations, observational studies with significant therapeutic effect;
-
Level of evidence C (Low) – RCTs with significant limitations, observational studies, case series, or published expert opinion.
When a level of evidence is not specifically supplied, recommendations reflect the consensus opinion of the authors.
Airway evaluation: anticipating the difficult airway
An airway evaluation should be performed on every patient requiring airway management (Strong recommendation for, level of evidence C). For the patient requiring tracheal intubation, an airway evaluation is performed primarily to help decide if intubation can be safely performed after the induction of general anesthesia (with or without maintenance of spontaneous ventilation) or if intubation should proceed with the patient awake. Even if a lack of patient cooperation precludes a complete airway evaluation or the option of awake intubation, performing this step serves as a “cognitive forcing strategy”16 to encourage appropriate planning and preparation for the airway intervention, however undertaken.
A complete airway evaluation should include an assessment of not only the predicted ease or difficulty of tracheal intubation (Tables 1 and 2) but also the predicted success of fallback options to achieve oxygenation, such as face mask ventilation (Table 3), SGD use (Table 4), and surgical airway (Table 5)1 (Strong recommendation for, level of evidence C). As the number of predictors of difficulty increases, so does the probability of actually encountering problems.17,18 In addition to physical examination and a history of prior difficulties provided by the patient, records of previous airway interventions, imaging studies, electronic databases and letters carried by the patient should be considered if time permits and records can be sourced. Other contextual issues must also be considered, including patient cooperation, the clinician’s skill and experience, availability of additional skilled help, and whether the desired equipment is accessible.19
Options when difficult tracheal intubation is anticipated
Avoiding tracheal intubation
When difficult tracheal intubation is anticipated in the surgical patient, it may be feasible to proceed without general anesthesia or with general anesthesia but without tracheal intubation. However, if general anesthesia with tracheal intubation would normally occur for the procedure, a careful risk-to-benefit assessment must be undertaken before proceeding without an airway secured by a tracheal tube. The following options can be considered:
Proceeding with regional or infiltration anesthesia: Regional (e.g., neuraxial or peripheral nerve block) or infiltration (local) anesthesia may be an option for surgery, with the following provisos:
-
Easy access to the airway during the case is advisable;
-
The nerve block must be compatible with the estimated duration of the surgical procedure;
-
Interrupting the surgery must be feasible in case an intraoperative awake intubation or re-do of the block is required;
-
The necessary equipment and expertise must be available to manage the airway in case complications of the block result in loss of consciousness or respiratory compromise.
If regional or local anesthesia is elected in the patient with anticipated difficult tracheal intubation, the surgical safety briefing should include the anesthesiologist’s planned strategy for conversion to general anesthesia, if required intraoperatively.
General anesthesia using SGD or face mask ventilation: Successful use of SGDs has been reported in patients who are known or suspected to be difficult to intubate by direct laryngoscopy.56-60 Nevertheless, the NAP4 study documented cases where inappropriate use of a SGD to avoid difficult tracheal intubation resulted in patient morbidity.61 If difficult tracheal intubation is predicted but intubation is not absolutely required for the safe conduct of general anesthesia, use of a SGD may be considered provided the patient is at low risk of aspiration and a plan has been made for managing intraoperative failure of ventilation or oxygenation.
Deferring surgery: For the elective surgical patient with predicted difficult tracheal intubation, the option of not proceeding with surgery at that time (or at all) should be considered. This choice may be especially relevant if working in unfavourable conditions (e.g., lacking access to difficult airway equipment and/or additional skilled help), as may be the case in some remote locations. Under such circumstances, airway management might be deferred until suitable equipment and/or expertise is in place.
The out-of-operating room (OR) emergency: Management of the emergency patient with known or presumed difficult tracheal intubation cannot be deferred. Nevertheless, it may be possible to sustain oxygenation using nasal cannulae with high flows of humidified oxygen, noninvasive ventilation (e.g., continuous or bilevel positive airway pressure), assisted face mask ventilation, or placement of a SGD pending the arrival of additional expertise or equipment for tracheal intubation. Occasionally, this may permit an underlying condition (e.g., congestive heart failure or acute respiratory failure) to be treated to the point that tracheal intubation is no longer required.62-65
Proceeding with tracheal intubation: options
When difficulty is predicted and tracheal intubation cannot be avoided, a number of options exist for how to proceed. Further details on the following options appear in subsequent sections.
Awake tracheal intubation: This can occur via the oral or nasal transglottic route, awake tracheotomy, or awake cricothyrotomy. This is generally facilitated by local anesthesia, with or without judicious sedation.
Tracheal intubation after induction of general anesthesia:
-
Induction with ablation of spontaneous ventilation using a bolus dose of sedative-hypnotic and optimizing intubating conditions with a neuromuscular blocking agent;
-
Induction while maintaining spontaneous ventilation via inhalation of volatile anesthetic or infusion of a sedative-hypnotic such as propofol.
Especially in out-of-OR settings for urgent or emergency cases, tracheal intubation is sometimes facilitated only by moderate to deep sedation. While often successful, this approach may result in patient apnea, suboptimal intubating conditions (including reflex glottic closure with airway instrumentation), and regurgitation/aspiration due to gag reflex activation.
Very rarely, establishing femorofemoral cardiopulmonary bypass under local anesthesia may be indicated prior to induction of general anesthesia, chiefly when disease intrinsic66-68 or extrinsic69,70 to the tracheal lumen threatens complete tracheal obstruction with the onset of general anesthesia.
Deciding on awake or post-induction tracheal intubation
With anticipated difficult tracheal intubation that cannot be avoided, the clinician must decide if intubation can proceed safely after induction of general anesthesia or if it would be achieved more safely in the awake patient. Although complications up to and including loss of the airway can occur during attempted awake intubation,71-73 an awake approach can potentially confer a safety benefit by having the patient maintain airway patency, gas exchange, and protection of the airway against aspiration of gastric contents or blood during the intubation process.
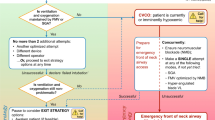
The following discussion and accompanying flow diagram (Figure) attempt to identify the relevant factors that must be weighed when creating a patient-specific airway strategy. Neither discussion nor flow diagram is meant to be prescriptive. Many factors impact the decision, including patient cooperation, consent, and the clinician’s expertise.
Two primary questions should be addressed:
If general anesthesia is induced, is tracheal intubation predicted to succeed with the chosen technique(s)? Guidance to help answer this question comes from the published studies on predictors of difficult tracheal intubation. Most of these studies relate to direct laryngoscopy (Table 1). Fewer studies have been published on the predictors of difficulty using alternative techniques such as video laryngoscopy (Table 2). Thus, if the intended “Plan A” or “Plan B” intubation technique includes the use of an alternative to direct laryngoscopy, the clinician must estimate the probability of success in his or her hands under the prevailing conditions.
Data from within74-76 and outside the operating room (OR)77-81 point to increasing morbidity with multiple intubation attempts. Any doubt about whether tracheal intubation will succeed in the anesthetized patient in a maximum of three attempts using direct laryngoscopy or an alternative to direct laryngoscopy would favour an awake approach.
If tracheal intubation fails, will oxygenation by face mask or SGD succeed? When difficult tracheal intubation is predicted, evaluation of the probable success of fallback oxygenation by face mask or SGD ventilation is especially warranted. Predictors of difficult face mask (Table 3) and SGD (Table 4) ventilation have been studied and published. In most situations, significant predicted difficulty with both tracheal intubation and face mask or SGD ventilation should be taken as a strong signal to consider awake intubation, particularly in the cooperative elective surgical patient (Strong recommendation for, level of evidence C).
It should be emphasized that overlap exists between some predictors of difficult direct and video laryngoscopy and those of difficult face mask ventilation. As such, when difficult laryngoscopy is predicted, a careful and deliberate assessment of predicted ease of face mask ventilation should occur. Consideration should also be given to the probability that successful ventilation by face mask or SGD may diminish with repeated intubation attempts.
Other patient or contextual issues may impact the decision of whether to proceed with tracheal intubation before or after induction of general anesthesia, and these issues should be considered19 (Strong recommendation for, level of evidence C). Although not an exhaustive list, if any of the following issues coincide with predicted difficult intubation, an awake approach may be most prudent:
Anticipated short safe apnea time: With the onset of apnea, rapid oxygen desaturation can be anticipated in the patient with decreased functional residual capacity, increased oxygen consumption, or low starting oxygen saturation. This will shorten the available time for intubation attempts before oxygen desaturation supervenes. Patients with respiratory or metabolic acidosis may also be less tolerant of apnea.
Significant risk of aspiration: When practical, awake intubation should be considered for the patient with predicted difficult tracheal intubation who is also at increased risk of regurgitation and aspiration of gastric contents.
Presence of obstructing airway pathology: Significant intrinsic, extrinsic, or incipient obstructing airway pathology should prompt consideration of awake management. In the NAP4 study, a number of cases were documented where attempted post-induction tracheal intubation resulted in serious patient morbidity in the presence of obstructing airway pathology.76
Additional skilled help not available: Skilled assistance during the management of a difficult airway is of considerable importance. Its absence should elevate the option of awake management (although this too may necessitate additional assistance).
