Abstract
Purpose
There is evidence that cyclic adenosine monophosphate (cAMP) transduction is involved in nociceptive processing. We previously showed that intrathecal injection of an adenylate cyclase inhibitor attenuated tactile allodynia caused by partial sciatic nerve ligation (PSNL) in rats. The present study investigates the pre-emptive effects of spinal cAMP transduction on nociceptive processing in a chronic neuropathic pain model.
Methods
Intrathecal catheterization and PSNL were performed in male Sprague-Dawley rats. Nociceptive responses to mechanical and thermal stimuli were evaluated at the hindpaw at 2 hr and at 3, 7, and 14 days after PSNL. The pre-emptive effects of the intrathecal adenylate cyclase inhibitor, SQ22536 (0.7 μmol · L−1, 30 min before or after nerve ligation) were assessed. Also, the spatial and temporal expression profiles and immunoreactivity in the spinal cord of the cAMP response element binding protein (CREB) and its phosphorylated proteins (CREB-IR and p-CREB-IR) were analyzed.
Results
Compared with the rats treated with the vehicle, allodynia and hyperalgesia were significantly attenuated at 1–3 days by the intrathecal injection of SQ22536 performed either before or after ligation. The expression of CREB was significantly higher after ligation (P < 0.05), but differences were not observed between groups. Intrathecal injection of SQ22536, either before or after ligation, partially reduced p-CREB-IR protein expression in comparison with the vehicle control, especially after the first 3 days (P < 0.05).
Conclusion
Our results show a possible association between the increase in p-CREB and PSNL-induced neuropathic pain. However, a pre-emptive effect of adenylate cyclase inhibitor administered before surgery was not observed.
Résumé
Objectif
Des données soutiennent que la transduction de l’adénosine monophosphate cyclique (AMPc) est impliquée dans le traitement des signaux nociceptifs. Nous avons précédemment montré que l’injection intrathécale d’un inhibiteur de l’adénylcyclase atténuait l’allodynie tactile causée par une ligature partielle du nerf sciatique (PSNL) chez le rat. Cette étude explore les effets préventifs de la transduction d’AMPc rachidienne sur le traitement des signaux nociceptifs dans un modèle de douleur neuropathique chronique.
Méthode
Un cathétérisme intrathécal et une PSNL ont été réalisés sur des rats mâles Sprague-Dawley. Les réactions nociceptives aux stimuli mécaniques et thermiques ont été évaluées à la patte arrière à 2 heures et à 3, 7 et 14 jours post-PSNL. Les effets préventifs de l’inhibiteur intrathécal de l’adénylcyclase, le SQ22536, (0.7 μmol·L−1, 30 min avant ou après la ligature du nerf) ont été évalués. De plus, les profils d’expression spatiale et temporelle et l’immunoréactivité dans la moelle épinière de la protéine CREB (élément de réponse liant l’AMPc) et ses protéines phosphorylées (CREB-IR et p-CREB-IR) ont été analysés.
Résultats
Par rapport aux rats traités avec le véhicule, l’allodynie et l’hyperalgésie ont été atténuées de façon significative à 1 et 3 jours par l’injection intrathécale de SQ22536 réalisée avant ou après la ligature. L’expression de CREB était significativement plus élevée après la ligature (P < 0.05), mais aucune différence n’a été observée entre les groupes. L’injection intrathécale de SQ22536, réalisée avant ou après la ligature, a partiellement réduit l’expression de la protéine p-CREB-IR par rapport à l’injection témoin, particulièrement après les 3 premiers jours (P < 0.05).
Conclusion
Nos résultats montrent une association possible entre l’augmentation des p-CREB et la douleur neuropathique induite par PSNL. Toutefois, nous n’avons pas observé d’effet préventif de l’inhibiteur de l’adénylcyclase administré avant la chirurgie.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Compared with physiologic pain, transmission of pathologic pain signals evoked by extensive and intense tissue injury leads to sensitization of the peripheral and central pain pathways.1–3 Pre-emptive or preventive analgesia is a treatment that is initiated before the injury to reduce this sensitization.4,5 Recent studies have shown that pre-treatment, not post-treatment, with a tumour necrosis factor-α or a phospho-p38 antagonist attenuates mechanical allodynia induced by spinal nerve ligation.6,7 Attempts to produce pre-emptive analgesia have been made with opioids, non-steroidal anti-inflammatory drugs (NSAIDs), local anesthetics, and N-methyl-d-aspartic acid receptor antagonists.8–10 Other potentially beneficial agents are under investigation.
