Abstract
Purpose of the Review
The purpose of this review is to address the rising incidence of cerebral metastases in breast cancer patients, which is now estimated to affect 30–40% of advanced breast cancer (ABC) patients.
Recent Findings
Magnetic resonance imaging (MRI) remains the gold standard for brain metastases (BM) diagnosis, with follow-up scans recommended every 3 months. Treatment options for BM include neurosurgery, stereotactic radiosurgery (SRS), stereotactic fractionated radiation therapy (SFRT), or whole brain radiation therapy (WBRT), selected based on BM number, size, and location. Local therapies like SRS or neurosurgery are preferred for single or oligo metastases, while SRS or WBRT may be used for multiple BM. Concurrent systemic treatment tailored to tumor biology is crucial, particularly with recent advancements in HER2-positive patient management..
Summary
Symptomatic BM warrants local treatment alongside systemic therapy, considering patient condition and prognosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
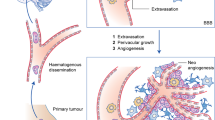
Breast cancer is the most frequent malignancy in women worldwide. Relative 5-year survival rate of patients suffering metastatic breast cancer is currently 30% [1]. The most common localization for metastases is bone, lung, and liver, but breast cancer also metastasizes in the brain [2]. Following lung cancer, breast cancer is the second most common cause for brain metastases (BM) [3].
BM can be asymptomatic or symptomatic, with neurological symptoms, such as headaches, vomiting, nausea or epileptic seizures [4]. Furthermore, motor deficits such as hemiparesis, hemisensory loss, personality changes, aphasia, visual disturbances or symptoms and other signs of raised intracranial pressure can occur [5]. The symptoms essentially depend on the location of the metastases and the size or number. The metastases are most often located at the cerebral hemispheres (75%), more rarely in the cerebellum (21%) or brain stem (3%) [5]. When BM occur, more than 50% of the patients already have multiple cerebral lesions [6]. The treatment and prognosis of BM has drastically changed in recent years. While only whole-brain radiation therapy (WBRT) and treatment with corticosteroids were standard in the 1970s, today the diagnostic and therapeutic options are manifold [7]. In the current review, we want to summarize the incidence, histological subtypes, as well as diagnostics, therapy options, and current research for breast cancer patients with BM.
Main Text
Incidence
The incidence of BM in breast cancer patients is increasing in recent years and it is currently assumed that nearly 30–40% of all advanced breast cancer patients will develop BM in the course of their disease [3, 8]. Whereas 7% of metastatic breast cancer patients already suffer of BM at diagnosis [9]. One possible explanation for the increasing number of BM might be the prolonged disease-free and overall survival in patients due to new drugs and more effective treatment strategies [10, 11]. On the other hand, increasing availability and quality of imaging (especially MRI), might lead to earlier discovery of BM [12].
Overall survival with BM was classified as extremely poor in former studies, with a median survival rate of 1 to 2 months if the patient was not treated at all [3]. The survival rate increased up to six months if the patient was treated accordingly [3]. Due to new medications and treatment strategies, survival rates increased depending on the source up to a median overall survival of 9 to 16 months [13,14,15].
A tool to estimate survival in patients with BM, was established with the diagnosis-specific Graded Prognostic Assessment (GPA) [13, 16]. The score predicts median overall survival dependent on tumor biology, Karnofsky performance status scale, age, disease burden, number of BM and extracranial metastasis in breast cancer patients, see Table 1 [13]. However, the score has also its limitations, as it does not consider the extracranial tumor burden, e.g., diffuse liver metastases. It just asks if extracranial metastases are present. In a validation of the GPA on the German Breast Cancer (BMBC) registry, it was further demonstrated that the GPA had low sensitivity in prediction of short-term OS (< 3 months), and long-term (> 12 months) OS [14].
Histological Subtypes
It is already known that the tumor subtype influences the prognosis of breast cancer patients and that some tumor subtypes are more likely to metastasize in the brain than others [8]. For example, hormone receptor (HR) positive tumors metastasize in the bone more frequently than in other organs, while HER2 positive and triple negative tumors are more likely to metastasize in lung, liver, or brain [17]. A 2021 meta-analysis of 41,958 patients proved, that especially HER2 positive patients and triple negative patients have a high incidence of BM with nearly one third of these subtypes developing BM (31% for HER2 positive patients and 32% of triple negative patients) [8]. In contrast, patients with HR positive, HER2 negative advanced breast cancer develop BM in only 15% of cases [8]. One possible explanation for the high incidence in HER2 positive patients might be the longer progression and overall survival due to antibody treatment strategies including trastuzumab and several others that changed the treatment landscape in the last years [18]. However, trastuzumab has a limited blood brain penetration and this might lead to the increase in BM in HER2 positive patients [18].
Another problem might be the receptor discordance between primary tumor and BM. Multiple studies reported receptor switch between primary tumor and BM in up to 20–35% [19,20,21,22]. Therefore, patients might not receive adequate therapy depending on histological subtype. A gain of hormone receptor was reported in up to 25%, and a gain in HER2 was reported in up to 13%, respectively [19]. Whereas a loss of hormone receptor occurred in 24%, and HER2 loss in 7% of patients [19]. However, not only a change in the subtype has been described, but new mutations at the molecular level have also been discovered in BM compared to the primary tumor [23]. This makes it even more difficult to find an effective therapy for the patients, as the tumor cells might have already developed various resistance mechanisms. However, with new detected mutations, there are also possible future drug targets [23].
