Abstract
Introduction
Nonessential central venous catheters (CVCs) should be removed promptly to prevent adverse events. Little is known about effective strategies to achieve this goal. The present study evaluates the effectiveness of a quality improvement (QI) initiative to remove nonessential CVCs in the intensive care unit (ICU).
Methods
A prospective observational study was performed in two ICUs following a QI intervention that included a daily checklist, education, and reminders. During 28 consecutive days, all CVCs were identified and the presence of ongoing indications for CVC placement was recorded. The proportions of nonessential CVCs and CVC days were compared with pre-intervention proportions and between the participating units. Rates of central line-associated bloodstream infections (CLABSI) were measured separately through Ontario’s Critical Care Information System.
Results
One hundred and ten patients and 159 CVCs were reviewed. Eighty-eight (11%) of 820 catheter days showed no apparent indication for CVC placement, and compared with the pre-intervention period, the proportion of patients with any number of nonessential CVC days decreased from 51% to 26% (relative risk 0.51; 95% confidence interval 0.34 to 0.74; P < 0.001). There was no significant difference in the proportion of nonessential catheter days between participating units. Reported rates of CLABSI decreased substantially during the intervention.
Discussion
A checklist tool supported by a multifaceted QI intervention effectively ensured prompt removal of nonessential CVCs in two ICUs.
Résumé
Introduction
Les cathéters veineux centraux (CVC) non essentiels doivent être retirés rapidement afin de prévenir les effets indésirables. On ne sait que peu de choses sur les stratégies efficaces permettant d’atteindre cet objectif. La présente étude évalue l’efficacité d’une initiative d’amélioration de la qualité pour supprimer les CVC non essentiels dans une unité de soins intensifs (USI).
Méthode
Une étude observationnelle prospective a été menée dans deux USI suite à une intervention d’amélioration de la qualité; elle incluait une liste de vérification quotidienne, de l’éducation et des rappels. Pendant 28 jours consécutifs, tous les CVC ont été identifiés et l’existence d’indications en cours a été notée. Les pourcentages de CVC non essentiels et de jours avec CVC ont été comparés aux pourcentages avant l’intervention et entre les unités participantes. L’incidence d’infections hématogènes associées à des voies veineuses centrales a été mesurée séparément au travers du Système d’information sur les soins intensifs de l’Ontario.
Résultats
Cent dix patients et 159 CVC ont été analysés. Sur 820 jours avec cathéters, 88 (11 %) n’avaient aucune indication apparente. Comparativement à la période préintervention, la proportion de patients ayant un nombre de journées avec CVC non essentiels a diminué de 51 % à 26 % (risque relatif: 0.51; intervalle de confiance à 95 %: 0,34 à 0,74, P < 0.001). Il n’y a pas eu de différence significative entre les deux USI participant à l’étude quant aux pourcentages de jours avec cathéters non essentiels. L’incidence d’infections hématogènes signalées a diminué substantiellement au cours de l’intervention.
Discussion
Un outil sous forme de liste de contrôle, soutenu par une intervention d’amélioration de la qualité aux multiples facettes s’est avérée efficace pour un retrait rapide des CVC non essentiels dans deux USI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Central venous catheters (CVCs) are commonly utilized in the intensive care unit (ICU). Indications for CVC placement and usage include intravenous therapies (e.g., vasopressors and inotropes, total parenteral nutrition, and long-term antibiotics), monitoring (e.g., transduction of pressure and measurement of hemoglobin oxygen saturation in the central venous blood), hemodialysis, cardiac pacing, lack of (i.e., inability to obtain) peripheral venous access, and the need for frequent blood draws.1
Central venous catheters are associated with important adverse events, including central line-associated bloodstream infections (CLABSI), which substantially impact morbidity, mortality, and cost.2-5
Several evidence-based practices to eliminate CLABSI have been promoted; some are applicable to insertion of CVCs and others to CVC maintenance. As the risk for CLABSI increases with the CVC’s duration, one of the key preventative measures is the removal of a catheter as soon as it is no longer necessary.6,7
Establishing a reliable system for prevention of CLABSI is challenging and requires a system-based approach.7-9 Moreover, reliable compliance with infection prevention practices is even more challenging for CVC maintenance than for CVC insertion, which should be provided during a defined procedure. For example, aseptic access to the CVC needs to be ensured each time the catheter is accessed. Similarly, assessing whether the CVC is still essential (i.e., the catheter is used for therapeutic purposes) needs to occur on a daily basis.