In previous reports, the role of the cyclic adenosine monophosphate (cAMP) transduction cascade nociceptive processing has been recognized. Gene transcription was induced through the activation of protein kinase A (PKA) with the subsequent phosphorylation of the transcription factor, cAMP response element-binding protein (CREB).11 The role of CREB is to mediate the effects of the activation of the cAMP pathway in the transcriptional regulation of a large number of proteins and peptides. The phosphorylation of CREB contributes to the gene transcription by allowing CREB to bind the CRE promoter, which is found in a number of pain-related genes, including c-fos, c-jun, somatostatin, and neurokinin 1 receptors.11 In addition, several lines of evidence showed an increase in CREB phosphorylation in the superficial dorsal horn of neurons following partial sciatic nerve ligation (PSNL).12 Recent evidence also supported the notion that intrathecal injection of CREB antisense oligonucleotide attenuated PSNL-induced tactile allodynia.13 According to our previous study, allodynia and hyperalgesia in the hindpaw, as well as CREB phosphorylation that was induced by PSNL, were only partially attenuated after postoperative injection of an adenylate cyclase inhibitor.14
Therefore, we addressed the hypothesis that preventing activation of the cAMP-CREB pathway before the pain stimulus rather than inhibiting its activity after injury would better prevent progression to chronic pain. This study was designed to assess the pre-emptive efficacy of the adenylate cyclase inhibitor, SQ22536, when administered before surgical incision in a chronic neuropathic pain model in rats.
Methods
The protocol for the study was approved by the Committee of Institutional Animal Care and Use. One hundred twenty-eight adult male Sprague-Dawley rats from the National Animal Centre (weighing 250–300 g) were used and housed in pairs. All animals were placed in a prone position and anesthetized with 1.5–2% isoflurane that was dispensed via a nose cone. After local sterilization, a small incision was made and a polyethylene catheter (PE-5, Becton, Dickinson and Company, Sparks, MD, USA) was inserted from the atlanto-occipital membrane for an approximate length of 8.5 cm. The external portion of the catheter was fixed at the back of the neck, and the wound was closed with sutures. The animals were allowed to recover in their cages. Before sciatic nerve ligation, animals with any signs of neurological deficits were excluded from the study. The function of the catheter was confirmed by an intrathecal injection of 2% lidocaine 10 μL, and the catheter was judged to be appropriately placed if loss of motor power in the lower extremities was observed.
One week after intrathecal catheterization, all rats were tested for baseline nociceptive responses to von Frey filaments as well as for radiant heat stimulation at the proximal part of the hindpaw, according to the method in our previous report.14 To minimize bias, one of the investigators (FCL) who was blinded to the group assignment performed all nociceptive tests in an isolated room. Withdrawal responses to mechanical stimulation were determined using a calibrated Electronic von Frey Anesthesiometer (Model 2290CE, IITC Life Science, Inc., Woodland Hills, CA, USA) that was applied to the distal portion of the plantar aspect of the hindpaw. Stimulation began at 0 g and continued until a withdrawal response was observed or a cut-off value (70 g) was reached. This procedure was repeated three times with at least a 5-min test-free period between trials. The average of the force was considered the withdrawal threshold and the conventional flexible von Frey filaments above and below it were tested to verify the withdrawal threshold. For heat stimulation, rats were placed individually on an elevated plastic mesh floor and were assessed using a focused radiant heat source (Model-33 Tail Flick Analgesia Meter, IITC Life Science Inc., CA, USA). The heat stimulus, a 50-W projector lamp with an aperture diameter of 6 mm, was applied from beneath a heat-tempered glass floor on the distal plantar portion of the hindpaw. The cut-off time was 15 sec to avoid tissue damage. Paw withdrawal latencies were measured to the nearest 0.1 sec. Three trials, 5–10 min apart, were used to obtain the average paw withdrawal latency.
