Abstract
Purpose
This dose-response study aimed to determine the optimal dose of remifentanil combined with propofol 2.5 mg·kg−1 iv in order to achieve excellent conditions for laryngeal mask airway (LMA™) insertion in 95% of adult female patients.
Methods
Sixty-eight adult premedicated female patients, American Society of Anesthesiologists (ASA) physical status I and II requiring anesthesia for ambulatory surgery, were randomly allocated to one of four remifentanil dose groups (0.25, 0.5, 1, or 2 µg·kg−1). Induction of anesthesia was achieved with one of the four blinded doses of remifentanil infused over 60 sec and simultaneously co-administered with propofol 2.5 mg·kg−1 iv infused over 45 sec. Insertion of the LMA was attempted 150 sec after the beginning of the induction sequence. Insertion conditions were assessed using a six-category score according to resistance to mouth opening and insertion, swallowing, coughing and gagging, movement, and laryngospasm. A probit analysis was performed to calculate the effective dose for insertion of the LMA in 95% of patients (efficient dose [ED]95). The changes in heart rate (HR) and mean arterial blood pressure (MAP) in response to LMA insertion were recorded and compared as secondary outcome variables.
Results
The ED95 of remifentanil was 1.32 (95% confidence interval [CI] 0.99-2.46) µg·kg−1. Changes in heart rate and mean arterial pressure were modest and similar over time across groups, with maximum decreases in heart rate and mean arterial pressure < 30% each during induction of anesthesia.
Conclusions
The required dose of remifentanil is 1.32 (95% CI 0.99-2.46) µg·kg−1 to achieve excellent LMA insertion conditions in 95% of patients when co-administered with propofol 2.5 mg·kg−1 in healthy premedicated female patients undergoing elective ambulatory surgery.
Résumé
Objectif
L’objectif de cette étude de dose-réponse était de déterminer la dose optimale de rémifentanil lors de l’administration conjointe de propofol 2,5 mg·kg−1 iv afin d’obtenir des conditions excellentes pour l’insertion d’un masque laryngé (LMA®) chez 95 % de patientes adultes.
Méthode
Soixante-huit patientes adultes ASA I et II prémédiquées et nécessitant une anesthésie pour une chirurgie ambulatoire ont été randomisées en quatre groupes selon la dose de rémifentanil (0,25, 0,5, 1 ou 2 µg·kg−1). L’induction de l’anesthésie a été réalisée avec l’une des quatre doses de rémifentanil en aveugle perfusée sur 60 secondes, administrée simultanément à du propofol 2,5 mg·kg−1 iv perfusé sur 45 secondes. L’insertion du LMA a été essayée 150 sec après le début de la séquence d’induction. Les conditions d’insertion ont été évaluées à l’aide d’une note en six catégories selon la résistance à l’ouverture de la bouche et à l’insertion, la déglutition, la toux et le réflexe pharyngé, le mouvement et le laryngospasme. Une analyse par la méthode des probits a été réalisée de façon à calculer la dose efficace nécessaire à une insertion du LMA chez 95 % des patientes [dose efficace (DE)95]. Les changements de fréquence cardiaque et de tension artérielle moyenne en réaction à l’insertion du LMA ont été enregistrés et comparés comme variables secondaires.
Résultats
La DE95 de rémifentanil était de 1,32 [intervalle de confiance (IC) 95 % : 0,99-2,46] µg·kg−1. Les changements au niveau de la fréquence cardiaque et de la tension artérielle moyenne étaient modestes et semblables au fil du temps dans les groupes, avec des diminutions maximales de la fréquence cardiaque et de la tension artérielle moyenne < 30 % chacune pendant l’induction de l’anesthésie.