Little is known about the fate of CVCs in the ICU after they have been inserted.10,11 Furthermore, the utility of currently promoted interventions to ensure prompt removal of nonessential CVCs (e.g., the use of checklists as a reminder) is not well known. In a previous observational study in the ICUs of Kingston General Hospital, Ontario, Canada, we discovered that 51% of the patients had at least one CVC day with no apparent indication for CVC placement.12
After discovering the high proportion of nonessential CVC days, a multifaceted quality improvement (QI) process was designed and introduced to our ICUs to ensure that nonessential catheters are identified and removed promptly.
The objective of the current study was to evaluate the effectiveness of this QI process by comparing the proportion of nonessential CVCs before and after the intervention. A secondary objective was to compare the two participating ICUs with respect to the intervention’s effectiveness. Ongoing monitoring of the rates of CLABSI in the participating ICUs informed the specific merit of this intervention.
Methods
This study was approved by Queen’s University Research Human Ethics Board, and this report follows the Standards for Quality Improvement Reporting Excellence (SQUIRE) guidelines.13
Setting
This prospective observational study took place at a tertiary care teaching centre, a 456-bed hospital with a catchment area of over 500,000 persons. The adult critical care program consists of a 24-bed level-3 unit and an 18-bed level-2 unit. The level-2-unit provides care for patients who require continuous cardiac and vital sign monitoring, low doses of vasoactive medications, and noninvasive positive pressure ventilation. The level-3 unit provides the full range of ICU care, including mechanical ventilation. The level-3 unit is a closed unit with primary comprehensive care provided by designated critical care physicians. The level-2 unit operates as an open unit where patient care is provided by various clinical services. Both units have dedicated critical care nursing staff. The usual nurse to patient ratio is 1:1 in the level-3 unit and 1:2 in the level-2 unit. In the level-3 unit, bedside rounds occur twice a day, with formal administrative rounds – a conference with senior physicians and nursing staff – each weekday. In the level-2 unit, individual services determine rounding structures. Patients might stay in both units during the same admission. Alternatively, usual practice in the level-3 ICU is to discharge recovering patients to the level-2 ICU. In addition, the level-3 unit provides overflow capacity for the level-2 unit.
Planning the intervention
The QI intervention was designed and carried out by the critical care program’s Patient Safety and Quality Improvement Committee. This multidisciplinary committee includes representatives of the ICU’s physicians, nurses, pharmacists, respiratory therapists, dietitians and physiotherapists, an infection control practitioner, and a volunteer. The framework for clinical practice improvement and behavioural change followed the principles of the Model for Improvement 14 with several iterations of the Plan-Do-Study-Act cycle.15 The intervention was based on the Critical Care Team Reminders, a checklist of routine evidence-based practices for consideration by the multidisciplinary teams during daily rounds. One of the items reminds users to remove any CVC if it is no longer necessary. Additional items on the checklist address reporting of safety concerns,16 prevention of ventilator-associated pneumonia, sedation practices, prescription of antibiotics, as well as adequacy of nutritional support. Committee members selected the checklist items and decided the wording for each item based on commonly promoted and previously reported interventions.7,17,18 In preparation for the introduction of the checklist, instructions were given to all ICU team members via existing mechanisms (e.g., clinical educators through meetings with nurses) as well as by committee members through group and individual meetings in the ICUs. For the level-3 (closed-model) unit only, residents rotating in the ICU (usually four to six residents for two-month blocks) received a short introduction to the checklist during their orientation day, and early in each rotation, the residents were given a specific 45-min seminar dedicated to patient safety. Each morning from then on, one of the residents was designated as the daily “Safety Checklist Champion” with the responsibility to prompt the team to consider the checklist items during rounds. Due to the multitude of clinical services involved in patient care in the level-2 unit, instruction for residents in the open unit was not possible. Throughout the project, committee members frequently reminded groups and individuals, mostly nursing staff, in both ICUs to use the checklist. The intervention was introduced in both ICUs 12 months prior to the study, and it was regularly maintained as described. Additional measures for prevention of CLABSI were already practiced in these ICUs prior to the QI intervention, including prepackaged CVC kits with supplies for a complete sterile barrier, use of chlorhexidine-based prep solution, and education of nurses regarding dressing and aseptic handling of the CVCs. No additional practices were introduced during the study.