All rats were re-anesthetized with isoflurane and then were randomly assigned into four different study groups (pre-ligation or post-ligation treatment with vehicle or SQ22536; n = 10 per group) and 4 different control groups (sham surgery with pre-ligation or post-ligation treatment with vehicle or SQ22536; n = 10 per group). An adenylate cyclase inhibitor, SQ22536, 0.7 μmol · L−1 dissolved and diluted with distilled water (Biomol International, LP, Plymouth Meeting, PA, USA) or the same volume of distilled water (vehicle) was administered intrathecally 30 min before or after nerve ligation. According to our previous study and other published research, the dosage of 0.7 μmol · L−1 was chosen for these experiments.14,15 The right sciatic nerve was exposed at a high level of the thigh, and half of the nerve was tightly ligated with 6-0 polydioxanone suture, as previously described by Seltzer et al. 16 Similar surgery was performed in the sham group exposing the sciatic nerve but without ligation. After a 2-hr recovery time, responses to the mechanical stimulus and radiant heat were retested. The responses to these stimuli were determined for the next 3, 7, and 14 days in all groups.
Different groups of animals (n = 4–5 per group) were sacrificed for immunohistochemical analysis at 2 hr and at 3, 7, and 14 days after nerve ligation. The rats were deeply anesthetized with intraperitoneal pentobarbital 100 mg · kg−1 and were perfused with cold saline followed by 4% intracardiac paraformaldehyde in phosphate buffer saline (PBS) 0.1 M, pH 7.4. The spinal cord segments were removed and fixed in the same fixative for 3–6 hr. The tissues were cryoprotected by transferring to 30% sucrose in PBS 0.1 M at 4°C for 24 hr. Subsequently, the spinal cord segments (L4–L5) were cut on a cryostat to a 20 μm thickness, and the free-floating sections were blocked with 3% normal goat serum (NGS) (Vector Laboratories, Burlingame, CA, USA) in 0.3% Triton X-100 and 0.3% hydrogen peroxide (H2O2) for 1 hr at room temperature. Sections were incubated for 36 hr at 4°C in a rabbit polyclonal anti-CREB antibody and anti-p-CREB antibody (1:1000) (New England Biolabs, Beverly, MA, USA) and were processed in biotinylated goat antirabbit IgG (1:200) using the Vectastain® Elite ABC kit (Vector Laboratories, Burlingame, CA, USA). Finally, the immunoprecipitates were developed with 0.05% diaminobenzidine in PBS for 5 min. Six to 8 of the L4–L5 spinal cord sections were randomly selected from each rat. Images of both ipsilateral and contralateral dorsal horns were captured at 250× magnification using a digital camera, Nikon® DXM 1200, with the Nikon® Eclipse E800 microscope (Nikon® Corporation, Tokyo, Japan). Anatomical landmarks in grey matter and standard anatomical drawings were used to identify the laminar borders. The numbers of digitized pixels overlaid onto the CREB-immunoreactive (CREB-IR) and p-CREB-IR cells in the superficial laminae of the dorsal horn were measured automatically using image analysis software, Image-Pro Plus® (Media Cybernetics®, Inc., Silver Spring, MD, USA).
Before the study, the sample size was determined using measures of variance from previous reports.12,14 Ten rats in each group would be adequate to provide an 80% power to detect a conservative alteration in nociceptive responses when the criterion for significance level (alpha) was set at 0.05. The results were averaged for each group and values were expressed as mean ± SD. Repeated-measures analysis of variance (ANOVA) tests were performed with group as a between-subjects factor and time after nerve ligation as a within-subjects factor. The Bonferroni test was examined post hoc for multiple comparisons at individual time points between groups. Statistical evaluation was performed with Prism® statistical software version 5.0 (GraphPad Software, Inc., San Diego, CA, USA). For all tests P < 0.05 was considered statistically significant.
Results
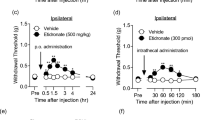
All animals were tested for baseline nociceptive response to von Frey filaments as well as for radiant heat stimulation before nerve ligation. Baseline values were not different between the groups. The response times to nociceptive stimuli of the ipsilateral hindpaws at the nerve lesion site were observed and compared with baseline values collected before nerve ligation (Fig. 1a, b). Compared with baseline, approximately 94% of the rats developed tactile allodynia and thermal hyperalgesia in the ipsilateral hindpaw for at least 14 days after PSNL.