Diagnostics
The gold standard for detecting and evaluating primary, as well as metastatic brain tumors is magnetic resonance imaging (MRI) with and without contrast agent administration carried out with at least 1.5 T-fold strength [5, 12]. There have been great advances in MRI diagnostics in recent years. However, challenges in the discrimination of metastases from primary brain tumors or infections, detection of small metastases or in discriminating treatment response from tumor recurrence and progression, remain [12]. At minimum, the diagnostic should include a cranial MRI with pre- and post-contrast T1-weighted, T2-weighted and/or T2-fluid-attenuated inversion recovery (FLAIR) and diffusion-weighted imaging (DWI) sequences [5]. Additional information can be achieved by magnetic resonance spectroscopy or functional MRI [24]. In case of contraindications for MRI, a cranial computed tomography (CT) or a positron emission tomography using [18F]-2-fluoro-2-deoxy-D-glucose (FDG–PET) can be performed [5]. Nevertheless, sensitivity of CTs and PET is reduced compared to MRI. As FDG uptake in normal brain tissue is high, detection of BM is more difficult [5]. With the use of new tracers, like amino acids, sensitivity of PET increased [25]. But still, PET is limited in the detection of small metastases- especially if they are smaller than one centimeter [25].
For follow-up diagnostic, a 3-month interval is recommended or at any clinically indicated time with progressive neurological symptoms [5]. For response and progression assessment the RANO group (Response Assessment in Neuro-Oncology Brain Metastases) has defined several criteria (see Table 2), based on the RECIST 1.1 criteria [26, 27]. Nevertheless, distinction between therapy-related changes and pseudo-progression, radionecrosis or tumor progression is not always clear [26].
Regarding the screening for BM in asymptomatic patients, no additional benefits have been demonstrated [28, 29]. Though early treatment of BM with radiotherapy led to a numeric decrease in brain metastases in asymptomatic patients, it did not prolong overall survival compared to symptomatic patients [28, 29]. Therefore, national, and international guidelines do not currently recommend performing cerebral imaging in asymptomatic patients in clinical routine [30, 31]. Nevertheless, a low threshold for MRI diagnostics is recommended in special subtypes, for example in HER2 positive patients [32]. Multiple prospective trials are currently underway to evaluate routine cerebral imaging in breast cancer patients and verify previous study results. For example, the randomized controlled trial from the Dana-Farber Cancer Institute (NCT04030507). The trial contains four cohorts: 1. Triple negative, 2. HR positive / HER2 negative, 3. HER2 positive and 4. inflammatory breast cancer patients. The cohorts 2 & 3 will be randomized to cerebral MRI yes/no, whereas cohort 1 & 4 will undergo cerebral MRI as a routine care [33]. Another trial is conducted by the Yonsei University (NCT03617341). Metastatic HER2 positive and triple negative patients are screened with cerebral MRI at time of diagnosis, as well as at time of first- and second-line treatment failure in order to detect BM before onset of symptoms [34].
In addition to neuroimaging, neurological symptoms and clinical assessment of the symptoms should be considered and checked during follow-ups. For this purpose, Neurologic Assessment in Neuro-Oncology (NANO) scale, evaluating 9 relevant neurologic domains (e.g., muscle strength, vision) can be used [35].
Prognostic Factors for OS with Cerebral Metastasis
In an analysis of 968 breast cancer patients with BM from the SEER (Surveillance, Epidemiology, and End Results) database of the National Cancer Institute in the United States, triple negative patients showed the worst survival rate (6 months) [15]. Whereas HR positive HER2 positive subtype showed the best median survival of 21 months [15]. Similar results have been demonstrated by another retrospective study. It showed worse median overall survival (7months) in triple negative patients compared to other tumor subtypes (16—26 months) [36]. Besides tumor biology, other identified prognostic factors for metastatic disease are high histological grade [37], size and location of extracerebral metastases [38, 39], as well as young age (diagnosis of metastatic breast cancer before 35/40 years) [39, 40] and short time from diagnosis of cancer to metastatic disease [39]. Furthermore, Ryberg et al. showed an elevated risk for BM, when lactate dehydrogenase (LDH) concentration in serum was above the upper normal limits before start of treatment [41]. Another important factor is the general condition of the patient. It could be shown that a Karnofsky Performance Status ≤ 60 was associated with a significantly poorer PFS, compared to a score > 60 [42•]. As a prognostic tool, the diagnosis-specific Graded Prognostic Assessment (GPA) was established, which takes these values into account (see Table 1) [13, 16].
Therapy Options
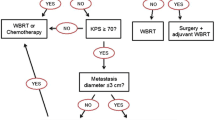
Treatment of brain metastasis is very complex and there are different options with surgery, stereotactic radiosurgery (SRS), stereotactic fractionated radiotherapy (SFRT), whole brain radiation therapy (WBRT), and systemic therapy, like chemotherapy, molecularly targeted therapeutics, and immunotherapy. Also, a combination of therapies is possible. Therefore, an interdisciplinary therapy recommendation should be made. Every patient should receive the best treatment depending on localization, size and number of BM, but also depending on previous illnesses and previous therapy lines [31]. Patients should be informed about the disease and the prognosis so that a participative decision can be made. Delay of neurological deterioration and prolonged survival with good quality of life are the main treatment goals [5]. In general, one or multiple local therapies (SRS, SFRT, surgery) should be offered to patients suffering from symptomatic brain metastases [9]. When disease progression occurs, change in systemic therapy is recommended as well. However, it can be omitted if it is the initial diagnosis of BM, extracranial disease is stable, and adequate local treatment of BM seems feasible [30]. Switching systemic therapy alone (without adding local therapy) is only an option for patients with HER2-positive, asymptomatic brain metastases and should be decided in an interdisciplinary tumor board [30].
Furthermore, as survival of patients with BM increased in recent years, patients should be encouraged for participation in clinical trials- especially if they have an expected survival of at least 50% for one year calculated by the GPA (see Table 1) [13].
Single and Oligo Metastases
The number and localization of BM has an important influence on therapy recommendation. Oligo metastases is defined as four or less BM or a cumulative tumor volume of < 15 ml in 5 to 10 BM, respectively [30]. The most common therapy approach for single, or oligo BM are SRS, SFRT or surgery. It has been shown that additional WBRT had an unnecessary negative impact on the neurocognitive function and quality of life, whereas overall survival did not increase [43, 44]. Therefore, additional WBRT is not recommended in patients suffering single or oligo BM (one to four metastases) [43]. The individual therapy options and their use depending on the size, number (single / oligo / multiple) and localization of metastases are presented separately below.