Clinician inexperienced with planned technique or device not available: The clinician must be competent and experienced with the planned intubation technique(s) when a post-induction approach is contemplated, and the preferred device(s) must be readily available.
Thus, for the patient with anticipated difficult tracheal intubation, a post-induction approach may be considered if successful intubation is anticipated with the chosen technique(s) within three attempts, successful fallback oxygenation by face mask or SGD ventilation is predicted, and other patient and contextual issues are favourable.
Conversely, if there is a significant risk that tracheal intubation may require more than three attempts despite optimized conditions, face mask ventilation or SGD ventilation is also predicted to be difficult, or other patient and contextual issues are unfavourable (e.g., lack of additional skilled help), the risk of failed oxygenation is elevated and an awake approach is prudent (Figure).
The elective surgical patient with a difficult airway
The cooperative elective surgical patient must be optimized preoperatively and managed in the safest way possible. When difficult tracheal intubation is anticipated in this population, proceeding with post-induction tracheal intubation should occur only with an estimated margin of safety equivalent to that of an awake intubation (Strong recommendation for, level of evidence C). Perceived time (“production”) pressure must not be allowed to impact the decision.
The uncooperative patient with a difficult airway
A lack of patient cooperation may preclude the option of awake tracheal intubation. This subsection refers to the actively uncooperative patient (as with many pediatric patients or adults with cognitive impairment, brain injury, or hypoxemia) and not patient refusal or clinician discomfort with awake techniques. Patient refusal of an awake intubation is unusual when the technique and its rationale are advanced with confidence and empathy, along with the risks of the alternatives.
All options for proceeding with anticipated difficult tracheal intubation of the uncooperative patient involve risk: the clinician’s job is to manage the risk. The benefit of proceeding with tracheal intubation at that time must exceed the risk of deferring intubation. If proceeding, even with an experienced airway manager in attendance, the location of additional skilled help should be established.
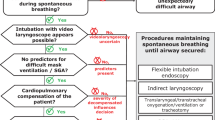
When significant difficulty is predicted and a lack of patient cooperation precludes the provider’s usual awake intubation technique(s), one of the following options can be considered to facilitate tracheal intubation:
Maintenance of spontaneous ventilation
-
Blind or bronchoscopic-aided nasal intubation (if not contraindicated), with or without use of gentle physical restraint, and application of local anesthesia as the situation permits;
-
Judicious sedation with a pharmacologic agent less likely to have an adverse impact on airway tone or respiratory effort (e.g., ketamine, dexmedetomidine, or haloperidol), with application of local anesthesia as the situation permits;
-
Induction of general anesthesia while maintaining spontaneous ventilation using inhalation of volatile anesthetic or an intravenous infusion of sedative-hypnotic.
Ablation of spontaneous ventilation
Occasionally, intravenous induction of general anesthesia using a bolus of sedative-hypnotic and neuromuscular blockade (e.g., rapid sequence intubation [RSI]) must be considered in the uncooperative patient with a difficult airway if techniques maintaining spontaneous ventilation have failed or are predicted to fail. This situation demands appropriate preparation, including a “double setup airway intervention”, whereby personnel and equipment are standing by to enable immediate cricothyrotomy in the event of failed oxygenation. See the section titled “The double setup airway intervention”.
The emergency patient with a difficult airway
Within or outside the OR, management of the critically ill emergency patient with a difficult airway is particularly challenging. Such patients generally have limited reserves, may be hypoxemic at presentation, difficult to adequately pre-oxygenate, and can rapidly desaturate with the onset of apnea. They must be assumed to be at increased risk of aspiration of gastric contents. Outside the OR, the risk of difficult tracheal intubation is higher and is associated with greater morbidity if multiple intubation attempts are required.77-81 There may be difficulties with access to the patient and optimum positioning. Manual in-line stabilization of the cervical spine and cricoid pressure may interfere with insertion of the laryngoscope, laryngeal exposure, or insertion of SGDs. In some centres, non-anesthesiologists may have few opportunities for airway management. This can be compounded by a limited selection of equipment and lack of access to additional skilled help. Airway management generally cannot be cancelled or deferred, and poor patient cooperation can adversely impact both the completeness of an airway assessment and options (e.g., awake intubation) for tracheal intubation.
The foregoing factors place emergency patients at higher risk of complications during attempted airway management; however, the principles outlined in the preceding sections remain applicable. While the need for tracheal intubation is often urgent in the critically ill patient, when difficulty is anticipated, there is often time to achieve topical airway anesthesia for awake intubation or to enlist additional skilled help. When rapid sequence intubation is required and difficulty is anticipated, requisite preparations should occur (see Preparation section below).
Evidence that adverse events escalate with multiple intubation attempts in the critically ill population77-81 suggests that the most expert airway manager available should perform airway interventions in the emergency patient.
Implementation – proceeding with anticipated difficult tracheal intubation
Awake tracheal intubation
Clinicians who manage difficult airways should be competent in awake tracheal intubation (Strong recommendation for, level of evidence C). For awake intubation, an antisialagogue is helpful prior to application of topical airway anesthesia, unless contraindicated. Adequate anesthesia of the pharynx, larynx, and trachea – and nasal cavity if nasal intubation is planned – can be applied topically or with nerve blocks. The semi-sitting or sitting position may provide greater airway patency and patient comfort and is recommended for the procedure when feasible. Sedation should be limited in an effort to retain airway patency and patient cooperation – amnesia is not necessarily a goal during awake intubation. Supplemental oxygen is useful and can be administered by nasal cannulae. Awake intubation in the elective surgical patient will most often proceed using a flexible intubating bronchoscope, but it can also occur with other devices alone or in combination (e.g., video laryngoscopes, optical stylets, light wands, or SGDs used as a conduit for bronchoscopic intubation). Direct laryngoscopy can be used for awake tracheal intubation (as may occur for the patient with relatively favourable airway anatomy and significant hemodynamic instability). Awake tracheotomy or cricothyrotomy performed under local anesthesia is an option and may be the safest approach in patients with symptomatic obstructing airway pathology.
Failed awake intubation
An awake intubation attempt may fail due to inadequate oropharyngeal or laryngeal airway anesthesia, excessive secretions or blood, very difficult patient anatomy, lack of patient cooperation, oversedation, or operator inexperience. If inadequate local anesthesia is the problem, before additional agent is administered, the total dose of local anesthetic already administered should be determined to avoid toxicity. If local anesthetic toxicity is a worry and the surgery is elective, the case may be deferred. The clinician must not feel compelled to proceed with post-induction intubation following failed awake intubation in elective surgical patients, as this has resulted in cases of major morbidity and death.74 In contrast, for the emergency patient, if additional expertise is unavailable for another awake intubation attempt, with appropriate preparation, post-induction tracheal intubation must sometimes be undertaken.
Inadvertent loss of the airway during attempted awake intubation
Case reports have been published of complete airway obstruction occurring during attempted awake intubation.71,72,82 This occurs most often in the setting of obstructing airway pathology;74 possible etiologies include natural disease progression, excess sedation, reflex glottic closure, trauma from intubation attempts, or a direct adverse effect of local anesthetic on upper airway patency.83,84 The latter phenomenon is infrequent, but it is important to be aware of this occurrence. This does not imply that awake transglottic intubation should be avoided in all patients with obstructing airway pathology, but it does mandate readiness to proceed rapidly with surgical access if oxygenation fails.
Post-induction tracheal intubation when difficulty is predicted
Preparation
When difficulty is predicted and the decision is made for tracheal intubation after induction of general anesthesia, the following preparations should occur (Strong recommendation for, level of evidence C):
-
The patient should be placed in an optimum position with adequate pre-oxygenation;
-
Equipment should be prepared for the primary intubation approach (Plan A);
-
A familiar alternative intubation device should also be immediately on hand (Plan B);
-
A suitably sized SGD should be prepared for use;
-
The location and availability of additional skilled help should be established;
-
An “exit strategy” plan for failed tracheal intubation should be articulated to those participating in the patient’s care. Such a pre-emptive briefing should be encouraged and does not suggest an expectation of failure; rather, it increases the likelihood of a coordinated and effective response by those involved. The exit strategy is the plan to engage if tracheal intubation is unsuccessful within a maximum of three attempts. It exists to alert the clinician to avoid further potentially harmful attempts at tracheal intubation. In the adequately oxygenated patient, exit strategies include awakening the patient (if not an emergency), temporizing with face mask or SGD ventilation, obtaining more expertise or equipment for a further careful intubation attempt (if this has a high probability of success), or very rarely, a surgical airway.13
Pre- and peri-intubation oxygenation
All patients with an anticipated difficult tracheal intubation and planned post-induction intubation should be pre-oxygenated with 100% oxygen for three minutes of tidal volume breathing, eight vital capacity breaths over 60 sec,85 or until FEO2 exceeds 90%86 (Strong recommendation for, level of evidence B). There is evidence that oxygen desaturation with apnea can be further postponed if pre-oxygenation is undertaken with the patient in the semi-seated (Fowler’s) position or with the stretcher or table in the reverse Trendelenburg position.87-91 Apneic oxygenation92 via nasopharyngeal catheter93,94 or nasal cannulae95 may also be beneficial during attempted tracheal intubation.