Behavioural changes to thermal (a) and mechanical (b) stimulations following partial sciatic nerve ligation in rats, with intrathecal injection of cAMP inhibitor (SQ22536) given 30 min before ligation, 30 min after ligation, or vehicle. * P < 0.05 with respect to baseline. # P < 0.05 compared with vehicle group. Mean ± SD, n = 10 in each group
The response times of the ipsilateral hindpaws at the nerve lesion site to nociceptive stimuli were compared between groups, and the pre-emptive effects of intrathecal SQ22536 were assessed. Compared with the vehicle, the allodynia and hyperalgesia in the ipsilateral hindpaw were significantly attenuated at 1–3 days, whether the intrathecal injection of SQ22536 occurred before or after treatment (Fig. 1a, b). However, differences were not observed between the groups that received the intrathecal injection before and after PSNL.
As shown in Fig. 2a1–a4, immunoreactivity to the anti-CREB antibody was detected in the spinal cords of the animals receiving PSNL. The expression of CREB-IR in the spinal cords of normal and sham surgery animals was very weak, yet detectable. Increases in the CREB-IR cell expression profiles were found in the dorsal horn of the L4–L5 spinal cord, predominantly in the superficial layers after PSNL (Fig. 2a2). The mean pixel number of CREB-IR cells in all groups was significantly higher than that of the baseline before injury, whether the intrathecal injection contained vehicle or SQ22536 before or after ligation (P < 0.05, Fig. 3), but differences were not observed between groups.
Immunohistochemistry staining pattern of total cAMP response element-binding protein-immunoreactivity (CREB-IR) (a1–a4), and phosphorylated CREB-immunoreactivity (p-CREB-IR) (b1–b4) cells in the dorsal horn of L4–L5 spinal cord of rats. Vehicle: treatment with vehicle before nerve ligation (a2, b2); pre-SQ22536: treatment with intrathecal SQ22536 before nerve ligation (a3, b3); post-SQ22536: treatment with intrathecal SQ22536 after nerve ligated (a4, b4). Scale bar = 50 μm
Mean pixel number of total cAMP response element-binding protein-immunoreactivity (CREB-IR) cells in the dorsal horn with SQ22536 before (pre-SQ22536) or after (post-SQ22536) nerve ligation, or vehicle. * P < 0.05 compared with baseline. No significant difference in the total CREB-IR cell count was observed between SQ22536- and vehicle-treated rats. Mean ± SD, n = 8 in each group
The expression patterns of p-CREB-IR were also analyzed by immunohistochemistry. As shown in Figs. 2b1–b4 and 4, immunoreactivity to anti-p-CREB-IR was detected in the spinal cords of animals receiving PSNL, and lower levels of p-CREB-IR were observed in the control group. Therefore, animals that received PSNL demonstrated some upregulation of p-CREB-IR expression in the corresponding spinal cord segment. Both pre- and post-treatment intrathecal injection of SQ22536 partially reduced p-CREB-IR protein expression profiles, but this attenuation was not observed after injection of the vehicle in the ipsilateral dorsal horn (Fig. 4). Quantitatively, the mean pixel number after intrathecal injection of SQ22536 was significantly decreased in comparison with the vehicle control, especially after the first 3 days (P < 0.05, Fig. 4).