Surgery
Especially patients with controlled systemic disease may benefit of neurosurgical resection of single brain metastases [5, 9]. Regarding the extinct of resection, complete resection correlates with better local control and en bloc resection may result in lower recurrence rates [45, 46]. In patients with multiple BM, surgical resection might only be a choice if a large BM causes increased intracranial pressure or neurological symptoms with significant limitations for the patient [5]. Another indication for a surgical intervention might be big size (> 4cm), cystic or necrotic BM, since SRS does not work so well in these patients compared to solid tumors [45, 47]. Additional radiotherapy after neurosurgical resection is recommended as significant lower local recurrence rates were shown [48]. In these cases, stereotactic radiotherapy should be preferred as it showed a better cognitive function and similar overall survival compared to adjuvant WBRT after surgical resection [49, 50]. Another reason for neurosurgical treatment is obtaining new histology. For example, when a switch in tumor type or new mutations are suspected and systemic therapy can be adjusted accordingly [5].
Radiotherapy
Stereotactic radiosurgery (SRS) is the gold standard in today’s clinical practice [9, 51]. Using stereotactic and image guidance, the target accuracy is around one millimeter [5]. In most cases, single fraction technique using 15 to 24 Gy is applied [5]. Multiple fractions (27 Gy in three fractions or 30 Gy in five fractions) are not commonly used, only in patients with larger BM, pre-irradiation or lesions close to risk structures (e.g., brain stem) [5, 52]. SRS alone is normally recommended in oligo (one to four), unresected BM or after surgical resection in the surgical cavity [9, 53]. Furthermore, especially patients with BM smaller than 4cm seem to benefit of SRS compared to WBRT [54]. An analysis from Harvard medical school further showed, that re-treatment with WBRT or SRS after initial SRS is only necessary in 55% of patients and takes place after 6 months on average [55]. The authors therefore conclude that the initial treatment of oligo BM with SRS is very efficient as only 11% of patients died due to cerebral disease progression during the study period [55].
As alternative, WBRT (20–30 Gy in 5–10 fractions) can be used as a primary therapy especially in patients with multiple BM who are not eligible for SRS [5, 9]. Furthermore, WBRT still plays an important role in the treatment of leptomeningeal metastases [56]. In contrast, the use of post-operative WBRT after neurosurgical resection or after SRS should not be done routinely as no survival benefit could be seen [43, 44, 57, 58]. A randomized clinical trial in patients with oligo BM (1 to 3 metastases) showed, that additional WBRT after SRS compared to SRS alone had higher rates of cognitive deterioration while overall survival did not differ between the two groups [43].
While treatment recommendations with SRS for single and oligo BM are relatively “clear”, examination of SRS efficacy in patients with multiple BM (more than four BM) is subject of current research. Dana-Farber Cancer Institute carries out a phase III randomized trial, comparing WBRT with hippocampal sparing to stereotactic radiation in patients with 5–20 BM (NCT03075072) [59]. Quality of life and overall survival will be recorded [59]. The results are eagerly awaited as they may indicate a switch from standard therapy (WBRT) in patients with multiple BM (5–20 BM) to stereotactic radiation. A Japanese study (JLGK0901), already showed promising results and proved the non-inferiority of SRS compared to WBRT in patients with multiple BM [60].
If WBRT is indicated, hippocampal area should be avoided since cognitive function and patient-reported symptoms are improved compared to “classic” WBRT [53, 61]. Furthermore, it was previously shown that memantine, a NMDA-receptor antagonist, can lead to better cognitive function and delayed cognitive decline in patients undergoing WBRT [62]. In order to increase local tumor control, a simultaneous or sequential dose escalation to the metastases may also be performed alongside hippocampal sparing [63].
Nevertheless, the therapy decision should be made on an individual basis, considering all different aspects like size, number, and localization of the BM as well as the general condition and neurological symptoms of the patient [9]. Therefore, international guidelines recommend that no radiotherapy in asymptomatic patients should be carried out, when Karnofsky Performance Status is ≤ 50, or < 70 and no systemic therapy options are available for additional treatment [9].
Medication
The systemic therapy of BM seems challenging due to multiple factors. Since many patients only metastasize cerebrally at an advanced stage, it is possible that patients have already received several previous therapies [13]. Therefore, poor respond rates and drug resistance must be considered. Furthermore, the blood–brain and brain-tumor penetrability can be responsible for reduced drug efficacy in BM [64]. Since systemic treatment essentially depends on the tumor subtype, we would like to consider this separately below. Furthermore, Table 3 presents studies that have significantly changed the treatment of ABC patients with BM.
HER2 Positive
New advances in drug therapy were recently made in HER2 positive patients. Namely, the HER2Climb study which compared the combination of trastuzumab and capecitabine with or without tucatinib in HER2 positive, ABC patients [65, 66••, 67]. A total of 291 patients with stable and active brain metastases at baseline were enrolled in the study [65, 66••]. The combination showed an improved intracranial objective response rate, as well as improved progression free survival (5.7 months) and a longer median overall survival of 9.1 months [65, 66••].
Another promising HER2 antibody drug conjugate is trastuzumab deruxtecan (T-DXd). T-DXd showed promising results in HER2 positive, heavily pretreated, ABC patients with progression-free survival of 16.4 months (95% CI, 12.7 to not reached) [68]. Furthermore, T-DXd showed high intracranial response rate (73.3%) in TUXEDO-1 trial in stable asymptomatic brain metastases [69]. Nevertheless, in this phase 2 trial, only 15 patients were included [69]. In comparison of T-DXd to trastuzumab emtansine (T-DM1), patients treated with T-DXd had significantly better PFS [70•]. The study contained 114 patients with BM, see Table 3 [70•]. A retrospective study further showed a promising activity of T-DXd in active BM and leptomeningeal carcinomatosis [71]. An overall response rate of 64% in patients with asymptomatic BM, receiving T-DXd was seen in a systematic meta-analysis in 2022 [72].