Conclusion
La dose de rémifentanil nécessaire pour obtenir des conditions excellentes pour l’insertion du LMA chez 95 % des patientes lorsqu’il est administré conjointement à du propofol 2,5 mg·kg−1 chez des patientes saines prémédiquées et subissant une chirurgie ambulatoire non urgente est de 1,32 (IC 95 % : 0,99-2,46) µg·kg−1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Successful insertion of the laryngeal mask airway (LMA™) requires adequate mouth opening and sufficient depth of anesthesia to prevent untoward events of coughing, gagging, movements, and laryngospasm. It has been shown that propofol 2.5-3.5 mg·kg−1, rather than thiopental 4-5 mg·kg−1,1-3 combined with alfentanil, rather than fentanyl4, provides superior conditions for the insertion of the LMA. Thus, the optimal alfentanil dosage providing excellent insertion conditions in 95% of patients when co-administered with 2.5 mg·kg−1 propofol was previously assessed.5
Remifentanil is an ultra-short-acting selective μ-opioid receptor agonist that is 20-30 times more potent than alfentanil. Due to its pharmacokinetic properties, the use of remifentanil is of particular interest whenever maintenance of sufficient analgesia level is required during the procedure.6 Its combination with propofol significantly improved the ease of insertion of the LMA in comparison with propofol alone.7,8 The optimum effect-site concentration (EC) of remifentanil for LMA insertion during target-controlled infusion of propofol was recently determined through use of a modified Dixon’s up-and-down method followed by a probit analysis for the EC95 estimation.9 However, the effective dose of remifentanil providing excellent insertion conditions in 95% of patients (efficient dose [ED]95) when co-administered with a single standard dose of propofol has not been assessed.
Therefore, using an optimized sequence for induction of anesthesia, we conducted a dose-response study to determine the effective dose of remifentanil co-administered with propofol 2.5 mg.kg−1 that would provide excellent insertion conditions in 95% of patients (ED95) undergoing ambulatory elective surgery.10 The secondary outcome variables included calculation of the ED50, qualitative analysis of the criteria used for the assessment of the insertion conditions of the LMA according to the remifentanil dosages, and hemodynamic tolerance (heart rate [HR] and mean arterial pressure [MAP]).
Methods
The study took place in our department of anesthesia for gynecological surgery at the Édouard Herriot Hospital, Lyon, France. After Hospital Ethics Committee approval of the study and after obtaining the patients’ written consent, we enrolled caucasian female patients who were scheduled for elective outpatient gynecological surgery requiring the use of LMA. Inclusion criteria included patients aged 18 to 70 yr who were American Society of Anesthesiologists (ASA) physical status I and II and had a body mass index < 30 kg·m−2. Exclusion criteria included patients with a history or evidence of a difficult airway (combination of the Mallampati score 3 or 4, thyromental distance < 60 mm,11,12 and mouth opening of < 35 mm) and patients with risk factors for pulmonary aspiration of gastric contents.
Anesthetic protocol
All patients were premedicated with alprazolam 0.5 mg and hydroxyzine 1 mg·kg−1 orally given one hour before induction of anesthesia.
Patients were randomly allocated by a computer-generated list in a single sequence of random assignment with no restriction (simple randomization) to receive one of four doses of remifentanil (0.25, 0.5, 1, or 2 μg·kg−1). The range of doses of remifentanil for the insertion of the LMA was selected based on the following assumptions: the required dose of remifentanil would be less than twice the dose required to moderate hemodynamic responses to tracheal intubation and more than one-fourth of a 4 μg·kg−1 dose determined from a recent study in a similar population,13 and as was previously reported when using alfentanil in combination with propofol.5 The randomization list was generated by a physician who was not involved in the enrollment of patients into the study. Allocation concealment was ensured by the use of coded sealed opaque envelopes. Remifentanil was diluted with 0.9% saline 50 μg·mL−1 into a 50 mL syringe.
Following application of routine monitors in the operating room, the patients were pre-oxygenated until their end-tidal oxygen fractions were > 90%, then anesthesia was induced. At time T0, the chosen dose of remifentanil was infused over 60 sec using a programmable syringe pump, Pilote Anesthésie 2®, (Fresenius Vial, Brezins, France) hidden behind a sheet, followed by a continuous infusion of 0.15 μg·kg−1·min−1. Propofol 2.5 mg·kg−1 was simultaneously administered over 45 sec. Ventilation via facemask was initiated once loss of eyelash reflex occurred.