Discussion
This study failed to demonstrate a pre-emptive effect on neuropathic pain with adenylate cyclase inhibitor administered before surgery. According to previous reports, activation of the cAMP pathway in the spinal cord is implicated in the mediation of nociceptive processing.17,18 Several immediate-early genes, such as c-fos and c-jun, and some late effector genes, including dynorphin and substance P receptor, were subsequently induced through the phosphorylation of the transcription factor, CREB.11,19,20 Our previous study demonstrated that allodynia and hyperalgesia in the hindpaw, as well as CREB phosphorylation after PSNL, were only partially attenuated after postoperative injection of adenylate cyclase inhibitor.14 According to the concept of pre-emptive analgesia, we expected that inhibiting cAMP-CREB pathway activation before pain stimulus would prevent progression to chronic pain more effectively than inhibiting its activity after injury. Our results showed that CREB is inducibly expressed in the spinal cord, and the increase in the immunopositive signal for p-CREB observed after PSNL indicated that spinal CREB is phosphorylated as a result of peripheral stimulation. Whether adenylate cyclase inhibitor was administered before or after injury, CREB activation after PSNL showed a gradual reduction in strength resulting in an attenuation of pain behaviour in the first 3 days. However, the onset of pathologic pain was simply delayed, and adenylate cyclase inhibitor failed to produce a significant pre-emptive effect.
The definitions of pre-emptive analgesia are far from uniform. According to recent definitions,21,22 our results represent a pre-emptive effect only in a narrow sense, because intervention began before surgery and covered a relatively short period. This is not adequate to fit a broader interpretation of pre-emptive analgesia in which peripheral and central sensitization caused by nerve injury with incisional and inflammatory stimuli is prevented from being established. Several possibilities may explain the lack of significant preventive effect of adenylate cyclase inhibitor on chronic neuropathic pain in our study. First, we speculate that a single dose of intrathecal adenylate cyclase inhibitor lacks the analgesic effect needed to cover all of the components of persistent postoperative noxious inputs generated by tissue injury and inflammation. Pain can be divided by mechanism into nociceptive, inflammatory, and neurogenic pain.5 In addition to the incisional damage to the skin and various other tissues, the nociceptive barrage during surgery is followed by a protracted inflammatory state in the postoperative period, both of which may contribute to central sensitization. Surprisingly, the information regarding the effect of adenylate cyclase inhibition on the inflammatory response was scanty. Previous studies reported that the expression of phosphorylated CREB was involved in the temporal effects of hyperalgesia in inflammatory pain.23 For instance, an increase in the expression of p-CREB occurred in the hindpaw of rats after subcutaneous injection of carrageenan, formalin, or complete Freund’s adjuvant.19,23,24 Those rats who suffered from neuropathic pain demonstrated a similar phenomenon.12,25 In contrast, Miletic et al. reported that there were no differences in CREB content between control and study animals.25 In fact, the effect of adenylate cyclase inhibitor on inflammatory response remains a topic for investigation.
Second, the route of administration of the drug in our study may have been suboptimal. It is widely believed that spinally mediated hyperalgesia is a frequent component of the pain experience after tissue injury. This spinal sensitization reflects a cascade of events that is initiated in part by persistent sensory input generated by tissue injury and inflammation.1,26 However, the causes of neuropathic pain as a consequence of peripheral nerve injury are very complex, and many mechanisms involving both the peripheral and the central nervous systems are implicated.2,27–29 Central activation in the spinal cord as well as peripheral inflammation in neuropathic rats have been reported.30 Many studies report on postoperative noxious inputs, including those arising from the ectopic neural activity and inflammatory response.28,31,32 Previous studies have demonstrated that the analgesic effects of NSAIDs have been attributed to their peripheral anti-inflammatory actions, but the spinal effects of NSAIDs are not as well documented.10,33 Thus, compared with central neuroinflammation, peripheral immune responses to nerve injury may play a partial role in nerve-induced pain hypersensitivity. Crucial research on the adenylate cyclase inhibitor still needs to be conducted, including acquisition of dose–response data, minimum effective dose, pharmacokinetics, bioavailability, and its ability to penetrate the blood–brain barrier. In the present study, the analgesic effects of adenylate cyclase inhibitor are limited to their central actions because it was administered intrathecally.