Other drugs and drug combinations, like T-DM1 [73], lapatinib plus capecitabine [74], neratinib plus capecitabine [75], neratinib plus paclitaxel [76], were examined in ABC with BM. Unfortunately, these combinations were not able to show overwhelming results, for example T-DM1 only showed a best overall response rate of 21.4% in patients with BM [73]. Moreover, PATRICIA trial, a phase 2 study, assessed the combination of high dose trastuzumab with pertuzumab [77]. Patients had only a cerebral overall response rate of 11% [77].
Further research is needed, and new trials are already ongoing, as the DESTINY-B12 (NCT04739761), a multicenter, phase 3b/4 study analyzing the use of T-DXd in patients with ABC (with or without baseline BM) who were previously treated with more than 2 regimens for HER 2 positive ABC [78]. Moreover, HER2Climb-05 (NCT05132582), evaluating the combination of tucatinib or placebo with pertuzumab and trastuzumab [79]. The trial is recruiting HER2 positive advanced breast cancer (ABC) patients with or without asymptomatic BM [79]. Another phase II trial is planning to test the combination of tucatinib with T-DXd (NCT04539938) in patients with HER2 positive ABC and BM [80]. The results are eagerly awaited as they might contribute to new guideline recommendations.
HER2 Negative
Regarding the subtypes of triple negative and HR positive, HER2 negative patients, fewer clinical studies have been conducted and less therapeutic successes have been achieved compared to the group of HER2 positive patients. Furthermore, most studies have not been conducted exclusively on patients with BM. Next to “traditional” chemotherapy in combination with bevacizumab, a VEGF inhibitor (if applicable), targeted therapies, e.g., PARP inhibitors for BRCA positive patients or immunotherapy for PD-L1 positive tumors play an important role for the treatment of triple negative ABC.
For example, the EMBRACA study who led to the approval of talazoparib, a PARP inhibitor, included patients with brain metastases (see Table 3) [81]. Patients with HER2 negative ABC and BRCA mutation were randomized to talazoparib or standard therapy and the study showed a higher progression free survival (PFS) in the talazoparib group (8.6 versus 5.6 months) [81]. Regrading triple negative patients, immunotherapy has gained importance in recent years, with the approval of atezolizumab and pembrolizumab for treatment of ABC [82, 83]. Both approval studies contained patients with asymptomatic BM (pembrolizumab 27 patients, atezolizumab 61 patients) [82, 83]. Currently, Weill Medical College of Cornell University evaluates the use of pembrolizumab in combination with SRS in patients with BM (NCT03449238). Patients with 2–10 BM will be included and tumor response rate, as well as OS will be assessed [84].
Regarding HR positive, HER2 negative ABC patients, therapy with CDK4/6 inhibitors is the standard first-line therapy. The effectiveness and safety was demonstrated in the approval studies and in the real-world setting [85, 86]. Of the three available CDK4/6 inhibitors, only abemaciclib was tested in a phase II study in 58 patients with active BM or leptomeningeal disease [87]. Primary endpoint was not met, with a low intracranial objective response rate of 5.2% [87]. However, intracranial clinical benefit rate was 24% and therapeutic concentrations of Abemaciclib in brain metastases tissue was demonstrated [87]. Currently, a new trial examining the combination of Abemaciclib and SRS is recruiting patients (NCT04923542). Intracranial, as well as extracranial PFS and OS will be analyzed in patients with less than 15 BM, eligible for SRS [88].
After publication of DESTINY-Breast04 trial, T-DXd is a new option for HER2 low ABC patients (HER2 low is defined as a score of 1 + on immunohistochemical [IHC] analysis or as an IHC score of 2 + and negative results on in situ hybridization) [89]. This might be especially interesting for HER2 low ABC patients with BM, as Krabaji et al. found inhibition of tumor growth and longer survival rates due to T-DXd in HER2 low orthotopic PDX models of breast cancer brain metastases [90]. Clinical studies investigating T-DXd in HER2 low ABC patients with BM are already on the way, like the HER2 low cohorts of the DEBBRAH trial (NCT04420598) [91].
Symptomatic therapy
Symptomatic therapy, symptom-related interventions, as well as psychosocial and supportive care are one of the main components in the treatment of ABC patients with BM [31]. When symptoms of intracranial pressure occur, therapy with corticosteroids is recommended. By EANO/ESMO corticosteroids should be used in the lowest dose and in the shortest time possible while the use of anticonvulsants is limited to the occurrence of seizures and should not be used as routine treatment [5]. Radiation necrosis is a side effect that can occur after radiotherapy. Application of bevacizumab in these patients as a symptomatic therapy is part of current phase I studies in different tumor entities [92, 93]. Results are promising and showing improved neurological symptoms compared to placebo, but further research is needed before bevacizumab can be incorporated as a routine care in these patients [92, 93].
Conclusion
To date, it has not been demonstrated that screening for BM in asymptomatic patients is beneficial [28, 29]. Though early treatment of BM with radiotherapy led to a numeric decrease in brain metastases in asymptomatic patients but did not prolong overall survival compared to symptomatic patients [28, 29]. Therefore, it is currently not recommended to perform cerebral imaging in asymptomatic patients [30, 31]. Nevertheless, especially triple negative and HER2 positive patients are at risk to develop BM, so the threshold to perform cerebral imaging should be low [32]. New studies are currently evaluating this clinical practice.