Planning the study
This was a prospective observational study with data collection occurring during 28 consecutive days in April and May 2010. The study was designed to provide 80% power at a two-sided alpha of 0.05 to detect a 20% decrease in the proportion of patients with any nonessential CVC days after the intervention. Since the pre-intervention proportion of patients with any nonessential CVC days was 51% based on 81 patients, 110 patients were required for the study. Given the patient flow and occupancy patterns in the participating units, we were able to obtain data on 110 patients in four weeks. Workflow was similar to the pre-intervention study, which has been published separately.12 Each patient admitted to the level-3 and level-2 ICUs during the study period was assessed daily by a research assistant, a second-year medical student, for the presence of CVCs. For each identified CVC, all definite indications for placement over the preceding 24 hr were pursued by means of one or more bedside inspections, a focused chart review, and nursing staff interviews. The primary author trained the research assistant, supervised the assistant closely during the first days of data collection, and directly supported any of the assistant’s questions and concerns throughout the study. Indications for CVC placement included any appropriate and long-term intravenous therapy, such as vasopressors, monitoring (including central venous pressure and hemoglobin oxygen saturation), hemodialysis, cardiac pacing, lack of (i.e., inability to obtain) peripheral venous access, and the need for frequent blood draws (arbitrarily defined as more than three separate blood samples per day). If any indication for CVC placement was identified within the preceding 24 hr, it was deemed a necessary CVC day. Conversely, if the CVC was not used within the preceding 24 hr for any of the definite indications, it was deemed a nonessential CVC day. Physicians were not consulted as part of the data collection process.
Methods of evaluation and statistical analysis
The proportions of nonessential CVC days were ascertained for all patients as well as separately for different catheter types and the two ICUs. We calculated the proportions as the number of nonessential CVC days divided by the overall CVC days. Given the unique and commonly intermittent utilization of hemodialysis catheters, the calculation was performed after excluding nonessential hemodialysis catheter days.
Using the Chi square test, a comparison was made between post-intervention vs pre-intervention with respect to the proportion of patients with at least one nonessential CVC day. The log-binomial model was used to estimate the relative risks of nonessential CVC days by comparing the total number in the level-3 ICU vs the level-2 ICU, and by comparing patients (sex, mechanical ventilation), catheter type (temporary CVC, dialysis catheter, peripherally inserted CVC), and catheter site (internal jugular, subclavian, femoral, arm) characteristics. Since patients could contribute multiple nonessential CVC days, generalized estimating equations (GEE) clustered by patient with a compound symmetric working correlation were used to account appropriately for the over-dispersed log-binominal model. The log-binomial GEE model was estimated by the GENMOD procedure of SAS® version 9.2 (SAS Institute Inc., Cary, NC, USA).
In addition, the incidence of CLABSI was monitored continuously, prior to and during the intervention, according to the guidelines of Ontario’s Critical Care Information System (CCIS).19 The CCIS is a performance measurement tool that supports decision-making at the provincial, regional, and hospital levels. It captures patient-specific data on the utilization of resources and allows for analysis of trends in ICUs across hospitals and regions. The nurse caring for the patient collects the data and then the unit clerk inputs the data. Accuracy checks are completed monthly, as applicable, by an advanced nurse practitioner. Interrater reliability checks are completed for individuals who are new to the inputting feature. As part of this system, CVC use is recorded for all patients by the bedside nurses. Reporting CLABSI in the CCIS is based on documentation of this diagnosis in the patient’s record by the ICU physician; however, physicians are not involved directly in data collection or in the reporting process. The CCIS performs the analysis and reporting of CLABSI rates as follows: CLABSI rate = (number of CLABSI incidents diagnosed after day two of ICU admission / number of CVC days) × 1,000.