A third possible explanation for the lack of pre-emptive effect of adenylate cyclase inhibitor in our study may be the existence of other signal transduction pathways aside from the cAMP pathway. In addition to the classical cAMP-PKA pathway, there are several intracellular messengers, such as calmodulin-dependent protein kinase (CaMK), nerve growth factor (NGF)-mediated Ras/Raf mitogen-activated protein kinase kinase-1/2 (MEK-1/2) and extracellular regulated kinase (ERK), c-Jun N-terminal kinase (JNK) and p38 MAPK, and other kinases that are activated through different upstream transduction chains and may contribute to the regulation of CREB phosphorylation.11,34 It has been shown that the ERK isoforms (ERK1/2) are phosphorylated in the spinal cord after peripheral inflammation and after PSNL.35,36 Inhibitory effects on formalin-induced flinching, comparable with those observed in a study using p38 MAPK inhibitors, were seen when an ERK inhibitor was given intrathecally.35 From this discussion, it is clear that neither the inhibition of ERK nor p38 MAPK completely abolished formalin-induced flinching, which may reflect a contribution of both the ERK and p38 MAPK pathways to pain behaviour.6,36 This means that the sole blockade of c-AMP activation in the spinal cord could not completely prevent CREB phosphorylation. Consequently, some persistent nociceptive input reaches the spinal cord, which may induce spinal activation.
In conclusion, this study failed to demonstrate a pre-emptive effect on neuropathic pain with adenylate cyclase inhibitor administered before injury. Our observation indicates that the relevant actions of cAMP in this model are partial and transient. Once the downstream targets are phosphorylated, the cascade of events leading to hyperalgesia is not reversed by spinal cAMP inhibition. However, the central and peripheral neural mechanisms leading to hyperalgesia are complex and not yet fully understood. Therefore, further studies are necessary to ascertain whether a combination of inhibitors of different pathways will completely prevent CREB phosphorylation as well as the most effective dosage, time frame, and route of administration, and whether blockade of c-AMP activation at the injured site would have additional efficacy in the prevention of neuropathic pain.
References
Yaksh TL. Regulation of spinal nociceptive processing: where we went when we wandered onto the path marked by the gate. Pain 1999; Suppl 6: S149–52.
Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science 2000; 288: 1765–9.
Zimmermann M, Herdegen T. Plasticity of the nervous system at the systematic, cellular and molecular levels: a mechanism of chronic pain and hyperalgesia. Prog Brain Res 1996; 110: 233–59.
Bromley L. Pre-emptive analgesia and protective premedication. What is the difference? Biomed Pharmacother 2006; 60: 336–40.
Dahl JB, Moiniche S. Pre-emptive analgesia. Br Med Bull 2004; 71: 13–27.
Svensson CI, Marsala M, Westerlund A, et al. Activation of p38 mitogen-activated protein kinase in spinal microglia is a critical link in inflammation-induced spinal pain processing. J Neurochem 2003; 86: 1534–44.
Schafers M, Svensson CI, Sommer C, Sorkin LS. Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J Neurosci 2003; 23: 2517–21.
Brill S, Gurman GM, Fisher A. A history of neuraxial administration of local analgesics and opioids. Eur J Anaesthesiol 2003; 20: 682–9.
McQuay HJ. Pre-emptive analgesia: a systematic review of clinical studies. Ann Med 1995; 27: 249–56.
Yaksh TL, Dirig DM, Malmberg AB. Mechanism of action of nonsteroidal anti-inflammatory drugs. Cancer Invest 1998; 16: 509–27.
Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron 2002; 35: 605–23.
Ma W, Quirion R. Increased phosphorylation of cyclic AMP response element-binding protein (CREB) in the superficial dorsal horn neurons following partial sciatic nerve ligation. Pain 2001; 93: 295–301.
Ma W, Hatzis C, Eisenach JC. Intrathecal injection of cAMP response element binding protein (CREB) antisense oligonucleotide attenuates tactile allodynia caused by partial sciatic nerve ligation. Brain Res 2003; 988: 97–104.
Liou JT, Liu FC, Hsin ST, Yang CY, Lui PW. Inhibition of the cyclic adenosine monophosphate pathway attenuates neuropathic pain and reduces phosphorylation of cyclic adenosine monophosphate response element-binding in the spinal cord after partial sciatic nerve ligation in rats. Anesth Analg 2007; 105: 1830–7.
Hoeger-Bement MK, Sluka KA. Phosphorylation of CREB and mechanical hyperalgesia is reversed by blockade of the cAMP pathway in a time-dependent manner after repeated intramuscular acid injections. J Neurosci 2003; 23: 5437–45.
Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain 1990; 43: 205–18.