The same experienced senior physician who was blinded to the anesthetic procedure performed all LMA (AuraOnce™, Ambu®, Ballerup, Denmark) insertions. A size 3 LMA was selected for patients < 65 kg, and a size 4 LMA was used for all other patients. At time T0 + 150 sec, the physician attempted to insert the LMA according to the technique recommended by Brain et al.,14 and assessed the quality of insertion as described further. If insertion conditions were judged to be uncomfortable, supplemental boluses of remifentanil 1 μg·kg−1 and/or propofol 0.5 mg·kg−1 were administered at the discretion of the inserting anesthesiologist.
Anesthesia was maintained with 1.5-2% end-tidal sevoflurane in 50% oxygen, and continuous infusion of remifentanil 0.15 μg·kg−1·min−1. The lungs were ventilated to normocapnia. No further stimulation was applied to the patient during the study period.
Monitors included an automated arterial pressure cuff, electrocardiogram, peripheral pulse oximeter, and capnometer. Control values of arterial pressure and HR were recorded before induction of anesthesia (pre-induction values), one, three, five, and ten minutes after the end of propofol infusion. If MAP or HR decreased more than 30% compared with corresponding pre-induction values, ephedrine 3 mg or atropine 0.5 mg was administered intravenously, as needed to reach at least 70% of the pre-induction value.
Assessment of the LMA insertion conditions
Insertion conditions were assessed with a scale used successfully in previous studies.4,5 Six variables were recorded: resistance to mouth opening, resistance to insertion of LMA, swallowing, coughing and gagging, head or body movement, and laryngospasm, as defined by prolonged obstruction with an apparently correctly placed LMA. Each of these variables was rated as excellent or intermediate or poor. The criteria for assigning values to each variable are set out in Table 1. The LMA insertion conditions were excellent if all criteria were scored as excellent, intermediate if all criteria were scored as either excellent or intermediate, and poor if a single criterion was scored as poor. The number of LMA insertion attempts was also recorded for each patient.
Statistical analysis
In a Cochran-Armitage test for trend in proportions, a sample size of 17 patients per group was obtained from four groups with remifentanil doses equivalent to 0.25, 0.5, 1, and 2 μg·kg−1 and proportions of excellent LMA insertion conditions equivalent to 0.40, 0.60, 0.80, and 0.90 μg.kg−1, respectively. A sample size of 68 subjects achieves 90% power to detect a linear trend using a two-sided Z test with continuity correction and a significance level of 0.05 (PASS 8.0.05 [NCSS, LCC, Kaysville, UT, USA]).15
The computer software package, Statistica 6.0 (Statsoft®, Tulsa, OK, USA), was used for analysis of the hemodynamic data. Median doses of ephedrine were analyzed using the Kruskal-Wallis H test. Repeated measures of hemodynamic values were analyzed by a two-way analysis of variance. The χ² test was used to analyze the incidence of requiring supplemental bolus doses of remifentanil and propofol, the incidence of requiring ephedrine, the overall LMA insertion conditions in the four patient groups distributed according to remifentanil dosages, and the quality of the six criteria used to assess the LMA insertion conditions. For each outcome measured, whenever the result of the χ² test reported a significant difference between groups (P < 0.05), a Bonferroni adjustment for multiple comparisons was made over the six between-dose comparisons, with a cut-off of 0.05/6 = 0.0083, which was considered to be statistically significant.
The event “success” or “failure” of excellent insertion conditions for each patient was analyzed using Statistical Package for Social Science version 16.0 (SPSS®, Chicago, IL, USA) for probit regression to calculate the effective dose of remifentanil required to provide excellent insertion conditions in 50% and 95% of patients (ED50 and ED95). Probit analysis is designed to model the probability of response to a stimulus in the setting of a dose-response experiment. Since the probability of an event must lie from 0 to 1, it is impractical to model probabilities with linear regression techniques, because the linear regression model allows the dependent variable to take values greater than 1 or less than 0. The probit analysis model is a type of generalized linear model that extends the linear regression model by linking the range of real numbers to the 0-1 range in a sigmoid relationship. Sigmoid relationships can be linearized by transformations such as logit and probit. The fitted model is assessed by statistics for heterogeneity which follow a Chi square distribution. If the heterogeneity statistics are significant, then the observed values deviate from the fitted curve too much for reliable inference to be made. Probit regression gives the effective levels of dose/stimulus with confidence intervals (CI) at the specified quantiles, e.g., ED50 or ED95, with the curve fitted by maximum likelihood estimation.