Therapy of BM consists of local and systemic treatment. Treatment decisions should always consider the patients’ general condition, as well as Karnofsky Performance Status and should be made by an interdisciplinary tumor board, considering the patients prognosis [31]. When symptomatic BM occur, local treatment is recommended [9]. For single or oligo BM, surgery or SRS are the treatment of choice, depending on localization and size of metastases [9]. When multiple BM occur, until now, WBRT with hippocampal sparing is mostly used. However, SRS is on the rise with new randomized controlled trials evaluating the safety and outcome of SRS in patients with multiple BM and showing promising results [59, 60]. Depending on the location and number of BM, SRS can therefore be a possible alternative for the treatment of multiple BM and could potentially replace WBRT in some categories of patients in the next few years.
Regarding systemic therapy, especially advances in HER2 positive tumors were achieved in recent years. The combination of trastuzumab, capecitabine and tucatinib showed the efficacy of a systemic therapy in active BM in breast cancer patients [65, 66••]. Furthermore, intracranial response rate of T-DXd seems to be very promising [69].
Abbreviations
- BM:
-
Brain metastases
- HR:
-
Hormone receptor
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- HER2:
-
Human epidermal growth factor 2
- ABC:
-
Advanced breast cancer
- T-DXd:
-
Trastuzumab deruxtecan
- TDM-1:
-
Trastuzumab emtansine
- PFS:
-
Progression free survival
- GPA:
-
Graded prognostic assessment
- MRI:
-
Magnetic resonance imaging
- CT:
-
Computed tomography
- PET:
-
Positron emission tomography
- SRS:
-
Stereotactic radiosurgery
- SRT:
-
Stereotactic radiotherapy
- WBRT:
-
Whole brain radiation therapy
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
American Cancer Society. Breast cancer survival rates by stage. https://www.cancer.org/cancer/types/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-survival-rates.html. Accessed 5/16/2024
Kim MY. Breast cancer metastasis. Adv Exp Med Biol. 2021;1187:183-204e.
Fidler IJ. The biology of brain metastasis. Cancer J. 2015;21(4):284–93.
Rostami R, Mittal S, Rostami P, Tavassoli F, Jabbari B. Brain metastasis in breast cancer: a comprehensive literature review. J Neurooncol. 2016;127(3):407–14.
Le Rhun E, Guckenberger M, Smits M, Dummer R, Bachelot T, Sahm F, et al. EANO–ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with brain metastasis from solid tumours. Ann Oncol. 2021;32(11):1332–47.
Wolpert F, Lareida A, Terziev R, Grossenbacher B, Neidert MC, Roth P, et al. Risk factors for the development of epilepsy in patients with brain metastases. Neuro Oncol. 2020;22(5):718–28.
Black P. Brain metastasis. Neurosurgery. 1979;5(5):617–31.
Kuksis M, Gao Y, Tran W, Hoey C, Kiss A, Komorowski AS, et al. The incidence of brain metastases among patients with metastatic breast cancer: a systematic review and meta-analysis. Neuro Oncol. 2021;23(6):894–904.
Vogelbaum MA, Brown PD, Messersmith H, Brastianos PK, Burri S, Cahill D, et al. Treatment for brain metastases: ASCO-SNO-ASTRO guideline. J Clin Oncol. 2022;40(5):492–516.
Miglietta F, Bottosso M, Griguolo G, Dieci MV, Guarneri V. Major advancements in metastatic breast cancer treatment: when expanding options means prolonging survival. ESMO Open. 2022;7(2):100409.
Meegdes M, Geurts SME, Erdkamp FLG, Dercksen MW, Vriens BEPJ, Aaldering KNA, et al. Real-world time trends in overall survival, treatments and patient characteristics in HR+/HER2− metastatic breast cancer: an observational study of the SONABRE Registry. Lancet Reg Health - Europe. 2023;26:100573.
Pope WB. Brain metastases: neuroimaging. Handb Clin Neurol. 2018;149: 89–112 .
Sperduto PW, Mesko S, Li J, Cagney D, Aizer A, Lin NU, et al. Survival in patients with brain metastases: summary report on the updated diagnosis-specific graded prognostic assessment and definition of the eligibility quotient. J Clin Oncol. 2020;38(32):3773–84.
Riecke K, Müller V, Weide R, Schmidt M, Park-Simon T-W, Möbus V, et al. Predicting prognosis of breast cancer patients with brain metastases in the BMBC registry—comparison of three different GPA prognostic scores. Cancers (Basel). 2021;13(4):844.
Martin AM, Cagney DN, Catalano PJ, Warren LE, Bellon JR, Punglia RS, et al. Brain metastases in newly diagnosed breast cancer. JAMA Oncol. 2017;3(8):1069.
Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419–25.
Soni A, Ren Z, Hameed O, Chanda D, Morgan CJ, Siegal GP, et al. Breast cancer subtypes predispose the site of distant metastases. Am J Clin Pathol. 2015;143(4):471–8.
Swain SM, Baselga J, Kim S-B, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372(8):724–34.
Sperduto PW, Mesko S, Li J, Cagney D, Aizer A, Lin NU, et al. Estrogen/progesterone receptor and HER2 discordance between primary tumor and brain metastases in breast cancer and its effect on treatment and survival. Neuro Oncol. 2020;22(9):1359–67.
Kaidar-Person O, Meattini I, Jain P, Bult P, Simone N, Kindts I, et al. Discrepancies between biomarkers of primary breast cancer and subsequent brain metastases: an international multicenter study. Breast Cancer Res Treat. 2018;167(2):479–83.
Timmer M, Werner JM, Röhn G, Ortmann M, Blau T, Cramer C, et al. Discordance and conversion rates of progesterone-, estrogen-, and HER2/neu-receptor status in primary breast cancer and brain metastasis mainly triggered by hormone therapy. Anticancer Res. 2017;37(9):4859–65.
Hulsbergen AFC, Claes A, Kavouridis VK, Ansaripour A, Nogarede C, Hughes ME, et al. Subtype switching in breast cancer brain metastases: a multicenter analysis. Neuro Oncol. 2020;22(8):1173–81.