Results
Patient and catheter characteristics
During the study’s four weeks, 75 of 110 patients were admitted to the level-3 unit and 35 were admitted to the level-2 unit; ten patients were admitted to both units at different times. The characteristics of patients and central lines reviewed in the pre- and post-intervention studies are shown in Table 1. One hundred fifty-nine CVCs were reviewed for a total of 820 CVC days, 631 (77.0%) days in the level-3 unit and 189 (23.0%) days in the level-2 unit. Table 2 shows the recorded indications for CVC days. Eighty-eight (10.7%) of all CVC days had no apparent indication for CVC placement; 48 (55%) of these were dialysis catheters, 30 (34%) were peripherally inserted central catheters (PICCs), and ten (11%) were other CVCs.
Nonessential CVC days
Fig. 1 shows the distribution of durations of nonessential CVC days among the study patients. Twenty-nine (26%) patients had at least one nonessential CVC day; 22 (76%) of these patients had only one or two such days. Fig. 2 shows the distribution of consecutive nonessential CVC days for various catheter types. Fifty (57%) of all nonessential CVC days were of one or two consecutive days. Only PICCs and dialysis catheters accounted for longer durations (i.e., ≥ three days) of consecutive nonessential CVC days. By accepting one or two consecutive unused CVC days, 50 of the 88 nonessential CVC days would be deemed necessary, and the overall proportion of nonessential CVC days would decrease from 10.7% to just 4.6% (38 out of 820 CVC days). Thirty-one of the 48 nonessential dialysis catheter days were of one or two consecutive days, and an additional 14 were of three consecutive days. Only three unused dialysis catheter days were more than three consecutive days, all in the same patient. By excluding dialysis catheter days, the overall proportion of nonessential CVC days would decrease to just 4.9% (40 out of 820 CVC days).
Pre/post intervention comparison
Compared with the pre-intervention period, the proportion of patients with any number of nonessential CVC days decreased by a factor of 0.51 (95% confidence interval [CI], 0.34 to 0.74; P < 0.001), from 51% to 26%.
Level-3 vs level-2 unit
Sixty-four (73%) of all CVC days with no apparent indication for CVC placement were recorded in the level-3 unit and 24 (27%) were recorded in the level-2 unit, representing 10.1% and 12.7% of all CVC days in these units, respectively, a non-significant difference (relative risk, 0.81; 95% CI, 0.45 to 1.47; P = 0.49).
Associations between nonessential CVC days and other variables
Associations between nonessential CVC days and unit (level-2 vs level-3), patient (sex, mechanical ventilation), and catheter (type and site) characteristics were examined using a generalized linear model. A statistically significant association with nonessential CVC days was found only for catheter type. Compared with temporary CVCs, the relative risk (95% CI) of nonessential CVC days for dialysis catheters and PICCs were 15.2 (5.9 to 39.2) and 4.5 (1.3 to 15.5), respectively (P = 0.002).
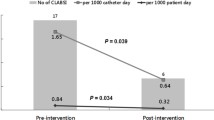
Rates of CLABSI
As shown in Fig. 3, CLABSI rates declined during the intervention, and no catheter-related infections have been reported in these ICUs for over a year now.
Discussion
After the introduction of a multifaceted QI intervention, the proportions of nonessential CVC days decreased substantially compared with pre-intervention rates and were similar in both participating ICUs. Dialysis catheters and PICCs accounted for the majority (54% and 34%, respectively) of nonessential CVC days. Concurrently, rates of CLABSI decreased substantially.