Results
Eighty-seven patients were screened for this study. Four were excluded because they refused to participate, and 15 did not meet the inclusion criteria. Sixty-eight patients were enrolled (17 patients per group) from May to December 2008. All included patients completed the study. Patients’ characteristics are shown in Table 2. All subjects were female.
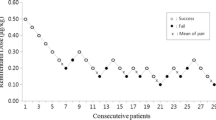
The overall and individual insertion scores are summarized in Figures 1 and 2. For eight patients (five from group 0.25 μg·kg−1 and three from group 0.5 μg·kg−1), insertion conditions were judged poor. Laryngeal mask airway insertion was achievable in all of the patients; however, six patients required more than one attempt at LMA placement. With Bonferroni adjustments for multiple comparisons, overall insertion conditions in group 0.25 μg·kg−1 were considerably different from group 2 μg·kg−1 (Figure 1). There was no significant difference between groups regarding coughing and gagging, head or body movements, and laryngospam (Figure 2). Concerning resistance to mouth opening and resistance to inserting the LMA (Figures 2a and d), there were statistically significant differences between group 0.25 μg.kg−1 and group 2 μg·kg−1, with group 0.25 μg.kg−1 having less excellent conditions. Nevertheless, there was no statistically significant difference between groups regarding the rate of undue force required. After Bonferroni adjustments for multiple comparisons, we were unable to locate the difference reported between groups regarding the rate of swallowing (Figure 2b).
Overall insertion conditions of the laryngeal mask airway (LMA) in the four groups of remifentanil dosages. P = 0.0023 between groups. * P = 0.0003 compared with group 2 μg·kg−1 regarding overall insertion conditions, and † P = 0.0002 compared with group 2 μg·kg−1 regarding excellent vs non-excellent conditions (Bonferroni adjustments for multiple comparisons)
Distribution of patients in the four groups of remifentanil dosages based on the quality of the six criteria used for assessing laryngeal mask airway (LMA) insertion conditions. a * P = 0.022 between groups. † P = 0.008 compared with group 2 μg·kg−1 regarding resistance to mouth opening, and ‡ P = 0.003 compared with group 2 μg·kg−1 regarding excellent vs non excellent conditions (Bonferroni adjustments for multiple comparisons). b ** P = 0.0046 between groups. d § P = 0.018 between groups. || P = 0.0053 compared with group 2 μg·kg−1 regarding resistance to insertion, and # P = 0.0012 compared with group 2 μg·kg−1 regarding excellent vs non excellent conditions (Bonferroni adjustments for multiple comparisons)
No difference was recorded between groups with respect to the requirement for supplemental administration of remifentanil and propofol (Table 3). From probit analysis, the ED50 of remifentanil was 0.44 μg·kg−1 (95% CI 0.11-0.64 μg·kg−1) and the ED95 was 1.32 μg·kg−1 (95% CI 0.99-2.46 μg·kg−1) (omnibus test of model coefficients: Chi = 24.28; df = 1; P < 0.01) (Figure 3).
Probit regression of the probability of excellent laryngeal mask airway (LMA) insertion conditions as a function of the induction dose of remifentanil. Horizontal bars denote 95% confidence interval for inserting efficient dose (ED) in 50% and 95% of patients (ED50 and ED95). Omnibus test of model coefficients: Chi = 24.28; df = 1; P < 0.01
For all four groups of patients, two-way analysis of variance of HR and MAP showed a significant decrease in HR and MAP following induction, without any significant difference between groups (Figure 4). The greatest decrease in mean values of HR and MAP was recorded five minutes after propofol administration, and the range across the four groups was 19-22% for HR and 27-29% for MAP (Figure 4). No statistical difference was found between groups with respect to ephedrine requirements (Table 3). No patient required atropine.