Morgan AJ, Giannoudis A, Palmieri C. The genomic landscape of breast cancer brain metastases: a systematic review. Lancet Oncol. 2021;22(1):e7-17.
Mohammadi M, Mohammadi S, Hadizadeh H, Olfati M, Moradi F, Tanzifi G, et al. Brain metastases from breast cancer using magnetic resonance imaging: a systematic review. J Med Radiat Sci. 2023. https://doi.org/10.1002/jmrs.715.
Galldiks N, Langen K-J, Albert NL, Chamberlain M, Soffietti R, Kim MM, et al. PET imaging in patients with brain metastasis—report of the RANO/PET group. Neuro Oncol. 2019;21(5):585–95.
Lin NU, Lee EQ, Aoyama H, Barani IJ, Barboriak DP, Baumert BG, et al. Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol. 2015;16(6):e270–8.
Schwartz LH, Seymour L, Litière S, Ford R, Gwyther S, Mandrekar S, et al. RECIST 1.1 – Standardisation and disease-specific adaptations: Perspectives from the RECIST Working Group. Eur J Cancer. 2016;62:138–45.
Niwińska A, Tacikowska M, Murawska M. The Effect of early detection of occult brain metastases in HER2-positive breast cancer patients on survival and cause of death. Int J Radiat Oncol Biol Phys. 2010;77(4):1134–9.
Kaplan MA, Inal A, Kucukoner M, Urakci Z, Ekici F, Firat U, et al. Cranial magnetic resonance imaging in the staging of HER2-positive breast cancer patients. Oncol Res Treat. 2013;36(4):176–81.
AGO Mamma (04/15/2023), https://www.ago-online.de, CNS-metastases
Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, André F, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol. 2020;31(12):1623–49.
Ramakrishna N, Anders CK, Lin NU, Morikawa A, Temin S, Chandarlapaty S, et al. Management of advanced human epidermal growth factor receptor 2–positive breast cancer and brain metastases: ASCO guideline update. J Clin Oncol. 2022;40(23):2636–55.
https://classic.clinicaltrials.gov/ct2/show/NCT04030507. Screening Magnetic Resonance Imaging of the Brain in Patients With Breast Cancer [Internet]. last assessed 9-5-2023
https://classic.clinicaltrials.gov/ct2/show/study/NCT03617341. Brain Monitoring for High Risk of Brain Metastases in Metastatic Breast Cancer [Internet]. last assessed 9-5-2023
Nayak L, DeAngelis LM, Brandes AA, Peereboom DM, Galanis E, Lin NU, et al. The Neurologic Assessment in Neuro-Oncology (NANO) scale: a tool to assess neurologic function for integration into the Response Assessment in Neuro-Oncology (RANO) criteria. Neuro Oncol. 2017;19(5):625–35.
Cho E, Rubinstein L, Stevenson P, Gooley T, Philips M, Halasz LM, et al. The use of stereotactic radiosurgery for brain metastases from breast cancer: who benefits most? Breast Cancer Res Treat. 2015;149(3):743–9.
Chow L, Suen D, Ma KK, Kwong A. Identifying risk factors for brain metastasis in breast cancer patients: implication for a vigorous surveillance program. Asian J Surg. 2015;38(4):220–3.
Slimane K, Andre F, Delaloge S, Dunant A, Perez A, Grenier J, et al. Risk factors for brain relapse in patients with metastatic breast cancer. Ann Oncol. 2004;15(11):1640–4.
Pestalozzi BC, Zahrieh D, Price KN, Holmberg SB, Lindtner J, Collins J, et al. Identifying breast cancer patients at risk for Central Nervous System (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG). Ann Oncol. 2006;17(6):935–44.
Evans AJ, James JJ, Cornford EJ, Chan SY, Burrell HC, Pinder SE, et al. Brain metastases from breast cancer: identification of a high-risk group. Clin Oncol. 2004;16(5):345–9.
Ryberg M, Nielsen D, Osterlind K, Andersen PK, Skovsgaard T, Dombernowsky P. Predictors of central nervous system metastasis in patients with metastatic breast cancer. A competing risk analysis of 579 patients treated with epirubicin-based chemotherapy. Breast Cancer Res Treat. 2005;91(3):217–25.
• Freeman M, Ennis M, Jerzak KJ. Karnofsky Performance Status (KPS) ≤60 is strongly associated with shorter brain-specific progression-free survival among patients with metastatic breast cancer with brain metastases. Front Oncol. 2022;12. https://doi.org/10.3389/fonc.2022.867462. General patient condition plays an important factor in outcome: It could be shown that a Karnofsky Performance Status ≤60 was associated with a significantly poorer PFS, compared to a score >60.
Brown PD, Jaeckle K, Ballman KV, Farace E, Cerhan JH, Anderson SK, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases. JAMA. 2016;316(4):401–9.
Kocher M, Soffietti R, Abacioglu U, Villà S, Fauchon F, Baumert BG, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J Clin Oncol. 2011;29(2):134–41.
Patel AJ, Suki D, Hatiboglu MA, Rao VY, Fox BD, Sawaya R. Impact of surgical methodology on the complication rate and functional outcome of patients with a single brain metastasis. J Neurosurg. 2015;122(5):1132–43.
Kamp MA, Rapp M, Slotty PJ, Turowski B, Sadat H, Smuga M, et al. Incidence of local in-brain progression after supramarginal resection of cerebral metastases. Acta Neurochir (Wien). 2015;157(6):905–11.
Jung T-Y, Kim I-Y, Jung S, Jang W-Y, Moon K-S, Park S-J, et al. Alternative treatment of stereotactic cyst aspiration and radiosurgery for cystic brain metastases. Stereotact Funct Neurosurg. 2014;92(4):234–41.
Mahajan A, Ahmed S, McAleer MF, Weinberg JS, Li J, Brown P, et al. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(8):1040–8.