Sluka KA. Stimulation of deep somatic tissue with capsaicin produces long-lasting mechanical allodynia and heat hypoalgesia that depends on early activation of the cAMP pathway. J Neurosci 2002; 22: 5687–93.
Sluka KA, Willis WD. The effects of G-protein and protein kinase inhibitors on the behavioral responses of rats to intradermal injection of capsaicin. Pain 1997; 71: 165–78.
Anderson LE, Seybold VS. Phosphorylated cAMP response element binding protein increases in neurokinin-1 receptor-immunoreactive neurons in rat spinal cord in response to formalin-induced nociception. Neurosci Lett 2000; 283: 29–32.
Sheng M, McFadden G, Greenberg ME. Membrane depolarization and calcium induce c-fos transcription via phosphorylation of transcription factor CREB. Neuron 1990; 4: 571–82.
Kissin I. Preemptive analgesia. Anesthesiology 2000; 93: 1138–43.
Gottschalk A, Ochroch EA. Preemptive analgesia: what do we do now? Anesthesiology 2003; 98: 280–1.
Ji RR, Rupp F. Phosphorylation of transcription factor CREB in rat spinal cord after formalin-induced hyperalgesia: relationship to c-fos induction. J Neurosci 1997; 17: 1776–85.
Wei F, Qiu CS, Kim SJ, et al. Genetic elimination of behavioral sensitization in mice lacking calmodulin-stimulated adenylyl cyclases. Neuron 2002; 36: 713–26.
Miletic G, Pankratz MT, Miletic V. Increases in the phosphorylation of cyclic AMP response element binding protein (CREB) and decreases in the content of calcineurin accompany thermal hyperalgesia following chronic constriction injury in rats. Pain 2002; 99: 493–500.
Yaksh TL, Hua XY, Kalcheva I, Nozaki-Taguchi N, Marsala M. The spinal biology in humans and animals of pain states generated by persistent small afferent input. Proc Natl Acad Sci USA 1999; 96: 7680–6.
Flor H. Phantom-limb pain: characteristics, causes, and treatment. Lancet Neurol 2002; 1: 182–9.
Willis WD. Role of neurotransmitters in sensitization of pain responses. Ann NY Acad Sci 2001; 933: 142–56.
Chen R, Cohen LG, Hallett M. Nervous system reorganization following injury. Neuroscience 2002; 111: 761–73.
DeLeo JA, Yezierski RP. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain 2001; 90: 1–6.
Myers RR, Campana WM, Shubayev VI. The role of neuroinflammation in neuropathic pain: mechanisms and therapeutic targets. Drug Discov Today 2006; 11: 8–20.
Moalem G, Tracey DJ. Immune and inflammatory mechanisms in neuropathic pain. Brain Res Rev 2006; 51: 240–64.
Katz J. Pre-emptive analgesia: importance of timing. Can J Anesth 2001; 48: 105–14.
Messersmith DJ, Kim DJ, Iadarola MJ. Transcription factor regulation of prodynorphin gene expression following rat hindpaw inflammation. Brain Res Mol Brain Res 1998; 53: 260–9.
Delghandi MP, Johannessen M, Moens U. The cAMP signalling pathway activates CREB through PKA p38 and MSK1 in NIH 3T3 cells. Cell Signal 2005; 17: 1343–51.
Ji RR, Baba H, Brenner GJ, Woolf CJ. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat Neurosci 1999; 2: 1114–9.
Acknowledgements
The authors thank Yeh Chiu-Ping (Department of Research, Buddhist Tzu Chi General Hospital Taipei Branch) for technical assistance and helpful suggestions.
Study funded by
This study was supported by a research grant (CMRPG 34028) to Jiin-Tarng Liou provided by Chang Gung Memorial Hospital and the Graduate Institute of Clinical Medical Sciences, Chang Gung University, Taoyuan, Taiwan.
Conflicts of interest
All authors declare no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liou, JT., Liu, FC., Mao, CC. et al. Adenylate cyclase inhibition attenuates neuropathic pain but lacks pre-emptive effects in rats. Can J Anesth/J Can Anesth 56, 763–769 (2009). https://doi.org/10.1007/s12630-009-9149-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-009-9149-z