Discussion
This dose-response study allowed us to determine the ED95 of remifentanil co-administered with propofol 2.5 mg·kg−1 in elective adult female patients.
Several previous studies demonstrated that adding an opioid to propofol significantly improved LMA insertion conditions. Thus, when co-administered with propofol, alfentanil was reported to be superior to fentanyl for reaching excellent insertion conditions,4 and the optimal dose of alfentanil was 10 μg·kg−1 when co-administered with propofol 2.5 mg·kg−1.5 However, compared with remifentanil, the context-sensitive half-times of alfentanil do not always allow prolonged infusion or repeated administration of this opioid due to the potential for alfentanil drug cumulation over time.6,16 Remifentanil is an ultra-short-acting opioid with a context-sensitive half-time that is independent of the duration of drug administration.6 Therefore, remifentanil has a pharmacokinetic profile well-suited for rapid recovery, even for more prolonged outpatient procedures requiring moderate or high levels of intraoperative analgesia. Its addition to propofol, remifentanil significantly improved LMA insertion conditions during induction of anesthesia.7,8 In a recent study, Kim et al. 9 reported that 3.79 ng·mL−1 is the optimum effect-site concentration of remifentanil for LMA insertion during target-controlled infusion of propofol 3.5 μg·mL−1. However, it remains difficult to compare these results with ours, since the model of administration of propofol and remifentanil was considerably different, and the modified Dixon’s up-and-down method followed by probit analysis methodology used in Kim et al.’s study for estimating the EC95 value10 resulted in a very large 95% CI for the EC95 (from 3.26 to 9.25 ng·mL−1).9
As expected, we observed a decrease in the ED95 of remifentanil for providing excellent LMA insertion conditions compared with the IED95 of remifentanil 4 μg·kg−1.13 This decrease was due to use of a carefully standardized anesthetic induction sequence and consideration of the unique pharmacokinetic and pharmacodynamic attributes of propofol and remifentanil such that LMA insertion occurred at the time when effect-site concentration peaked for both agents.17,18 In fact, insertion of the LMA constitutes a less noxious stimulus compared with laryngoscopy. Billard et al. observed a significant increase in systolic blood pressure (> 40%) during laryngoscopy and tracheal intubation after induction of anesthesia with propofol 2.5 mg·kg−1 given alone,19 whereas Hickey et al. 20 reported minor cardiovascular response to insertion of LMA after the same dose of propofol. Yu et al. 5 calculated that the optimum dose of alfentanil for inserting the classic LMA was 10 μg·kg−1 when co-administered with popofol 2.5 mg·kg−1, which corresponds to one-fourth of the required dose of alfentanil for tracheal intubation when co-administered with propofol 2 mg·kg−1.21 Therefore, it is not surprising that ED95 of remifentanil for LMA insertion is lower than the dose required for tracheal intubation.
Increasing doses of remifentanil significantly improved LMA insertion conditions, mainly by decreasing resistance to both mouth opening and insertion. In the study by Yu et al. 5 in which they assessed the ED95 of alfentanil combined with propofol for LMA insertion, the addition of alfentanil did not provide reliable mouth opening or a reduction in resistance to insertion. For these authors, these two assessments may have been influenced by anatomical variations of the upper airway, which are different in ethnic Chinese compared with the Caucasian population for whom the anatomic characteristcs of the LMA were originally designed.4 Only Caucasian patients were included in our study to minimize the impact of this feature as a potentially confounding variable. Mouth opening and resistance to insertion are essential criteria that must be optimized for ease of LMA insertion.