Equipment choice
No recommendation can be made for the use of a particular device or class of device for post-induction tracheal intubation when difficulty is predicted. Video laryngoscopes can be effective in enabling a view of the larynx and facilitating intubation when direct laryngoscopy has failed or is predicted to fail. Other classes of intubation device can be similarly effective when difficult tracheal intubation is predicted, including blind intubation with a lighted stylet or via the Fastrach™ laryngeal mask airway. Some clinicians may be facile in using the flexible bronchoscope for post-induction intubation, with or without use of a SGD as a conduit. Optical indirect laryngoscopes, such as the Airtraq™ or Bullard™ laryngoscope, are also effective and can be video enabled. Most important is the clinician's estimation that the chosen device will successfully address the anatomic reason for predicted difficulty with tracheal intubation, that he or she is experienced with its use, and that it is available.
Ablation or maintenance of spontaneous ventilation
Conditions for tracheal intubation are generally considered to be optimized with ablation of spontaneous ventilation by administration of a sedative-hypnotic and neuromuscular blocking agent. However, inhalational induction of general anesthesia has been suggested as a method to facilitate intubation when difficulty is anticipated. The theoretical safety advantage afforded by inhalational induction (or induction by infusion of a sedative-hypnotic, e.g., propofol) relates to maintenance of spontaneous ventilation and therefore oxygenation during the induction process.96 While inhalational induction is commonly used in the pediatric population, in adults, it can take time to attain a sufficiently deep plane of general anesthesia for airway instrumentation without provoking reflex glottic closure. Furthermore, as consciousness is lost during anesthetic induction, the activity of the upper airway dilator muscles is attenuated, rendering the pharynx vulnerable to collapse during inspiration.97,98 The tendency of an airway to collapse is compounded in the presence of negative intraluminal pressures generated on inspiration within a narrowed airway.97 If airway collapse occurs during induction with spontaneous ventilation, it can be somewhat mitigated by head extension99 and use of a nasopharyngeal airway while the patient is still in a light plane of anesthesia.83
Checking for efficacy of face mask ventilation after induction, before administration of a neuromuscular blocking agent
Before administering a neuromuscular blocking agent (NMBA), confirmation that face mask ventilation is possible following the induction of general anesthesia has been advocated as a patient safety measure.100-102 The theoretical advantage of withholding NMBAs until after successful face mask ventilation has been demonstrated is that if significantly difficult face mask ventilation is encountered, the patient could be allowed to awaken and the airway subsequently secured awake.103 However, a review by Calder and Yentis revealed that this recommendation was not based on published evidence when it was first mentioned by Stone and Gal in the third edition of Miller’s Anesthesia.102,103 Furthermore, data from three prospective studies suggest that neuromuscular blockade improves or has no effect on face mask ventilation, but never worsens it.104-106 Once the decision is made to proceed with tracheal intubation after the induction of general anesthesia with ablation of spontaneous ventilation, no recommendation can be made for or against the practice of checking for efficacy of face mask ventilation prior to administration of a NMBA. This applies to patients with both anticipated easy and difficult tracheal intubation.
Use of a short- or intermediate-acting NMBA
No recommendation can be made on whether to use a short- (e.g., succinylcholine) or intermediate-acting NMBA to facilitate tracheal intubation when difficulty is anticipated. In a failed oxygenation “cannot intubate, cannot oxygenate” (CICO)107 situation, there is theoretical evidence that even succinylcholine may not wear off in time to prevent hypoxic brain injury by allowing resumption of spontaneous ventilation.108 In addition, an argument can be made that short-acting NMBAs may not provide sufficient time for a smooth transition to a “Plan B” alternative intubation technique before the return of reflex glottic closure in response to airway instrumentation. Even with rapid reversal of an intermediate-acting non-depolarizing NMBA (e.g., reversal of rocuronium’s effects using sugammadex) in a failed oxygenation/CICO situation, case reports suggest that timely resumption of adequate spontaneous ventilation may not be guaranteed.109,110 With no assurance of a sufficiently early resumption of spontaneous ventilation with either short-acting NMBAs or rapid-reversal agents, the emphasis should not lie with the type of NMBA to use when difficulty is anticipated; rather, it should lie earlier in the decision process when deciding if awake intubation (or induction of general anesthesia with maintenance of spontaneous ventilation) will provide a greater margin of safety.
Cricoid pressure
The use of cricoid pressure remains controversial. Randomized controlled trials on its efficacy are lacking in patients at high risk of regurgitation111-113 and are unlikely to be forthcoming. Recently, investigators have identified that the esophagus is not completely obstructed by cricoid pressure114 and that the cricoid cartilage can collapse during the application of pressure, thus failing to compress the esophagus.115 The maneuver is often performed incorrectly116; it may attenuate lower esophageal sphincter tone,117 hinder face mask ventilation, interfere with placement of and ventilation through SGDs,118,119 and render laryngoscopy and tracheal intubation more difficult.120 Furthermore, there are reports that some anesthetists have seen regurgitation despite its application.121,122 Nevertheless, even if it results in incomplete esophageal occlusion, there is evidence that cricoid pressure still leads to compression of the post-cricoid hypopharynx, constituting at least some degree of physical barrier to the passive regurgitation of alimentary track contents.123 In addition, there are case reports and series of patients in whom significant regurgitation has occurred upon release of cricoid pressure after successful tracheal intubation.124,125
In the NAP4 study, aspiration was the most common cause of anesthesia-related mortality. Analysis of these cases suggests that there was a failure to employ a rapid sequence intubation technique when a significant risk of aspiration existed.126 As cricoid pressure is likely to have potential benefits,127 its continued use seems prudent during rapid sequence intubation in the patient at high risk of aspiration (Strong recommendation for, level of evidence C). However, if difficulty is encountered with face mask ventilation or tracheal intubation, or if SGD insertion is needed, progressive or complete release of cricoid pressure is justified.
Difficult tracheal intubation encountered in the unconscious patient
Difficulty with tracheal intubation will inevitably be encountered in some patients once unconscious. This may be expected, especially when post-induction intubation is elected in the patient with predictors of difficulty, or it may be unanticipated. Appropriate management is addressed in the first article of this two-part series.13
Obstructing airway pathology
The patient with significant obstructing airway pathology may be maintaining airway patency only with considerable effort. If time permits, consultation with the attending surgeon and review of recent imaging studies (e.g., CT scans) is advisable prior to airway management. Nasopharyngoscopy may provide useful current information about the extent, location, and nature of obstructing or distorting pathology in the pharynx and larynx.128 Such an examination may help identify patients in whom an awake technique is appropriate. Awake bronchoscopic intubation may be feasible for oral cavity and pharyngeal pathology, although effective topical airway anesthesia may be difficult to achieve, friable tumours may bleed easily, anatomic landmarks may be obscured by edematous tissues, and bronchoscope manipulation around obstructing lesions can be challenging. Many such patients will have received radiation therapy to the upper airway or neck, rendering tissues friable or less compliant. Bulky lesions of the larynx may accommodate passage of a bronchoscope, although complete airway obstruction by the bronchoscope or the combination of the bronchoscope and tracheal tube may occur. Thus, awake tracheotomy or cricothyrotomy should be strongly considered as a primary technique for significant obstructing airway pathology.
Management of mid- or lower tracheal obstruction remains controversial.96,129 Rigid bronchoscopy and a skilled operator should be immediately available in case tracheal intubation fails to establish oxygenation.83,129 Cricothyrotomy or tracheotomy cannot be relied on to rescue a more distal airway obstruction.
Inhalational induction with obstructing airway pathology
Inhalational induction has been used successfully in the setting of obstructing airway pathology. Nevertheless, apneic spells, hypoxemia, and hypercarbia can occur with this approach.96 Episodes of complete airway obstruction can also occur, following which the patient may not rapidly awaken as hypoxemia worsens.76 The use of inhalational induction in this context is controversial, with limited supporting evidence and varying expert opinion. Although the number of occasions during the study period in which the technique was used successfully is not known, the NAP4 data reveal serious episodes of failure.76 If awake bronchoscopic intubation or awake tracheotomy is not considered feasible in the presence of predicted difficult tracheal intubation due to obstructing airway pathology, a weak recommendation can be made for the cautious use of inhalational induction (Weak recommendation for, level of evidence C). Nevertheless, if complete obstruction occurs when using inhalational induction in this setting, an exit strategy other than awakening the patient must be in place to rescue the airway.76
The “double setup airway intervention”
A “double setup airway intervention” refers to the immediate availability of equipment and personnel capable of performing a surgical airway in the event that oxygenation fails for any reason during attempted tracheal intubation. Elements of the double setup include identification and marking of the cricothyroid membrane location, (sometimes with application of disinfectant solution to the neck and infiltration of local anesthetic into the overlying skin), ensuring cricothyrotomy equipment is in the room, and designation of an appropriately skilled individual to perform the procedure. In experienced hands, ultrasound may be helpful to identify the cricothyroid membrane, but there is no evidence to support its use in an emergency.