Brown PD, Ballman KV, Cerhan JH, Anderson SK, Carrero XW, Whitton AC, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(8):1049–60.
Kayama T, Sato S, Sakurada K, Mizusawa J, Nishikawa R, Narita Y, et al. Effects of surgery with salvage stereotactic radiosurgery versus surgery with whole-brain radiation therapy in patients with one to four brain metastases (JCOG0504): a phase III, noninferiority, randomized controlled trial. J Clin Oncol. 2018;36(33):3282–9.
Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. The Lancet. 2004;363(9422):1665–72.
Lehrer EJ, Peterson JL, Zaorsky NG, Brown PD, Sahgal A, Chiang VL, et al. Single versus multifraction stereotactic radiosurgery for large brain metastases: an international meta-analysis of 24 trials. Int J Radiat Onc Biol Phys. 2019;103(3):618–30.
Gondi V, Bauman G, Bradfield L, Burri SH, Cabrera AR, Cunningham DA, et al. Radiation therapy for brain metastases: an ASTRO clinical practice guideline. Pract Radiat Oncol. 2022;12(4):265–82.
Halasz LM, Uno H, Hughes M, D’Amico T, Dexter EU, Edge SB, et al. Comparative effectiveness of stereotactic radiosurgery versus whole-brain radiation therapy for patients with brain metastases from breast or non-small cell lung cancer. Cancer. 2016;122(13):2091–100.
Liu Y, Alexander BM, Chen Y-H, Horvath MC, Aizer AA, Claus EB, et al. Salvage whole brain radiotherapy or stereotactic radiosurgery after initial stereotactic radiosurgery for 1–4 brain metastases. J Neurooncol. 2015;124(3):429–37.
Le Rhun E, Weller M, Brandsma D, Van den Bent M, de Azambuja E, Henriksson R, et al. EANO–ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with leptomeningeal metastasis from solid tumours. Ann Oncol. 2017;28:iv84-99.
Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases. JAMA. 2006;295(21):2483.
Soon YY, Tham IWK, Lim KH, Koh WY, Lu JJ. Surgery or radiosurgery plus whole brain radiotherapy versus surgery or radiosurgery alone for brain metastases. Cochrane Database Syst Rev. 2014;2016(9). https://doi.org/10.1002/14651858.CD009454.pub2
Hippocampal Sparing Whole Brain Radiation Versus Stereotactic Radiation in Patients With 5-20 Brain Metastases: A Phase III RT (NCT03075072). https://clinicaltrials.gov/study/NCT03075072?cond=Breast%20Cancer%20Brain%20Metastases&term=randomized&aggFilters=status:not%20rec&rank=2. last assessed 9-13-2023
Yamamoto M, Serizawa T, Shuto T, Akabane A, Higuchi Y, Kawagishi J, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15(4):387–95.
Brown PD, Gondi V, Pugh S, Tome WA, Wefel JS, Armstrong TS, et al. Hippocampal avoidance during whole-brain radiotherapy plus memantine for patients with brain metastases: phase III trial NRG oncology CC001. J Clin Oncol. 2020;38(10):1019–29.
Brown PD, Pugh S, Laack NN, Wefel JS, Khuntia D, Meyers C, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15(10):1429–37.
Grosu A-L, Frings L, Bentsalo I, Oehlke O, Brenner F, Bilger A, et al. Whole-brain irradiation with hippocampal sparing and dose escalation on metastases: neurocognitive testing and biological imaging (HIPPORAD) – a phase II prospective randomized multicenter trial (NOA-14, ARO 2015–3, DKTK-ROG). BMC Cancer. 2020;20(1):532.
Lockman PR, Mittapalli RK, Taskar KS, Rudraraju V, Gril B, Bohn KA, et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res. 2010;16(23):5664–78.
Murthy RK, Loi S, Okines A, Paplomata E, Hamilton E, Hurvitz SA, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020;382(7):597–609.
•• Lin NU, Murthy RK, Abramson V, Anders C, Bachelot T, Bedard PL, et al. Tucatinib vs Placebo, Both in Combination With Trastuzumab and Capecitabine, for Previously Treated ERBB2 (HER2)-Positive Metastatic Breast Cancer in Patients With Brain Metastases. JAMA Oncol. 2023;9(2):197. The combination showed an improved intracranial objective response rate, as well as improved progression free survival (5.7 months) and a longer median overall survival of 9.1 month.
Lin NU, Borges V, Anders C, Murthy RK, Paplomata E, Hamilton E, et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. J Clin Oncol. 2020;38(23):2610–9.
Modi S, Saura C, Yamashita T, Park YH, Kim S-B, Tamura K, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382(7):610–21.
Bartsch R, Berghoff AS, Furtner J, Marhold M, Bergen ES, Roider-Schur S, et al. Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: a single-arm, phase 2 trial. Nat Med. 2022;28(9):1840–7.
• Cortés J, Kim S-B, Chung W-P, Im S-A, Park YH, Hegg R, et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. New England Journal of Medicine. 2022 Mar;386(12):1143–54. In comparison of T-DXd to trastuzumab emtansine (T-DM1), patients treated with T-DXd had significantly better PFS (the study contained 114 patients with BM).
Yamanaka T, Niikura N, Nomura H, Kusama H, Yamamoto M, Matsuura K, et al. Abstract PD7–01: tastuzumab deruxtecan for the treatment of patients with HER2-positive breast cancer with brain and/or leptomeningeal metastases: a multicenter retrospective study (ROSET-BM study). Cancer Res. 2023;83(5 Supplement):PD7-01-PD7-01.
Werter IM, Remmelzwaal S, Burchell GL, de Gruijl TD, Konings IR, van der Vliet HJ, et al. Systemic therapy for patients with HER2-positive breast cancer and brain metastases: a systematic review and meta-analysis. Cancers (Basel). 2022;14(22):5612.