No clinically significant muscle rigidity was observed in either group. Muscle rigidity was described after rapid infusion of large doses of opioids;22 therefore, in our study, remifentanil was administered over 60 sec. Similarly, no muscular rigidity was observed in previous studies with doses of remifentanil ranging from 1 to 4 μg·kg−1 administered over 90 sec in combination with propofol 2 mg·kg−1 or thiopental 5 mg·kg−1 for tracheal intubation.23,24 Moreover, alprazolam given 60 min prior to induction of anesthesia may also have contributed to minimizing the occurrence of muscle rigidity, since premedication with benzodiazepine is effective in preventing opioid-induced muscle rigidity.25
Hemodynamic tolerance was similar and acceptable in the four groups of our study. The maximum decrease in mean values of HR and MAP was less than 30%, and there was no significant increase in the requirement of ephedrine administration across the four groups. Several previous studies reported a similar acceptable decrease in the MAP and HR when using varying amounts of remifentanil (from 0.5 to 5 μg·kg−1) combined with propofol 2.0 or 2.5 mg·kg−1.23,26 In fact, in ASA I and II patients, it has been reported that co-administration of fentanyl (2 or 4 μg·kg−1) with increased doses of propofol (from 2 to 3.5 μg·kg−1) did not exacerbate a decrease of either blood pressure or HR while it attenuated the cardiovascular response to laryngoscopy in comparison with placebo.19 In the same way, the addition of remifentanil 0.25 or 0.5 μg·kg−1 to propofol 2.5 mg·kg−1 was well tolerated and moderated cardiovascular responsiveness to LMA insertion that were present when no opioid was co-administered with propofol.7,19,20
There are several limitations of our study. First, only female patients were included, as this study was conducted in a gynecological unit. Although a gender effect in opioid analgesic responsivenss remains controversial, it would be of interest to perform a similar dose response study in male patients.27,28 Second, our results are available for ASA I and II patients only, aged 18 to 70 yr. Hemodynamic tolerance in ASA III or IV patients may be different, particularly those suffering from serious heart disease. A further limitation of the study is that the sample size calculation was not based on the primary objective of the study, i.e., the estimation of ED95, limiting the precision of the ED95 estimate. Therefore, it would be of interest to perform a larger study focusing on a smaller dose range to more precisely determine the ED95.
In conclusion, our study investigated the optimal dose of remifentanil combined with propofol 2.5 mg·kg−1 to provide excellent LMA insertion conditions in 95% of healthy patients. Although the estimate of the ED95 value is not sufficiently precise to draw firm conclusions, we suggest using remifentanil 1.20 to 1.40 μg·kg−1 iv given over 60 sec and started 150 sec before insertion of the LMA when using propofol in healthy adult patients.
References
Brown GW, Patel N, Ellis FR. Comparison of propofol and thiopentone for laryngeal mask insertion. Anaesthesia 1991; 46: 771-2.
Scanlon P, Carey M, Power M, Kirby F. Patient response to laryngeal mask insertion after induction of anaesthesia with propofol or thiopentone. Can J Anaesth 1993; 40: 816-8.
Tanaka M, Nishikawa T. Propofol requirement for insertion of cuffed oropharyngeal airway versus laryngeal mask airway with and without fentanyl: a dose-finding study. Br J Anaesth 2003; 90: 14-20.
Hui JK, Critchley LA, Karmakar MK, Lam PK. Co-administration of alfentanil-propofol improves laryngeal mask airway insertion compared to fentanyl-propofol. Can J Anesth 2002; 49: 508-12.
Yu AL, Critchley LA, Lee A, Gin T. Alfentanil dosage when inserting the classic laryngeal mask airway. Anesthesiology 2006; 105: 684-8.
Kapila A, Glass PS, Jacobs JR, et al. Measured context-sensitive half-times of remifentanil and alfentanil. Anesthesiology 1995; 83: 968-75.
Lee MP, Kua JS, Chiu WK. The use of remifentanil to facilitate the insertion of the laryngeal mask airway. Anesth Analg 2001; 93: 359-62.
Grewal K, Samsoon G. Facilitation of laryngeal mask airway insertion: effects of remifentanil administered before induction with target-controlled propofol infusion. Anaesthesia 2001; 56: 897-901.
Kim MK, Lee JW, Jang DJ, Shin OY, Nam SB. Effect-site concentration of remifentanil for laryngeal mask airway insertion during target-controlled infusion of propofol. Anaesthesia 2009; 64: 136-40.
Pace NL, Stylianou MP. Advances in and limitations of up-and-down methodology: a precis of clinical use, study design, and dose estimation in anesthesia research. Anesthesiology 2007; 107: 144-52.