It should be emphasized that rapid cricothyrotomy is unlikely to succeed and cannot be regarded a prudent rescue option if access to the cricothyroid membrane is likely to be very difficult (e.g., in a patient with a very thick neck, previous neck radiation, or overlying tumour or inflammation). This situation may mandate awake tracheotomy under local anesthesia as the preferred primary technique, performed by a surgeon under controlled conditions.
A double setup airway intervention should be prepared whenever the clinician considers a significant possibility of encountering a failed oxygenation situation during attempted awake or post-induction airway management (Strong recommendation for, level of evidence C).
The morbidly obese patient
NAP4 reported a fourfold increase in major airway events in the morbidly obese population.130 Variously defined as a body mass index (BMI) > 35 or 40 kg·m−2, morbid obesity can portend difficulty with most aspects of airway management. Even below this level, a BMI > 26 or 30 kg·m−2 is an independent predictor of difficult face mask ventilation.40,42-44 Other conditions frequently accompanying morbid obesity, such as a thick neck, history of snoring or obstructive sleep apnea, are similarly associated with difficult face mask ventilation.40-44 Studies are contradictory on whether morbid obesity or its coexisting anatomic or pathophysiologic features are predictive of difficult direct laryngoscopy; although again, a thick neck does appear to portend difficulty.35,131-137 Appropriate positioning with “ramping” of the patient to align the external auditory meatus horizontally with the sternum will aid direct laryngoscopy.87,138,139 Increased BMI is a predictor of SGD failure,53 and landmark identification and execution can be challenging for cricothyrotomy (e.g., with a thick neck, standard tracheotomy or cricothyrotomy cannulae may fail to reach the trachea).130 Additionally, physiologic factors, such as rapid oxygen desaturation and increased risk of aspiration, must be considered. Thus, an especially careful airway evaluation is warranted in the morbidly obese patient. When difficult laryngoscopy or intubation is anticipated, given the potential for difficulty with fallback oxygenation options and the potentially short safe apnea time, an awake approach may be safest. Management of the severely obese patient has recently been reviewed in more detail elsewhere.140
Tracheal extubation in the patient with a difficult airway
Numerous reports emphasize the risks associated with extubation and subsequent loss of the airway.11,141-143 Such events account for a significant proportion of adverse respiratory outcomes and are sometimes catastrophic. While there has been a decrease in adverse respiratory events associated with tracheal intubation, the same has not been observed for extubation.74 Many of these outcomes can be avoided with proper planning and recognition of risk.144-147 Patients are at particular risk during emergence from anesthesia, relocation to a recovery area, and discontinuation of full monitoring. In the recovery area, recognition and correction of a deteriorating airway can potentially be delayed. Recovering patients may be under the influence of medications that depress their respiratory drive, reduce muscular power, and diminish their protective reflexes. Critically ill patients are at further risk because of limited physiologic reserves.
In contrast to tracheal intubation, extubation is almost always elective, and therefore careful planning is possible. This should include identification of patients at risk of failed tracheal extubation, and those with anatomic features that place them at higher risk of difficult re-intubation should this prove necessary.146,147 Examples include but are not limited to patients with a reduced functional residual capacity, increased work of breathing, reduced minute ventilation, increased dead space, swelling in or around the airway, a previously difficult airway, or an airway where accessibility is challenged.
Planning for extubation begins with ensuring optimal conditions, including adequate oxygenation and minute ventilation and intact protective reflexes, and excluding probable causes of airway obstruction. The patient should be hemodynamically stable and normothermic. Recovery from any administered neuromuscular blocking agents should be confirmed with a nerve stimulator, and reversal agents should be given when indicated. Tracheal extubation of at-risk patients requires expert judgement to ensure that appropriate circumstances and resources are in place to provide continuous post-extubation oxygenation. Premature extubation during emergence is more likely to be associated with complications such as breath-holding, aspiration, laryngospasm, and hypoxemia.
If tracheal intubation had been very difficult or circumstances now suggest that it would be so, short-term maintenance of tracheal access using an airway exchange catheter148 is recommended (Strong recommendation for, level of evidence C). Airway exchange catheters can also be used to exchange defective or inappropriate tracheal tubes. When used to retain tracheal access after extubation, the airway exchange catheter should not be removed prematurely, as re-intubation of an at-risk airway is much more likely to be associated with an adverse outcome after the device has been removed.144 When properly positioned above the carina and secured, smaller gauge (e.g., 11- or 14-French) airway exchange catheters are generally well tolerated and permit spontaneous ventilation, coughing, and talking. Generally, supplemental oxygen should be applied by face mask or nasal cannulae. Although the hollow lumen of airway exchange catheters can be used for oxygen insufflation149 and has been used for jet ventilation, fatal barotrauma has been reported with both modalities.150,151 When to remove an airway exchange catheter after extubation is the subject of much debate and should be individualized to the patient’s respiratory reserve, potential for difficult re-intubation, and anticipated clinical course. In the intensive care setting, the majority of patients requiring tracheal re-intubation undergo the procedure within two to ten hours after extubation.144
If tracheal re-intubation is required over an airway exchange catheter, success can be enhanced by using a laryngoscope to retract the tongue. Use of a video laryngoscope for this purpose holds the advantage of also allowing indirect visualization of tube passage and facilitating corrective maneuvers for any tube impingement on laryngeal structures.145 In addition, prior passage of an intermediate catheter (e.g., the Aintree catheter [Cook Medical, Bloomington, IN, USA]) over a smaller gauge airway exchange catheter may facilitate subsequent passage of the tracheal tube through the adult larynx by reducing the size discrepancy between the outer diameter of the catheter and the inner diameter of the tracheal tube.152
Summary of recommendations
Strong recommendation for, level of evidence B
-
1.
All patients with anticipated difficult tracheal intubation and planned post-induction intubation should be pre-oxygenated with 100% oxygen for three minutes of tidal volume breathing, eight vital capacity breaths over 60 sec, or until FEO2 exceeds 90%.
Strong recommendation for, level of evidence C
-
1.
A complete airway evaluation should be performed in every patient requiring airway management to assess for potential difficulty with tracheal intubation, face mask ventilation, SGD use, and surgical airway.
-
2.
When deciding if post-induction intubation can be safely undertaken, consideration must be given to face mask ventilation, SGD or surgical airway rescue, and other patient or contextual issues (e.g., safe apnea time, aspiration risk, availability of additional skilled help, presence of obstructing airway pathology, or clinician experience) as well as to anticipated success of tracheal intubation.
-
3.
Proceeding with post-induction tracheal intubation in the cooperative elective surgical patient with an anticipated difficult airway should only occur with an estimated margin of safety equivalent to that of an awake intubation.
-
4.
In most situations, significant predicted difficulty with both tracheal intubation and face mask or SGD ventilation should be taken as a strong signal to consider awake intubation, particularly in the cooperative elective surgical patient.
-
5.
Clinicians with responsibility for difficult airway management should be competent in performing awake tracheal intubation.
-
6.
Prior to proceeding with a post-induction tracheal intubation in the patient with known or suspected difficult intubation, the clinician should prepare equipment for both primary (“Plan A”) and alternative (“Plan B”) intubation approaches. In addition, an exit strategy for failed intubation should be clear in the clinician’s mind.
-
7.
As cricoid pressure does have potential benefits and the consequences of aspiration are significant, its use is recommended during rapid sequence intubation in the patient at high risk of aspiration.
-
8.
During attempted airway management by awake or post-induction approaches, whenever the clinician suspects a significant possibility of encountering a failed oxygenation “cannot intubate, cannot oxygenate” situation, a “double setup airway intervention” should be prepared.
-
9.
If tracheal intubation had been very difficult or circumstances now suggest it would be difficult, short-term maintenance of tracheal access using an airway exchange catheter is recommended upon extubation.
Weak recommendation for, level of evidence C
-
1.
Cautious use of inhalational induction can be considered in the presence of a difficult airway or obstructing airway pathology if awake options for tracheal intubation are impractical.
References
Murphy M, Hung O, Launcelott G, Law JA, Morris I. Predicting the difficult laryngoscopic intubation: are we on the right track? Can J Anesth 2005; 52: 231-5.
Rosenblatt WH, Wagner PJ, Ovassapian A, Kain ZN. Practice patterns in managing the difficult airway by anesthesiologists in the United States. Anesth Analg 1998; 87: 153-7.
Jenkins K, Wong DT, Correa R. Management choices for the difficult airway by anesthesiologists in Canada. Can J Anesth 2002; 49: 850-6.
Zugai BM, Eley V, Mallitt KA, Greenland KB. Practice patterns for predicted difficult airway management and access to airway equipment by anaesthetists in Queensland. Australia. Anaesth Intensive Care 2010; 38: 27-32.