In recent years, with major campaigns to ensure reliable compliance with infection prevention practices in hospitals,7,20 reported rates of CLABSI have decreased; however, they have not yet been eliminated.21 We addressed one of the key CLABSI prevention practices, prompt removal of nonessential CVCs.6 Although the use of checklists to achieve reliable compliance with this specific goal has been promoted, the effectiveness of this approach has been examined only by a single recent study from Chicago.22 However, the Chicago group relied on an individual external to the ICU team to provide prompting. Our QI initiative was based on participation of organic team members; therefore, our study suggests that removal of nonessential CVCs can be achieved reliably by ICU teams through the use of checklists.
The development of medical checklists requires consideration and skill;23 however, ensuring that a checklist is used reliably by clinicians might be an even greater challenge. Successful QI interventions based on checklists invested substantial efforts to ensure that the checklists would be utilized. For example, an Australian hospital has improved patient care by using checklists and reminders in certain clinical pathways. These tools were deliberately designed to be easily completed by staff and were incorporated into the patients’ medical records.24 Similarly, in the 2004 Johns Hopkins report on eliminating CLABSI in the ICU, which was part of a system-based approach and in addition to many other interventions, the question whether any catheters or tubes could be removed was added to a “daily goals form” that had already been in use in that ICU prior to that specific intervention.17 In our ICUs, the infrastructure to facilitate providers’ compliance with QI interventions was created concurrently with the intervention. It included leadership by the multidisciplinary Patient Safety and Quality Improvement Committee, design and introduction of the daily checklist, ongoing education and reminders, and designation of daily checklist “champions”. While the improvement strategy has not changed, ongoing measurement at the closed-model unit outside of this study has shown similar rates of nonessential CVC days, as evidence of sustainability.25
In the hospital environment, the risk of CLABSI from PICCs is comparable with that from other temporary CVCs2; therefore, the rule to remove a catheter as soon as it is no longer necessary should apply to PICCs as well. In our study, PICCs accounted for 34% of all nonessential CVC days, three times greater than other temporary CVCs. The reason for that is unknown; perhaps providers viewed these catheters as safer than other CVCs. In addition, the perceived “secured” intravenous access might have given providers a sense of safety when looking after critically ill patients. Alternatively, the peripheral insertion site of PICCs might have made these catheters less visible to providers, resulting in catheters being missed during rounds. Finally, it is possible that ICU clinicians decided to keep PICCs, as well as other CVCs, in place for reasons not captured by the research team. Regardless of the reasons, QI initiatives aimed at the removal of nonessential CVCs should specifically address PICCs.
Dialysis catheters are unique in their potentially alternating utilization: for a typical patient receiving three hemodialysis therapies per week, four of the seven weekly catheter days would be designated as “nonessential” (i.e., the catheter was not used). In addition, for patients with dialysis-dependent renal failure in recovery, it is hard to anticipate whether additional dialysis therapies would be required and difficult to determine the best timing for removing the catheter. Thus, a considerable number of “nonessential” catheter days are expected for this population. Accepting one or two consecutive unused catheter days, our results suggest that nonessential dialysis catheters were removed fairly promptly.
The initiative was equally effective in both participating ICUs. In the pre-intervention study, proportions of nonessential CVC days were much higher in the level-2 (open-model) unit compared with the level-3 unit (59.9% vs 13.0%, respectively, of all patient days with a CVC with no apparent indication for placement.12 Whereas proportions of nonessential CVC days decreased in both units, the dramatic improvement seen in the level-2 unit can be explained by an existing need that was met by the intervention, perhaps as an alternative to critical care physician-directed teams.26
This study has several strengths. Primarily, this novel study provides evidence that a checklist-based intervention directed specifically at removal of nonessential CVCs is effective. Second, repeating a similar study protocol at the same clinical setting allowed for improvements to the data collection process and resulted in a robust, reusable, and sharable methodology. Third, the inclusion of different ICU types, specifically closed- and open-model units, represents our critical care setting. Furthermore, similar to our previous experience with improving safety reporting practices,16 this initiative serves as substantiation for the concept of successful QI initiatives led by a dedicated patient safety committee. Fourth, the prospective and inclusive nature of data collection minimized the risk of sampling bias.