Mallampati SR, Gatt SP, Gugino LD, et al. A clinical sign to predict difficult tracheal intubation: a prospective study. Can Anaesth Soc J 1985; 32: 429-34.
Shiga T, Wajima Z, Inoue T, Sakamoto A. Predicting difficult intubation in apparently normal patients: a meta-analysis of bedside screening test performance. Anesthesiology 2005; 103: 429-37.
Bouvet L, Stoian A, Rimmele T, Allaouchiche B, Chassard D, Boselli E. Optimal remifentanil dosage for providing excellent intubating conditions when co-administered with a single standard dose of propofol. Anaesthesia 2009; 64: 719-26.
Brain AI, McGhee TD, McAteer EJ, Thomas A, Abu-Saad MA, Bushman JA. The laryngeal mask airway. Development and preliminary trials of a new type of airway. Anaesthesia 1985; 40: 356-61.
Nam JM. A simple approximation for calculating sample sizes for detecting linear trend in proportions. Biometrics 1987; 43: 701-5.
Shafer A, Sung ML, White PF. Pharmacokinetics and pharmacodynamics of alfentanil infusions during general anesthesia. Anesth Analg 1986; 65: 1021-8.
Glass PS, Gan TJ, Howell S. A review of the pharmacokinetics and pharmacodynamics of remifentanil. Anesth Analg 1999; 89: S7-14.
Minto CF, Schnider TW, Gregg KM, Henthorn TK, Shafer SL. Using the time of maximum effect site concentration to combine pharmacokinetics and pharmacodynamics. Anesthesiology 2003; 99: 324-33.
Billard V, Moulla F, Bourgain JL, Megnigbeto A, Stanski DR. Hemodynamic response to induction and intubation. Propofol/fentanyl interaction. Anesthesiology 1994; 81: 1384-93.
Hickey S, Cameron AE, Asbury AJ. Cardiovascular response to insertion of Brain’s laryngeal mask. Anaesthesia 1990; 45: 629-33.
Scheller MS, Zornow MH, Saidman LJ. Tracheal intubation without the use of muscle relaxants: a technique using propofol and varying doses of alfentanil. Anesth Analg 1992; 75: 788-93.
Thompson JP, Rowbotham DJ. Remifentanil–an opioid for the 21st century. Br J Anaesth 1996; 76: 341-3.
Stevens JB, Wheatley L. Tracheal intubation in ambulatory surgery patients: using remifentanil and propofol without muscle relaxant. Anesth Analg 1998; 86: 45-9.
Durmus M, Ender G, Kadir BA, Nurcin G, Erdogan O, Ersoy MO. Remifentanil with thiopental for tracheal intubation without muscle relaxants. Anesth Analg 2003; 96: 1336-9.
Sanford TJ Jr, Weinger MB, Smith NT, et al. Pretreatment with sedative-hypnotics, but not with nondepolarizing muscle relaxants, attenuates alfentanil-induced muscle rigidity. J Clin Anesth 1994; 6: 473-80.
Erhan E, Ugur G, Alper I, Gunusen I, Ozyar B. Tracheal intubation without muscle relaxants: remifentanil or alfentanil in combination with propofol. Eur J Anaesthesiol 2003; 20: 37-43.
Ciccone GK, Holdcroft A. Drugs and sex differences: a review of drugs relating to anaesthesia. Br J Anaesth 1999; 82: 255-65.
Olofsen E, Romberg R, Bijl H, et al. Alfentanil and placebo analgesia: no sex differences detected in models of experimental pain. Anesthesiology 2005; 103: 130-9.
Competing interests
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
Support was provided solely by institutional sources.
An erratum to this article can be found at http://dx.doi.org/10.1007/s12630-010-9326-0
Rights and permissions
About this article
Cite this article
Bouvet, L., Da-Col, X., Rimmelé, T. et al. Optimal remifentanil dose for laryngeal mask airway insertion when co-administered with a single standard dose of propofol. Can J Anesth/J Can Anesth 57, 222–229 (2010). https://doi.org/10.1007/s12630-009-9249-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-009-9249-9