Crosby ET, Cooper RM, Douglas MJ, et al. The unanticipated difficult airway with recommendations for management. Can J Anaesth 1998; 45: 757-76.
Henderson JJ, Popat MT, Latto IP, Pearce AC; Difficult Airway Society. Difficult Airway Society guidelines for management of the unanticipated difficult intubation. Anaesthesia 2004; 59: 675-94.
Apfelbaum JL, Hagberg CA, Caplan RA, et al. Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology 2013; 118: 251-70.
Petrini F, Accorsi A, Adrario E, et al. Recommendations for airway control and difficult airway management. Minerva Anestesiol 2005; 71: 617-57.
Boisson-Bertrand D, Bourgain JL, Camboulives J, et al. Difficult intubation. French Society of Anesthesia and Intensive Care. A collective expertise (French). Ann Fr Anesth Reanim 1996; 15: 207-14.
Braun U, Goldmann K, Hempel V, Krier C. Airway management. Guidelines of the German Society of Anesthesiology and Intensive Care. Anaesth Intensivmed 2004; 45: 302-06.
Cook T, Woodall N, Frerk C. 4th National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Major Complications of Airway Management in the United Kingdom. London: The Royal College of Anaesthetists; 2011 .
Shiga T, Wajima Z, Inoue T, Sakamoto A. Predicting difficult intubation in apparently normal patients: a meta-analysis of bedside screening test performance. Anesthesiology 2005; 103: 429-37.
Law JA, Broemling N, Cooper RM, et al.; for the Canadian Airway Focus Group. The difficult airway with recommendations for management – Part 1 – Difficult tracheal intubation encountered in an unconscious/induced patient. Can J Anesth 2013; 60: this issue. DOI:10.1007/s12630-013-0019-3.
Guyatt G, Gutterman D, Baumann MH, et al. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an American College of Chest Physicians Task Force. Chest 2006; 129: 174-81.
Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336: 924-6.
Croskerry P. Cognitive forcing strategies in clinical decisionmaking. Ann Emerg Med 2003; 41: 110-20.
Rocke DA, Murray WB, Rout CC, Gouws E. Relative risk analysis of factors associated with difficult intubation in obstetric anesthesia. Anesthesiology 1992; 77: 67-73.
Frerk CM. Predicting difficult intubation. Anaesthesia 1991; 46: 1005-8.
Hung O, Murphy M. Context-sensitive airway management. Anesth Analg 2010; 110: 982-3.
Khan ZH, Kashfi A, Ebrahimkhani E. A comparison of the upper lip bite test (a simple new technique) with modified Mallampati classification in predicting difficulty in endotracheal intubation: a prospective blinded study. Anesth Analg 2003; 96: 595-9.
Eberhart LH, Arndt C, Cierpka T, Schwanekamp J, Wulf H, Putzke C. The reliability and validity of the upper lip bite test compared with the Mallampati classification to predict difficult laryngoscopy: an external prospective evaluation. Anesth Analg 2005; 101: 284-9.
Eberhart LH, Arndt C, Aust HJ, Kranke P, Zoremba M, Morin A. A simplified risk score to predict difficult intubation: development and prospective evaluation in 3763 patients. Eur J Anaesthesiol 2010; 27: 935-40.
Savva D. Prediction of difficult tracheal intubation. Br J Anaesth 1994; 73: 149-53.
Mallampati SR, Gatt SP, Gugino LD, et al. A clinical sign to predict difficult tracheal intubation: a prospective study. Can Anaesth Soc J 1985; 32: 429-34.
Samsoon GL, Young JR. Difficult tracheal intubation: a retrospective study. Anaesthesia 1987; 42: 487-90.
Karkouti K, Rose K, Cohen M, Wigglesworth D. Models for difficult laryngoscopy. Can J Anaesth 2000; 47: 94-5.
el-Ganzouri AR, McCarthy RJ, Tuman KJ, Tanck EN, Ivankovich AD. Preoperative airway assessment: predictive value of a multivariate risk index. Anesth Analg 1996; 82: 1197-204.
Tse JC, Rimm EB, Hussain A. Predicting difficult endotracheal intubation in surgical patients scheduled for general anesthesia: a prospective blind study. Anesth Analg 1995; 81: 254-8.
Orozco-Diaz E, Alvarez-Rios JJ, Arceo-Diaz JL, Ornelas-Aguirre JM. Predictive factors of difficult airway with known assessment scales. Cir Cir 2010; 78: 393-9.
Arne J, Descoins P, Fusciardi J, et al. Preoperative assessment for difficult intubation in general and ENT surgery: predictive value of a clinical multivariate risk index. Br J Anaesth 1998; 80: 140-6.
Saghaei M, Safavi MR. Prediction of prolonged laryngoscopy. Anaesthesia 2001; 56: 1198-201.
Rose DK, Cohen MM. The airway: problems and predictions in 18,500 patients. Can J Anaesth 1994; 41(5 Pt 1): 372-83.
Reed MJ, Dunn MJ, McKeown DW. Can an airway assessment score predict difficulty at intubation in the emergency department? Emerg Med J 2005; 22: 99-102.
Wilson ME, Spiegelhalter D, Robertson JA, Lesser P. Predicting difficult intubation. Br J Anaesth 1988; 61: 211-6.
Brodsky JB, Lemmens HJ, Brock-Utne JG, Vierra M, Saidman LJ. Morbid obesity and tracheal intubation. Anesth Analg 2002; 94: 732-6.
Tremblay MH, Williams S, Robitaille A, Drolet P. Poor visualization during direct laryngoscopy and high upper lip bite test score are predictors of difficult intubation with the GlideScope videolaryngoscope. Anesth Analg 2008; 106: 1495-500.
Aziz MF, Healy D, Kheterpal S, Fu RF, Dillman D, Brambrink AM. Routine clinical practice effectiveness of the Glidescope in difficult airway management: an analysis of 2,004 Glidescope intubations, complications, and failures from two institutions. Anesthesiology 2011; 114: 34-41.
Hung OR, Pytka S, Morris I, et al. Clinical trial of a new lightwand device (Trachlight) to intubate the trachea. Anesthesiology 1995; 83: 509-14.
Hung OR, Pytka S, Morris I, Murphy M, Stewart RD. Lightwand intubation: II—Clinical trial of a new lightwand for tracheal intubation in patients with difficult airways. Can J Anaesth 1995; 42: 826-30.
Kheterpal S, Han R, Tremper KK, et al. Incidence and predictors of difficult and impossible mask ventilation. Anesthesiology 2006; 105: 885-91.
Kheterpal S, Martin L, Shanks AM, Tremper KK. Prediction and outcomes of impossible mask ventilation: a review of 50,000 anesthetics. Anesthesiology 2009; 110: 891-7.
Langeron O, Masso E, Huraux C, et al. Prediction of difficult mask ventilation. Anesthesiology 2000; 92: 1229-36.
Yildiz TS, Solak M, Toker K. The incidence and risk factors of difficult mask ventilation. J Anesth 2005; 19: 7-11.
Gautam P, Gaul TK, Luthra N. Prediction of difficult mask ventilation. Eur J Anaesthesiol 2005; 22: 638-40.
Langeron O, Semjen F, Bourgain JL, Marsac A, Cros AM. Comparison of the intubating laryngeal mask airway with the fiberoptic intubation in anticipated difficult airway management. Anesthesiology 2001; 94: 968-72.
Giraud O, Bourgain JL, Marandas P, Billard V. Limits of laryngeal mask airway in patients after cervical or oral radiotherapy. Can J Anaesth 1997; 44: 1237-41.
Salvi L, Juliano G, Zucchetti M, Sisillo E. Hypertrophy of the lingual tonsil and difficulty in airway control. A clinical case (Italian). Minerva Anestesiol 1999; 65: 549-53.
Asai T, Hirose T, Shingu K. Failed tracheal intubation using a laryngoscope and intubating laryngeal mask. Can J Anesth 2000; 47: 325-8.
Ishimura H, Minami K, Sata T, Shigematsu A, Kadoya T. Impossible insertion of the laryngeal mask airway and oropharyngeal axes. Anesthesiology 1995; 83: 867-9.
Kumar R, Prashast , Wadhwa A, Akhtar S. The upside-down intubating laryngeal mask airway: a technique for cases of fixed flexed neck deformity. Anesth Analg 2002; 95: 1454-8.
Brimacombe JR. Laryngeal Mask Anesthesia: Principles and Practice. 2nd ed. Philadelphia: Saunders; 2005 .
Li CW, Xue FS, Xu YC, et al. Cricoid pressure impedes insertion of, and ventilation through, the ProSeal laryngeal mask airway in anesthetized, paralyzed patients. Anesth Analg 2007; 104: 1195-8.
Ramachandran SK, Mathis MR, Tremper KK, Shanks AM, Kheterpal S. Predictors and clinical outcomes from failed Laryngeal Mask Airway Unique™: a study of 15,795 patients. Anesthesiology 2012; 116: 1217-26.