Our study has five known limitations. First, the intervention was performed in a single centre. While routine practices, including reasons for inserting a CVC and baseline incidence of nonessential catheters, may vary across centres, our findings may not be directly generalized to other organizations. Nevertheless, the general approach to QI as well as the intervention and measurement principles are described and could be easily replicated. Second, there was possible introduction of bias by the research team occasionally interacting with the providers, perhaps leading to change in practice. Furthermore, providers might have decided to keep CVCs in place for acceptable reasons that were not on our list of indications. Clearly, the very purpose of this QI initiative was to influence team members to remove nonessential CVCs when appropriate. Nevertheless, a similar measurement approach was taken at the pre-intervention study, which showed much higher proportions of nonessential CVC days. Third, as a result of learning from the earlier study, data collection tools were modified to gather richer information in the current follow-up study. This modification limited the comparison between the pre- and post-intervention periods. For example, it is likely that some nonessential CVC days were not captured in the pre-intervention study. However, this would result in an underestimation of the pre-intervention proportions of nonessential CVC days, and thus the actual effect size would be even greater than reported. Fourth, the reported CLABSI rates were based on data collected for and analyzed by Ontario’s CCIS outside of our study and were potentially subjected to sources of bias and inaccuracy such as underreporting by physicians. Given the small sample size and statistical power, we did not attempt to examine for association between nonessential CVC days and CLABSI. Generating accurate measures for CLABSI is challenging,22 and attention should certainly be paid to the establishment of reliable measurement procedures. Then again, the processes of data collection and analysis did not change during the intervention, and thus, the likelihood of systemic biases influencing the reported CLABSI rates is small. Finally, although no additional preventative measures were introduced to our units during this QI initiative, we cannot comment on the possibility that acceptance and implementation of existing parts of the CLABSI prevention bundle, as well as hand hygiene, have increased during the time. Therefore, the observed reduction in CLABSI may be related to multiple factors.
In conclusion, proportions of nonessential CVCs decreased substantially in two ICUs after the implementation of a multifaceted QI initiative led by a patient safety committee. The initiative included education, reminders, a daily checklist, and designation of daily “champions”. Peripherally inserted central catheters contributed to the count of nonessential CVC days more than any other catheter type. During the equivalent time, CLABSI rates decreased substantially as well. This study provides evidence that using a checklist, along with additional measures, is effective for ensuring prompt removal of CVCs when they are no longer necessary.
References
Irwin RS, Rippe JM, Lisbon A, Heard SO. Procedures, Techniques and Minimally Invasive Monitoring in Intensive Care Medicine. 4th ed. Philadelphia, USA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008.
Marschall J, Mermel LA, Classen D, et al. Strategies to prevent central line-associated bloodstream infections in acute care hospitals. Infect Control Hosp Epidemiol 2008; 29(Suppl 1): S22-30.
Renaud B, ICU-Bacteremia Study Group. Outcomes of primary and catheter-related bacteremia. A cohort and case-control study in critically ill patients. Am J Respir Crit Care Med 2001; 163: 1584-90.
Polderman KH, Girbes AR. Central venous catheter use. Part 1: mechanical complications. Intensive Care Med 2002; 28: 1-17.
Polderman KH, Girbes AR. Central venous catheter use. Part 2: infectious complications. Intensive Care Med 2002; 28: 18-28.
Yokoe DS, Mermel LA, Anderson DJ, et al. A compendium of strategies to prevent healthcare-associated infections in acute care hospitals. Infect Control Hosp Epidemiol 2008; 29(Suppl 1): S12-21.
Canadian Patient Safety Institute. Safer healthcare now! 2011; Available from URL: http://www.saferhealthcarenow.ca/EN/Pages/default.aspx (accessed September, 2012).
Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med 2006; 355: 2725-32.
Institute for Healthcare Improvement. 5 Million Lives – 2011; Available from URL: http://www.ihi.org/IHI/Programs/Campaign/ (accessed September, 2012).