Montemurro F, Delaloge S, Barrios CH, Wuerstlein R, Anton A, Brain E, et al. Trastuzumab emtansine (T-DM1) in patients with HER2-positive metastatic breast cancer and brain metastases: exploratory final analysis of cohort 1 from KAMILLA, a single-arm phase IIIb clinical trial☆. Ann Oncol. 2020;31(10):1350–8.
Bachelot T, Romieu G, Campone M, Diéras V, Cropet C, Dalenc F, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 2013;14(1):64–71.
Saura C, Oliveira M, Feng Y-H, Dai M-S, Chen S-W, Hurvitz SA, et al. neratinib plus capecitabine versus lapatinib plus capecitabine in HER2-positive metastatic breast cancer previously treated with ≥ 2 HER2-directed regimens: phase III NALA trial. J Clin Oncol. 2020;38(27):3138–49.
Awada A, Colomer R, Inoue K, Bondarenko I, Badwe RA, Demetriou G, et al. Neratinib plus paclitaxel vs trastuzumab plus paclitaxel in previously untreated metastatic ERBB2-positive breast cancer. JAMA Oncol. 2016;2(12):1557.
Lin NU, Pegram M, Sahebjam S, Ibrahim N, Fung A, Cheng A, et al. Pertuzumab plus high-dose trastuzumab in patients with progressive brain metastases and HER2-positive metastatic breast cancer: primary analysis of a phase II study. J Clin Oncol. 2021;39(24):2667–75.
A Study of T-DXd in Participants With or Without Brain Metastasis Who Have Previously Treated Advanced or Metastatic HER2 Positive Breast Cancer (DESTINY-B12) (NCT04739761). https://classic.clinicaltrials.gov/ct2/show/NCT04739761?term=NCT04739761&draw=2&rank=1 [Internet]. last assessed 9-13-2023
A Study of Tucatinib or Placebo With Trastuzumab and Pertuzumab for Metastatic HER2+ Breast Cancer (HER2CLIMB-05) (NCT05132582). https://clinicaltrials.gov/study/NCT05132582 [Internet]. last assessed 9-13-2023
A Study of Tucatinib Plus Trastuzumab Deruxtecan in HER2+ Breast Cancer (HER2CLIMB-04) (NCT04539938). https://clinicaltrials.gov/study/NCT04539938?cond=trastuzumab%20deruxtecan&rank=6 [Internet]. last assessed 9-13-2023
Litton JK, Rugo HS, Ettl J, Hurvitz SA, Gonçalves A, Lee K-H, et al. Talazoparib in patients with advanced breast cancer and a Germline BRCA Mutation. N Engl J Med. 2018;379(8):753–63.
Cortes J, Rugo HS, Cescon DW, Im S-A, Yusof MM, Gallardo C, et al. Pembrolizumab plus Chemotherapy in Advanced Triple-Negative Breast Cancer. N Engl J Med. 2022;387(3):217–26.
Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–21.
Pembrolizumab And Stereotactic Radiosurgery (Srs) Of Selected Brain Metastases In Breast Cancer Patients (NCT03449238). https://classic.clinicaltrials.gov/ct2/show/NCT03449238?cond=brain+metastases+breast+cancer&draw=4&rank=22 [Internet]. las assessed 9-13-2023
Müller C, Kiver V, Solomayer EF, Wagenpfeil G, Neeb C, Blohmer JU, et al. CDK4/6 inhibitors in advanced HR+/HER2-breast cancer: a multicenter real-world data analysis. Breast Care. 2023;18(1):31–41.
De Laurentiis M, Borstnar S, Campone M, Warner E, Bofill JS, Jacot W, et al. Full population results from the core phase of CompLEEment-1, a phase 3b study of ribociclib plus letrozole as first-line therapy for advanced breast cancer in an expanded population. Breast Cancer Res Treat. 2021;189(3):689–99.
Tolaney SM, Sahebjam S, Le Rhun E, Bachelot T, Kabos P, Awada A, et al. A phase II study of abemaciclib in patients with brain metastases secondary to hormone receptor-positive breast cancer. Clin Cancer Res. 2020;26(20):5310–9.
Stereotactic Radiation & Abemaciclib in the Management of HR+/HER2- Breast Cancer Brain Metastases (NCT04923542). https://classic.clinicaltrials.gov/ct2/show/NCT04923542?cond=brain+metastases+breast+cancer&draw=4&rank=24 [Internet]. last assessed 9-13-2023
Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387(1):9–20.
Kabraji S, Ni J, Sammons S, Li T, Van Swearingen AED, Wang Y, et al. Preclinical and clinical efficacy of trastuzumab deruxtecan in breast cancer brain metastases. Clin Cancer Res. 2023;29(1):174–82.
Trastuzumab deruxtecan in patients with central nervous system involvement from HER2-positive breast cancer: The DEBBRAH trial (NCT04420598). https://classic.clinicaltrials.gov/ct2/show/NCT04420598. last assessed 9/13/2023
Levin VA, Bidaut L, Hou P, Kumar AJ, Wefel JS, Bekele BN, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79(5):1487–95.
Khan M, Zhao Z, Arooj S, Liao G. Bevacizumab for radiation necrosis following radiotherapy of brain metastatic disease: a systematic review & meta-analysis. BMC Cancer. 2021;21(1):167.
Funding
Open Access funding enabled and organized by Projekt DEAL. CM is supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation, grant ID MU 4812/2–1:2).
Author information
Authors and Affiliations
Contributions
All authors made a substantial contribution to the concept, design, and interpretation of the current literature to the article. CM wrote the first draft of the manuscript. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Müller, C., Schmidt, G., Solomayer, EF. et al. Cerebral Metastases in Breast Cancer Patients: a Narrative Review. Curr Breast Cancer Rep (2024). https://doi.org/10.1007/s12609-024-00558-x
Accepted:
Published:
DOI: https://doi.org/10.1007/s12609-024-00558-x