Aslani A, Ng SC, Hurley M, McCarthy KF, McNicholas M, McCaul CL. Accuracy of identification of the cricothyroid membrane in female subjects using palpation: an observational study. Anesth Analg 2012; 114: 987-92.
Elliott DS, Baker PA, Scott MR, Birch CW, Thompson JM. Accuracy of surface landmark identification for cannula cricothyroidotomy. Anaesthesia 2010; 65: 889-94.
Joo HS, Kapoor S, Rose DK, Naik VN. The intubating laryngeal mask airway after induction of general anesthesia versus awake fiberoptic intubation in patients with difficult airways. Anesth Analg 2001; 92: 1342-6.
Ferson DZ, Rosenblatt WH, Johansen MJ, Osborn I, Ovassapian A. Use of the intubating LMA-Fastrach in 254 patients with difficult-to-manage airways. Anesthesiology 2001; 95: 1175-81.
Timmermann A, Russo SG, Rosenblatt WH, et al. Intubating laryngeal mask airway for difficult out-of-hospital airway management: a prospective evaluation. Br J Anaesth 2007; 99: 286-91.
Cros AM, Maigrot F, Esteben D. Fastrach laryngeal mask and difficult intubation (French). Ann Fr Anesth Reanim 1999; 18: 1041-6.
Parmet JL, Colonna-Romano P, Horrow JC, Miller F, Gonzales J, Rosenberg H. The laryngeal mask airway reliably provides rescue ventilation in cases of unanticipated difficult tracheal intubation along with difficult mask ventilation. Anesth Analg 1998; 87: 661-5.
Cook T. Supraglottic airway devices. In: Cook T, Woodall N, Frerk C, editors. 4th National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society Major Complications of Airway Management in the United Kingdom. London: The Royal College of Anaesthetists; 2011. p. 86-95.
Ricard JD. High flow nasal oxygen in acute respiratory failure. Minerva Anestesiol 2012; 78: 836-41.
Ward JJ. High-flow oxygen administration by nasal cannula for adult and perinatal patients. Respir Care 2013; 58: 98-122.
Williams TA, Finn J, Perkins GD, Jacobs IG. Prehospital continuous positive airway pressure for acute respiratory failure: a systematic review and meta-analysis. Prehosp Emerg Care 2013; 17: 261-73.
Lenglet H, Sztrymf B, Leroy C, Brun P, Dreyfuss D, Ricard JD. Humidified high flow nasal oxygen during respiratory failure in the emergency department: feasibility and efficacy. Respir Care 2012; 57: 1873-8.
Zhou YF, Zhu SJ, Zhu SM, An XX. Anesthetic management of emergent critical tracheal stenosis. J Zhejiang Univ Sci B 2007; 8: 522-5.
Jeon HK, So YK, Yang JH, Jeong HS. Extracorporeal oxygenation support for curative surgery in a patient with papillary thyroid carcinoma invading the trachea. J Laryngol Otol 2009; 123: 807-10.
Tyagi I, Goyal A, Syal R, Agarwal SK, Tewari P. Emergency cardiopulmonary bypass for impassable airway. J Laryngol Otol 2006; 120: 687-90.
Sendasgupta C, Sengupta G, Ghosh K, Munshi A, Goswami A. Femoro-femoral cardiopulmonary bypass for the resection of an anterior mediastinal mass. Indian J Anaesth 2010; 54: 565-8.
Belmont MJ, Wax MK, DeSouza FN. The difficult airway: cardiopulmonary bypass—the ultimate solution. Head Neck 1998; 20: 266-9.
McGuire G, el-Beheiry H. Complete upper airway obstruction during awake fibreoptic intubation in patients with unstable cervical spine fractures. Can J Anesth 1999; 46: 176-8.
Ho AM, Chung DC, To EW, Karmakar MK. Total airway obstruction during local anesthesia in a non-sedated patient with a compromised airway. Can J Anesth 2004; 51: 838-41.
Martin R, Girouard Y, Cote DJ. Use of a laryngeal mask in acute airway obstruction after carotid endarterectomy (letter). Can J Anesth 2002; 49: 890.
Peterson GN, Domino KB, Caplan RA, Posner KL, Lee LA, Cheney FW. Management of the difficult airway: a closed claims analysis. Anesthesiology 2005; 103: 33-9.
Bogod D, Popat M. Tracheal intubation. In: Cook T, Woodall N, Frerk C (Eds). Fourth National Audit Project of the Royal College of Anaesthetists and The Difficult Airway Society. Major Complications of Airway Management in the United Kingdom. London: The Royal College of Anaesthetists; 2011: 96-104.
Patel A, Pearce A, Pracy P. Head and neck pathology. In: Cook T, Woodall N, Frerk C (Eds). 4th National Audit Project of the Royal College of Anaesthetists and The Difficult Airway Society. Major Complications of Airway Management in the United Kingdom. London: The Royal College of Anaesthetists; 2011: 143-54.
Griesdale DE, Bosma TL, Kurth T, Isac G, Chittock DR. Complications of endotracheal intubation in the critically ill. Intensive Care Med 2008; 34: 1835-42.
Martin LD, Mhyre JM, Shanks AM, Tremper KK, Kheterpal S. 3,423 emergency tracheal intubations at a university hospital: airway outcomes and complications. Anesthesiology 2011; 114: 42-8.
Mort TC. Emergency tracheal intubation: complications associated with repeated laryngoscopic attempts. Anesth Analg 2004; 99: 607-13.
Hasegawa K, Shigemitsu K, Hagiwara Y, et al. Association between repeated intubation attempts and adverse events in emergency departments: an analysis of a multicenter prospective observational study. Ann Emerg Med 2012; 60: 749-54.e2.
Sakles JC, Chiu S, Mosier J, Walker C, Stolz U. The importance of first pass success when performing orotracheal intubation in the emergency department. Acad Emerg Med 2013; 20: 71-8.
Shaw IC, Welchew EA, Harrison BJ, Michael S. Complete airway obstruction during awake fibreoptic intubation. Anaesthesia 1997; 52: 582-5.
Mason RA, Fielder CP. The obstructed airway in head and neck surgery. Anaesthesia 1999; 54: 625-8.
Liistro G, Stanescu DC, Veriter C, Rodenstein DO, D’Odemont JP. Upper airway anesthesia induces airflow limitation in awake humans. Am Rev Respir Dis 1992; 146: 581-5.
Pandit JJ, Duncan T, Robbins PA. Total oxygen uptake with two maximal breathing techniques and the tidal volume breathing technique: a physiologic study of preoxygenation. Anesthesiology 2003; 99: 841-6.
Tanoubi I, Drolet P, Donati F. Optimizing preoxygenation in adults. Can J Anesth 2009; 56: 449-66.
Boyce JR, Ness T, Castroman P, Gleysteen JJ. A preliminary study of the optimal anesthesia positioning for the morbidly obese patient. Obes Surg 2003; 13: 4-9.
Dixon BJ, Dixon JB, Carden JR, et al. Preoxygenation is more effective in the 25 degrees head-up position than in the supine position in severely obese patients: a randomized controlled study. Anesthesiology 2005; 102: 1110-5.
Altermatt FR, Munoz HR, Delfino AE, Cortinez LI. Pre-oxygenation in the obese patient: effects of position on tolerance to apnoea. Br J Anaesth 2005; 95: 706-9.
Ramkumar V, Umesh G, Philip FA. Preoxygenation with 20 degrees head-up tilt provides longer duration of non-hypoxic apnea than conventional preoxygenation in non-obese healthy adults. J Anesth 2011; 25: 189-94.
Lane S, Saunders D, Schofield A, Padmanabhan R, Hildreth A, Laws D. A prospective, randomised controlled trial comparing the efficacy of pre-oxygenation in the 20 degrees head-up vs supine position. Anaesthesia 2005; 60: 1064-7.
Weingart SD, Levitan RM. Preoxygenation and prevention of desaturation during emergency airway management. Ann Emerg Med 2012; 59: 165-75.e1.
Baraka AS, Taha SK, Siddik-Sayyid SM, et al. Supplementation of pre-oxygenation in morbidly obese patients using nasopharyngeal oxygen insufflation. Anaesthesia 2007; 62: 769-73.
Taha SK, Siddik-Sayyid SM, El-Khatib MF, Dagher CM, Hakki MA, Baraka AS. Nasopharyngeal oxygen insufflation following pre-oxygenation using the four deep breath technique. Anaesthesia 2006; 61: 427-30.
Ramachandran SK, Cosnowski A, Shanks A, Turner CR. Apneic oxygenation during prolonged laryngoscopy in obese patients: a randomized, controlled trial of nasal oxygen administration. J Clin Anesth 2010; 22: 164-8.
Patel A, Pearce A. Progress in management of the obstructed airway. Anaesthesia 2011; 66(Suppl 2): 93-100.
Hillman DR, Platt PR, Eastwood PR. The upper airway during anaesthesia. Br J Anaesth 2003; 91: 31-9.