Trick WE, Vernon MO, Welbel SF, Wisniewski MF, Jernigan JA, Weinstein RA. Unnecessary use of central venous catheters: The need to look outside the intensive care unit. Infect Control Hosp Epidemiol 2004; 25: 266-8.
Voges KA, Webb D, Fish LL, Kressel AB. One-day point-prevalence survey of central, arterial, and peripheral line use in adult inpatients. Infect Control Hosp Epidemiol 2009; 30: 606-8.
Cload B, Day AG, Ilan R. Evaluation of unnecessary central venous catheters in critically ill patients: a prospective observational study. Can J Anesth 2010; 57: 830-5.
Davidoff F, Batalden P, Stevens D, Ogrinc G, SQUIRE Development Group. Publication guidelines for quality improvement studies in health care: Evolution of the SQUIRE project. Qual Saf Health Care 2008; 17(Suppl 1): i3-9.
Langley GL, Nolan KM, Nolan TW, Norman CL, Provost LP. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. 2nd ed. California, USA: Jossey-Bass Publishers; 2009.
Institute for Healthcare Improvement. Improvement Methods – 2012; Available from URL: http://www.ihi.org/IHI/Topics/Improvement/ImprovementMethods/Tools/ (accessed September, 2012).
Ilan R, Squires M, Panopoulos C, Day A. Increasing patient safety event reporting in 2 intensive care units: a prospective interventional study. J Crit Care 2011; 26: 431.e11-8.
Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med 2004; 32: 2014-20.
Anonymous. Checkoffs play key role in SICU improvement. Healthcare Benchmarks Qual Improv 2003; 10: 113-5.
Ontario Ministry of Health and Long-Term Care. Critical care information system (CCIS); Available from URL: http://www.health.gov.on.ca/english/providers/program/critical_care/cct_infosystem.html (accessed September, 2012).
Berwick DM, Calkins DR, McCannon CJ, Hackbarth AD. The 100,000 lives campaign: setting a goal and a deadline for improving health care quality. JAMA 2006; 295: 324-7.
Centers for Disease Control and Prevention (CDC). Vital signs: central line associated blood stream infections—United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep 2011; 60: 243-8.
Weiss CH, Moazed F, McEvoy CA, et al. Prompting physicians to address a daily checklist and process of care and clinical outcomes: a single-site study. Am J Respir Crit Care Med 2011; 184: 680-6.
Hales B, Terblanche M, Fowler R, Sibbald W. Development of medical checklists for improved quality of patient care. Int J Qual Health Care 2008; 20: 22-30.
Wolff AM, Taylor SA, McCabe JF. Using checklists and reminders in clinical pathways to improve hospital inpatient care. Med J Aust 2004; 181: 428-31.
Ilan R, Heyland D, Muscedere J, Harris GA. Patient safety coordinator to improve adherence to evidence based practices in the ICU: an interventional study in an academic centre. Critical Care Canada Forum 2011 (abstract).
Gutsche JT, Kohl BA. Who should care for intensive care unit patients? Crit Care Med 2007; 35(2 Suppl): S18-23.
Funding
This study did not receive research funding.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was performed at the Critical Care Program, Kingston General Hospital, Kingston, ON, Canada.
Author contributions
Roy Ilan conceived and designed the study, supervised data acquisition, and drafted the article. John Doan and Bruce Cload contributed to the study’s design. John Doan and Mae Squires contributed to the acquisition of data. Roy Ilan, John Doan, Bruce Cload, Mae Squires, and Andrew Day participated in data analysis and interpretation. John Doan, Bruce Cload, Mae Squires, and Andrew Day critically revised the article.
All authors participated in the revision process.
APPENDIX: Critical care team reminders
APPENDIX: Critical care team reminders

Rights and permissions
About this article
Cite this article
Ilan, R., Doan, J., Cload, B. et al. Removing nonessential central venous catheters: evaluation of a quality improvement intervention. Can J Anesth/J Can Anesth 59, 1102–1110 (2012). https://doi.org/10.1007/s12630-012-9794-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-012-9794-5