Hillman DR, Walsh JH, Maddison KJ, et al. Evolution of changes in upper airway collapsibility during slow induction of anesthesia with propofol. Anesthesiology 2009; 111: 63-71.
Walsh JH, Maddison KJ, Platt PR, Hillman DR, Eastwood PR. Influence of head extension, flexion, and rotation on collapsibility of the passive upper airway. Sleep 2008; 31: 1440-7.
Xue FS, Liao X, Wang Q, Yuan YJ, Xiong J, Liu JH. Is it unnecessary to confirm successful facemask ventilation before administration of a neuromuscular blocking agent? Anaesthesia 2011; 66: 519-20; author reply 520.
Pandit JJ. Checking the ability to mask ventilate before administering long-acting neuromuscular blocking drugs. Anaesthesia 2011; 66: 520-2; author reply 523-4.
Stone DJ, Gal TJ. Airway management. In: Miller RD, editor. Anesthesia. 3rd ed. New York: Churchill Livingstone; 1990. p. 1265-92.
Calder I, Yentis SM. Could ‘safe practice’ be compromising safe practice? Should anaesthetists have to demonstrate that face mask ventilation is possible before giving a neuromuscular blocker? Anaesthesia 2008; 63: 113-5.
Amathieu R, Combes X, Abdi W, et al. An algorithm for difficult airway management, modified for modern optical devices (Airtraq laryngoscope; LMA CTrach™): a 2-year prospective validation in patients for elective abdominal, gynecologic, and thyroid surgery. Anesthesiology 2011; 114: 25-33.
Goodwin MW, Pandit JJ, Hames K, Popat M, Yentis SM. The effect of neuromuscular blockade on the efficiency of mask ventilation of the lungs. Anaesthesia 2003; 58: 60-3.
Warters RD, Szabo TA, Spinale FG, DeSantis SM, Reves JG. The effect of neuromuscular blockade on mask ventilation. Anaesthesia 2011; 66: 163-7.
Walls RM. The emergency airway algorithms. In: Walls RM, Murphy MF, Luten RC, Schneider RE, editors. Manual of Emergency Airway Management. Philadelphia: Lippincott Williams & Wilkins; 2004. p. 8-21.
Benumof JL, Dagg R, Benumof R. Critical hemoglobin desaturation will occur before return to an unparalyzed state following 1 mg/kg intravenous succinylcholine. Anesthesiology 1997; 87: 979-82.
Kyle BC, Gaylard D, Riley RH. A persistent ‘can’t intubate, can’t oxygenate’ crisis despite rocuronium reversal with sugammadex. Anaesth Intensive Care 2012; 40: 344-6.
Curtis R, Lomax S, Patel B. Use of sugammadex in a ‘can’t intubate, can’t ventilate’ situation. Br J Anaesth 2012; 108: 612-4.
Ellis DY, Harris T, Zideman D. Cricoid pressure in emergency department rapid sequence tracheal intubations: a risk-benefit analysis. Ann Emerg Med 2007; 50: 653-65.
Brimacombe JR, Berry AM. Cricoid pressure. Can J Anaesth 1997; 44: 414-25.
Neilipovitz DT, Crosby ET. No evidence for decreased incidence of aspiration after rapid sequence induction. Can J Anesth 2007; 54: 748-64.
Smith KJ, Dobranowski J, Yip G, Dauphin A, Choi PT. Cricoid pressure displaces the esophagus: an observational study using magnetic resonance imaging. Anesthesiology 2003; 99: 60-4.
Palmer JH, Ball DR. The effect of cricoid pressure on the cricoid cartilage and vocal cords: an endoscopic study in anaesthetised patients. Anaesthesia 2000; 55: 263-8.
Clark RK, Trethewy CE. Assessment of cricoid pressure application by emergency department staff. Emerg Med Australas 2005; 17: 376-81.
Garrard A, Campbell AE, Turley A, Hall JE. The effect of mechanically-induced cricoid force on lower oesophageal sphincter pressure in anaesthetised patients. Anaesthesia 2004; 59: 435-9.
Aoyama K, Takenaka I, Sata T, Shigematsu A. Cricoid pressure impedes positioning and ventilation through the laryngeal mask airway. Can J Anaesth 1996; 43: 1035-40.
Asai T, Barclay K, McBeth C, Vaughan RS. Cricoid pressure applied after placement of the laryngeal mask prevents gastric insufflation but inhibits ventilation. Br J Anaesth 1996; 76: 772-6.
Hartsilver EL, Vanner RG. Airway obstruction with cricoid pressure. Anaesthesia 2000; 55: 208-11.
Howells TH, Chamney AR, Wraight WJ, Simons RS. The application of cricoid pressure. An assessment and a survey of its practice. Anaesthesia 1983; 38: 457-60.
Cook TM, Godfrey I, Rockett M, Vanner RG. Cricoid pressure: which hand? Anaesthesia 2000; 55: 648-53.
Rice MJ, Mancuso AA, Gibbs C, Morey TE, Gravenstein N, Deitte LA. Cricoid pressure results in compression of the postcricoid hypopharynx: the esophageal position is irrelevant. Anesth Analg 2009; 109: 1546-52.
Sellick BA. Cricoid pressure to control regurgitation of stomach contents during induction of anaesthesia. Lancet 1961; 2: 404-6.
Neelakanta G. Cricoid pressure is effective in preventing esophageal regurgitation. Anesthesiology 2003; 99: 242.
Cook T, Frerk C. Aspiration of gastric contents and blood. In: Cook T, Woodall N, Frerk C (Eds). 4th National Audit Project of the Royal College of Anaesthetists and The Difficult Airway Society. Major Complications of Airway Management in the United Kingdom. London: The Royal College of Anaesthetists 2011: 155-64.
Ovassapian A, Salem MR. Sellick’s maneuver: to do or not do. Anesth Analg 2009; 109: 1360-2.
Rosenblatt W, Ianus AI, Sukhupragarn W, Fickenscher A, Sasaki C. Preoperative endoscopic airway examination (PEAE) provides superior airway information and may reduce the use of unnecessary awake intubation. Anesth Analg 2011; 112: 602-7.
Cook TM, Morgan PJ, Hersch PE. Equal and opposite expert opinion. Airway obstruction caused by a retrosternal thyroid mass: management and prospective international expert opinion. Anaesthesia 2011; 66: 828-36.
Woodall N, Rangasami J. Obesity. In: Cook T, Woodall N, Frerk C (Eds). 4th National Audit Project of the Royal College of Anaesthetists and The Difficult Airway Society. Major Complications of Airway Management in the United Kingdom. London: The Royal College of Anaesthetists; 2011: 165-73.
Hekiert AM, Mick R, Mirza N. Prediction of difficult laryngoscopy: does obesity play a role? Ann Otol Rhinol Laryngol 2007; 116: 799-804.
Lundstrom LH, Moller AM, Rosenstock C, Astrup G, Wetterslev J. High body mass index is a weak predictor for difficult and failed tracheal intubation: a cohort study of 91,332 consecutive patients scheduled for direct laryngoscopy registered in the Danish Anesthesia Database. Anesthesiology 2009; 110: 266-74.
Gonzalez H, Minville V, Delanoue K, Mazerolles M, Concina D, Fourcade O. The importance of increased neck circumference to intubation difficulties in obese patients. Anesth Analg 2008; 106: 1132-6.
Juvin P, Lavaut E, Dupont H, et al. Difficult tracheal intubation is more common in obese than in lean patients. Anesth Analg 2003; 97: 595-600.
Mashour GA, Kheterpal S, Vanaharam V, et al. The extended Mallampati score and a diagnosis of diabetes mellitus are predictors of difficult laryngoscopy in the morbidly obese. Anesth Analg 2008; 107: 1919-23.
Ezri T, Medalion B, Weisenberg M, et al. Increased body mass index per se is not a predictor of difficult laryngoscopy. Can J Anesth 2003; 50: 179-83.
Neligan PJ, Porter S, Max B, Malhotra G, Greenblatt EP, Ochroch EA. Obstructive sleep apnea is not a risk factor for difficult intubation in morbidly obese patients. Anesth Analg 2009; 109: 1182-6.
Rao SL, Kunselman AR, Schuler HG, DesHarnais S. Laryngoscopy and tracheal intubation in the head-elevated position in obese patients: a randomized, controlled, equivalence trial. Anesth Analg 2008; 107: 1912-8.
Collins JS, Lemmens HJ, Brodsky JB, Brock-Utne JG, Levitan RM. Laryngoscopy and morbid obesity: a comparison of the “sniff” and “ramped” positions. Obes Surg 2004; 14: 1171-5.
Cullen A, Ferguson A. Perioperative management of the severely obese patient: a selective pathophysiological review. Can J Anesth 2012; 59: 974-96.
Rose DK, Cohen MM, Wigglesworth DF, DeBoer DP. Critical respiratory events in the postanesthesia care unit. Patient, surgical, and anesthetic factors. Anesthesiology 1994; 81: 410-8.
Cheney FW, Posner KL, Lee LA, Caplan RA, Domino KB. Trends in anesthesia-related death and brain damage: a closed claims analysis. Anesthesiology 2006; 105: 1081-6.
Auroy Y, Benhamou D, Pequignot F, Bovet M, Jougla E, Lienhart A. Mortality related to anaesthesia in France: analysis of deaths related to airway complications. Anaesthesia 2009; 64: 366-70.
Mort TC. Continuous airway access for the difficult extubation: the efficacy of the airway exchange catheter. Anesth Analg 2007; 105: 1357-62.
Mort TC. Tracheal tube exchange: feasibility of continuous glottic viewing with advanced laryngoscopy assistance. Anesth Analg 2009; 108: 1228-31.
Difficult Airway Society Extubation Guidelines Group; Popat M, Mitchell V, Dravid R, et al. Difficult Airway Society Guidelines for the management of tracheal extubation. Anaesthesia 2012; 67: 318-40.
Cooper RM, Khan SM. Extubation and reintubation of the difficult airway. In: Hagberg CA, editor. Benumof and Hagberg’s Airway Management. 3rd ed. Philadelphia: Elsevier-Saunders; 2012. p. 1018-46.
McDonnell NJ, Paech MJ, Clavisi OM, Scott KL, ANZCA Trials Group. Difficult and failed intubation in obstetric anaesthesia: an observational study of airway management and complications associated with general anaesthesia for caesarean section. Int J Obstet Anesth 2008; 17: 292-7.
Cooper RM. The use of an endotracheal ventilation catheter in the management of difficult extubations. Can J Anaesth 1996; 43: 90-3.
Cooper RM, Cohen DR. The use of an endotracheal ventilation catheter for jet ventilation during a difficult intubation. Can J Anaesth 1994; 41: 1196-9.
Duggan LV, Law JA, Murphy MF. Brief review: Supplementing oxygen through an airway exchange catheter: efficacy, complications, and recommendations. Can J Anesth 2011; 58: 560-8.
Higgs A, Swampillai C, Dravid R, et al. Re-intubation over airway exchange catheters – mind the gap (letter). Anaesthesia 2010; 65: 859-60.
Acknowledgments
Supported in part by the Department of Anesthesia, Dalhousie University.
Conflicts of interest
Dr. J. Adam Law is co-director of and royalty recipient from the Airway Interventions and Management in Emergencies (AIME) course and a recipient of equipment (as loan or gift) from Ambu A/S.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
(Please see Appendix for all authors’ affiliations, attributions, and disclosures).
This article is accompanied by an editorial. Please see Can J Anesth 2013; 60: this issue.
Appendix: Authorship Affiliations, Attribution, and Disclosures
Appendix: Authorship Affiliations, Attribution, and Disclosures
Author | Affiliation |
|---|---|
J. Adam Law, MD | Department of Anesthesia, Pain Management and Perioperative Medicine, Queen Elizabeth II Health Sciences Centre; Dalhousie University. 1796 Summer Street, Halifax, Nova Scotia B3H 3A7, Canada. E-mail: jlaw@dal.ca |
Natasha Broemling, MD | Department of Pediatric Anesthesia, BC Children’s Hospital; University of British Columbia |
Richard M. Cooper, MD | Department of Anesthesia and Pain Management, University Health Network, Toronto General Hospital Site; University of Toronto |
Pierre Drolet, MD | Département d’anesthésiologie, Hôpital Maisonneuve-Rosemont; Université de Montréal |
Laura V. Duggan, MD | Department of Anesthesiology, Pharmacology and Therapeutics, Royal Columbian Hospital; University of British Columbia |
Donald E. Griesdale, MD, MPH | a. Department of Anesthesia, Pharmacology and Therapeutics, University of British Columbia, Vancouver BC, Canada |
b. Department of Medicine, Division of Critical Care Medicine, University of British Columbia, Vancouver BC, Canada | |
c. Centre for Clinical Epidemiology and Evaluation, Vancouver Coastal Health Research Institute, Vancouver BC, Canada | |
Orlando R. Hung, MD | Department of Anesthesia, Pain Management and Perioperative Medicine, Queen Elizabeth II Health Sciences Centre; Dalhousie University. |
Philip M. Jones, MD, MSc | Department of Anesthesia and Perioperative Medicine, University Hospital, London Health Sciences Centre; Western University |
George Kovacs, MD, MHPE | Department of Emergency Medicine, Queen Elizabeth II Health Sciences Centre; Dalhousie University. |
Simon Massey, MB BCh | Department of Anesthesiology, Pharmacology and Therapeutics, BC Women’s Hospital and Health Centre; University of British Columbia |
Roanne Preston, MD | Department of Anesthesiology, Pharmacology and Therapeutics; Faculty of Medicine; University of British Columbia |
Ian R. Morris, MD | Department of Anesthesia, Pain Management and Perioperative Medicine, Queen Elizabeth II Health Sciences Centre; Dalhousie University. |
Timothy Mullen, MD | Department of Anesthesia, Pain Management and Perioperative Medicine, Queen Elizabeth II Health Sciences Centre; Dalhousie University. |
Michael F. Murphy, MD | Department of Anesthesiology and Pain Medicine, Walter Mackenzie Health Sciences Centre; University of Alberta |
Viren N. Naik, MD, MEd | Department of Anesthesiology, The Ottawa Hospital; University of Ottawa |
Jeanette Scott, MB ChB, FANZCA | Department of Anesthesia and Pain Medicine, Middlemore Hospital, Auckland, New Zealand |
Shean Stacey, MD | Department of Anesthesia, Foothills Medical Centre; University of Calgary |
Timothy P. Turkstra, MD, MEng | Department of Anesthesia and Perioperative Medicine; Western University |
David T. Wong, MD | Department of Anesthesia, University Health Network, Toronto Western Hospital Site; University of Toronto |
Author | Attribution(s) | Disclosure(s) |
|---|---|---|
J. Adam Law, MD | Focus group chair; data acquisition, analysis, and interpretation; writing and critically revising article. | Work supported by the Department of Anesthesia, Dalhousie University. Co-director of and royalty recipient from Airway Interventions and Management in Emergencies (AIME) course. Recipient of equipment (as loan or gift) from Ambu A/S. |
Natasha Broemling, MD | Data acquisition, analysis, and interpretation; critically revising article. | None |
Richard M. Cooper, MD | Data acquisition, analysis, and interpretation; writing and critically revising article. | Unpaid consultant to Verathon. Recipient of equipment (as loan or gift) from Clarus, McGrath, Prodol, Verathon, Karl Storz. |
Pierre Drolet, MD | Data acquisition, analysis, and interpretation; critically revising article. | None |
Laura V. Duggan, MD | Data acquisition, analysis, and interpretation; writing and critically revising article. | None |
Donald E. Griesdale, MD, MPH | Data acquisition, analysis, and interpretation; critically revising article. | Funding sources: Institutional: Clinician Scientist Award from the Vancouver Coastal Health Research Institute Departmental: Vancouver Hospital Department of Anesthesia |
Orlando R. Hung, MD | Data acquisition, analysis, and interpretation; critically revising article. | Consultant to Covidien and King Systems |
Philip M. Jones, MD, MSc | Data acquisition, analysis, and interpretation; critically revising article. | None |
George Kovacs, MD, MHPE | Data acquisition, analysis, and interpretation; critically revising article. | Co-director of and royalty recipient from Airway Interventions and Management in Emergencies (AIME) course. |
Simon Massey, MB BCh | Data acquisition, analysis, and interpretation; critically revising article. | None |
Roanne Preston, MD | Data acquisition, analysis, and interpretation; critically revising article. | None |
Ian R. Morris, MD | Data acquisition, analysis, and interpretation; writing and critically revising article. | None |
Timothy Mullen, MD | Data acquisition, analysis, and interpretation; critically revising article. | None |
Michael F. Murphy, MD | Data acquisition, analysis, and interpretation; critically revising article. | Owner of Airway Management Education Center (the Difficult Airway Course™ Anesthesia and Emergency); and First Airway (The Difficult Airway Course: EMS™ and Fundamentals of Airway Management™) |
Viren N. Naik, MD, MEd | Data acquisition, analysis, and interpretation; writing and critically revising article. | Work supported by University of Ottawa Skills and Simulation Centre |
Jeanette Scott, MB ChB | Data acquisition, analysis, and interpretation; writing and critically revising article. | None |
Shean Stacey, MD | Data acquisition, analysis, and interpretation; critically revising article. | None |
Timothy P. Turkstra, MD, M. Eng | Data acquisition, analysis, and interpretation; writing and critically revising article. | None |
David T. Wong, MD | Data acquisition, analysis, and interpretation; critically revising article. | Supported in part by the Department of Anesthesia, Toronto Western Hospital, University of Toronto |
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Law, J.A., Broemling, N., Cooper, R.M. et al. The difficult airway with recommendations for management – Part 2 – The anticipated difficult airway. Can J Anesth/J Can Anesth 60, 1119–1138 (2013). https://doi.org/10.1007/s12630-013-0020-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-013-0020-x